Adaptation is one of the longest-studied phenomena in perception and neuroscience. Adaptation generally results in negative perceptual aftereffects: after prolonged exposure to a specifc feature, perception of a neutral stimulus is biased in the opposite direction [1,2]. A recent paper in Current Biology[3] challenged this view by proposing that, additionally, adaptation biases perception in the same direction as features observed over a relatively long time from the past. This fnding challenges dominant theories of visual adaptation; however, here we argue that long-term positive correlations are not due to neural or perceptual processes but arise due to short-term negative aftereffects. Thus, existing models of adaptation remain unchallenged, and critical evaluations of how adaptation could predictively aid perception are still needed.
Chopin and Mamassian [3] presented observers with binocularly rivalrous oriented gratings within series of non-rivalrous gratings [4]. Their analysis correlates observers' responses with stimuli presented in windows of different durations and at different time points (lags) in the past (their Figure 2). Perception of rivalrous gratings was biased opposite to previously shown non-rivalrous gratings. In addition to this negative aftereffect, observers' responses were positively correlated with stimuli from a ‘reference window’ in the past. The same held true in a tilt-aftereffect experiment [5].
However, short-term negative aftereffects alone account for this pattern of correlations. We simulated an artificial observer whose responses were determined by a noisy short-term negative aftereffect (see Supplemental Information). Performing the same analysis, we found significant positive correlations for large window durations and time lags (Figure 1A), similar to results in human observers (Figure 2 in [3]). Different parameters for aftereffect length and noise yielded similar results (Supplemental Figure S1). Positive correlations arise because of an interaction between the short-term negative aftereffect and random fluctuations in the stimulus sequence. Any random sequence will exhibit fluctuations in the proportion of left or right stimuli. Because of the short-term aftereffect, responses are correlated negatively with the stimuli in recent history and thus show similar fluctuations in counterphase. Increasing the time lag of stimulus windows amounts to shifting the timecourse relative to observers' responses. Because of the fluctuations in both timecourses, some phase shifts will necessarily lead to positive correlations (Figure 1B). Indeed, because averaging of long stimulus windows reduces the number of statistically independent samples, mathematical considerations predict more positive than negative correlations (see Supplemental Information).
Figure 1.
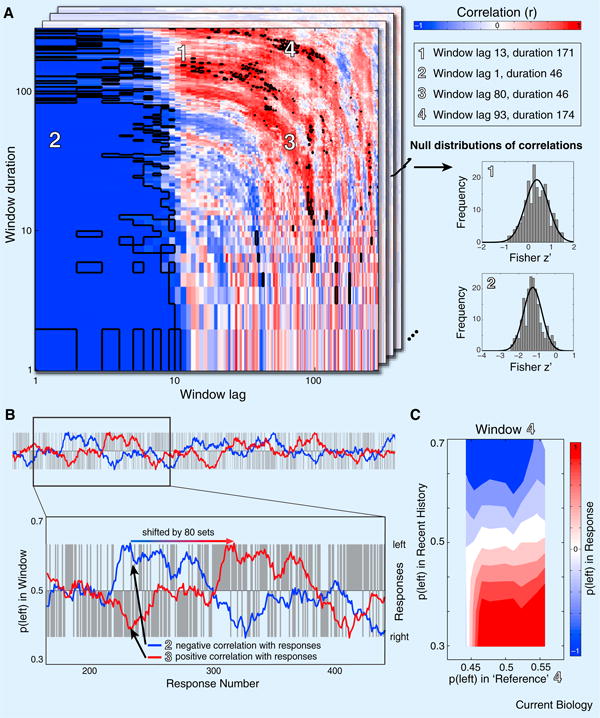
A short-term negative aftereffect can produce seeming capture of responses by long-term stimulus history. (A) Correlations between responses of simulated observers and stimulus windows of varying durations and lags lead to remarkably similar results as human observers in [3]. Signifcant correlations are indicated by black outlines. Repeating this simulation many times generates a null distribution for correlations expected from just a negative aftereffect (shown for two example windows on the right; also see Supplemental Figure S2). (B) Simulated timecourses of responses (gray bars) and stimuli in a sliding average window (blue, 2 in A). Because of the short-term aftereffect, responses are negatively correlated with the averaged stimulus timecourse. Increasing the window's lag (red, 3 in A) relative to the responses can turn these negative correlations to positive. (C) Simulated responses as a function of stimuli in the recent history and a selected ‘reference window’ (4 in A). A positive relationship between reference window and responses (as shown for a selected window in Figure 3 of [3]) can occur by chance due to noise in the observer.
Demonstrating a genuine long-term positive aftereffect necessitates statistically comparing empirical correlations with a null distribution generated by assuming just a short-term aftereffect (rather than that no correlations exist). We generated such null distributions by repeating our simulation many times on random sequences and found positive correlations for large window durations and lags, all of which resulted from only the short-term negative aftereffect (Figures 1A and Supplemental Figure S2).
To explain the long-term assimilative effect, Chopin and Mamassian [3] proposed a model of how recent stimulus history and a long-term ‘reference’ window are taken into account in perceptual decision-making. This model predicts effects of both recent history and reference on observers' responses. For a selected reference window they showed such effects (their Figur;e 3). But the same analysis on simulated data revealed that similar interactions could occur by chance (Figure 1C), even though recent history and reference window do not independently infuence responses in our simulations. Again, additional infuence of long-term history – beyond that of short-term history – should be assessed by comparison to null distributions from simulations.
More consideration is needed regarding the proposal that long-term positive aftereffects could serve a ‘predictive’ purpose. Chopin and Mamassian [3] write: “Implicit predictions are based on the assumption that the distributions of orientations should match between recent history and the remote reference” (p. 625). This ‘gambler's fallacy’ model, however, assumes that the proportion of observable orientations in the world is static and unchanging over the period in question (empirically ~13 minutes for the stimuli in [3]). Considering the dynamic properties of the natural world, one could reasonably argue that the best predictions for the state of the world are based on its current or very recent state, not a remote past reference. Physical auto-correlations, by defnition, are strongest at short timescales. To overcome internal perturbations in the perceptual system, there is no reason to believe that an estimate from ~10 minutes ago is any more reliable or less biased than one based on more recent evidence.
Our simulations show that human perception and behavior can exhibit deceptive long-term temporal structure. While negative aftereffects in both rivalry [4] and tilt [5] are well established, the long-term assimilative effects in [3] and our simulation are spurious. Previous models of visual adaptation, including error correction, decorrelation, or Bayesian inference processes [1,2], can easily accommodate the apparent assimilative structure; they need no modifcation or new parameters.
Supplementary Material
Figure S1 Simulation results for a range of parameters of the artificial observer model, all based on the same pseudo-random stimulus sequence.
The observed pattern of correlations occurs consistently for a wide range of artificial observers with varying aftereffect lengths and noise weights in the integration process. Generally, longer aftereffects cause more negative correlations for windows with increasingly longer lags. Regardless of the aftereffect length, however, positive correlations occur for long window durations and long lags. The bottom row shows the same analysis for an artificial observer with a leaky integrator type aftereffect (see Supplemental Experimental Procedures).
Figure S2 Simulation results for different stimulus sequences.
A) Results for 9 iterations of the simulation with different pseudo-random stimulus sequences for an artificial observer with aftereffect length 36 and noise weight 0.33 (as in Figure 1A). Each sequence consists of the same number of stimuli that were presented to single observers in Chopin and Mamassian's study. The short-term aftereffect (blue) occurs consistently (because it is implemented in the artificial observer). Positive correlations occur for variable window lags and durations.
B) Mean correlations results of 200 iterations as in A. On average, a simulated observer with only a short-term aftereffect still produces responses that are positively correlated for long window durations and lags.
C) Null distribution of correlation coefficients for some selected window durations and lags. Distributions of r values (and Fisher z' values, which are normally distributed) are positively biased for large window durations and lags. To statistically assess whether correlations of human observers' responses with long-term stimulus history are higher than expected from just a short-term aftereffect, one should compare empirical correlations at each window position to corresponding null distributions, like those in panel C.
Acknowledgments
We thank A. Chopin, R. Denison, J. Fischer, S. Haroz, P. Mamassian, and T. Sweeny for thought-provoking discussions. Supported in part by grants NIH EY007043 (W.C.), NSF 1106400 (A.L.), and NIH EY018216 (D.W.).
Footnotes
Supplemental Information: Supplemental Information includes details of experimental procedures and two figures, and can be found with this article online at http://dx.doi.org/10.1016/j.cub.2013.03.024.
References
- 1.Clifford CWG, Webster MA, Stanley GB, Stocker AA, Kohn A, Sharpee TO, Schwartz O. Visual adaptation: neural, psychological and computational aspects. Vision Res. 2007;47:3125–3131. doi: 10.1016/j.visres.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 2.Kohn A. Visual adaptation: physiology, mechanisms, and functional benefts. J Neurophysiol. 2007;97:3155–3164. doi: 10.1152/jn.00086.2007. [DOI] [PubMed] [Google Scholar]
- 3.Chopin A, Mamassian P. Predictive properties of visual adaptation. Curr Biol. 2012;22:622–626. doi: 10.1016/j.cub.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 4.Blake R, Overton R. The site of binocular rivalry suppression. Perception. 1979;8:143–152. doi: 10.1068/p080143. [DOI] [PubMed] [Google Scholar]
- 5.Gibson JJ, Radner M. Adaptation, after-effect and contrast in the perception of tilted lines I Quantitative studies. J Exp Psychol. 1937;20:453–467. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Simulation results for a range of parameters of the artificial observer model, all based on the same pseudo-random stimulus sequence.
The observed pattern of correlations occurs consistently for a wide range of artificial observers with varying aftereffect lengths and noise weights in the integration process. Generally, longer aftereffects cause more negative correlations for windows with increasingly longer lags. Regardless of the aftereffect length, however, positive correlations occur for long window durations and long lags. The bottom row shows the same analysis for an artificial observer with a leaky integrator type aftereffect (see Supplemental Experimental Procedures).
Figure S2 Simulation results for different stimulus sequences.
A) Results for 9 iterations of the simulation with different pseudo-random stimulus sequences for an artificial observer with aftereffect length 36 and noise weight 0.33 (as in Figure 1A). Each sequence consists of the same number of stimuli that were presented to single observers in Chopin and Mamassian's study. The short-term aftereffect (blue) occurs consistently (because it is implemented in the artificial observer). Positive correlations occur for variable window lags and durations.
B) Mean correlations results of 200 iterations as in A. On average, a simulated observer with only a short-term aftereffect still produces responses that are positively correlated for long window durations and lags.
C) Null distribution of correlation coefficients for some selected window durations and lags. Distributions of r values (and Fisher z' values, which are normally distributed) are positively biased for large window durations and lags. To statistically assess whether correlations of human observers' responses with long-term stimulus history are higher than expected from just a short-term aftereffect, one should compare empirical correlations at each window position to corresponding null distributions, like those in panel C.


