Abstract
Objective
The purpose of this case report is to describe a therapeutic intervention for peroneal nerve paralysis involving the sciatic nerve.
Clinical features
A 24-year-old man presented with peroneal nerve paralysis with decreased sensation, severe pain in the popliteal fossa, and steppage gait, which occurred 3 days prior to the consultation. Magnetic resonance imaging and electromyography confirmed lumbar disk herniation with sciatic common peroneal nerve entrapment in the popliteal fossa.
Intervention and outcome
A combined treatment protocol of spinal and fibular head manipulation and neurodynamic mobilization including soft tissue work of the psoas and hamstring muscles was performed. Outcome measures were assessed at pretreatment, 1 week posttreatment, and 3-month follow-up and included numeric pain rating scale, range of motion, pressure pain threshold, and manual muscle testing. Treatment interventions were applied for 3 sessions over a period of 1 week. Results showed reduction of the patient’s subjective pain and considerable improvement in range of motion, strength, and sensation in his left foot, which was restored to full function.
Conclusion
A combined program of spinal and fibular head manipulation and neurodynamic mobilization reduced pain, increased range of motion and strength, and restored full function to the left leg in this patient who had severe functional impairment related to a compressed left common peroneal nerve.
Key indexing terms: Manual therapy, Peroneal neuropathies, Sciatic nerve
Introduction
Drop foot can arise from various musculoskeletal or neurologic etiologic processes. Leg pain, sensory loss, and weakness in ankle dorsiflexion comprise a symptom complex that is most frequently caused by degenerative disk disease of the lumbar spine.1 The condition involves the muscles of foot dorsiflexion (tibialis anterior, extensor hallucis longus, and extensor digitorum longus) and the nerves that supply them, primarily the common peroneal nerve. Common causes of drop foot include compartment syndrome, diabetes, stroke, lumbar disk protrusions, musculoskeletal compression, myopathies, neuropathies, and peripheral nerve injuries2-4 and corresponding steppage gait—also known as drop-foot gait.5 Peroneal nerve paralysis (PNP) is the most common mononeuropathy in the lower limb, and it is vulnerable to damage around the fibular head because of the anatomical position between the peroneal nerve and fibula.6 Hence, a lesion of the common peroneal nerve would result in foot drop.
The purpose of the current study is to describe the combination of care using spinal and fibular head manipulation for PNP with neurodynamic mobilization (NM).
Case report
A 24-year-old man presented with signs of PNP, decreased sensation, and motor weakness in the lateral aspect of his left leg and foot resulting in a steppage gait. The symptoms began 3 days prior to the consultation. Clinical examination no revealed a low back pain. His medical history revealed a single episode of sciatic pain and subsequent magnetic resonance imaging (MRI) indicating a lumbar disk herniation in the previous 4 months. Results of laboratory blood tests were unremarkable for metabolic, inflammatory, or infectious joint disease.
The patient’s subjective reports indicated increased severity of symptoms associated with his occupational tasks as an electrician, particularly kneeling for long periods. Pretreatment examination for manual muscle testing of the left tibialis anterior, extensor hallucis longus, extensor digitorum longus, and peroneus muscles revealed muscle strength at the level of 1/5, 2/5, 2/5, and 1/5, respectively. Diminished sensation in the first toe web space, an area of tenderness over the region of the fibular head, and a positive Tinel sign near the head of the fibula on the left leg were also detected during the examination. No mass lesion was palpable. Pain was located in the region of the fibular head and was described as a “constant achy feeling,” with occasional “sharp” pain with specific movements, and graded at 7 out of 10 on a numeric pain scale (NPS). Electromyography (EMG) was used to confirm the diagnosis2 the previous day in the hospital by the patient’s medical physician (neurologist). The EMG revealed a left common peroneal neuropathy below the branch leading to the short head of the biceps femoris muscle, which predominantly affected the common peroneal nerve.
The NPS was used to measure the patient’s subjective pain,7 and the Hospital Anxiety and Depression Scale was used to capture psychosocial adjustment.8,9 Pressure pain threshold (PPT) at the fibular head was measured by algometry, which has previously been shown to be a valid and reliable measure, with higher PPT values indicating less severe sensitivity.10,11 Manual muscle testing was performed to assess strength and based on the analysis of physical impairment assessment standard from the Guides to the Evaluation of Permanent Impairment.12
The above outcome measures were performed at pretreatment, 1 week posttreatment and 3-month follow-up. Before initiation of treatment, the patient was advised about the potential benefits of physiotherapy treatment as well as its potential adverse effects (ie, decreased sensation); and informed consent was obtained and documented.
During the 3 intervention sessions over the course of 1 week, the patient received spinal and fibular head manipulation and NM.
Method of application of manipulation
Lumbar manipulation in supine position
A long-lever rotary spinal manipulation technique was used with the patient positioned in a lateral recumbent or side-lying position with the superior or free hip and knee flexed and adducted across the midline. During the procedure, the clinician stabilized the participant’s free leg with his own leg while holding the participant’s superior shoulder; and the manipulative force was applied with the clinician’s forearm resting on the pelvis. The rotatory thrust on the pelvis was directed at a localized lumbar segment (L3-4) and was delivered by a quick, short, controlled movement of the shoulder and arm combined with a slight body drop. The manipulation force applied was localized to the dysfunctional vertebral segment using alignments of force vectors secondary to participant positioning (Fig 1).
Fig 1.
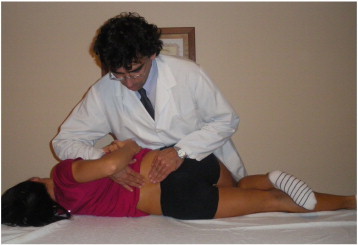
Schematic illustration of the high-velocity, low-amplitude spinal manipulation technique.
Fibular head manipulation
The patient was positioned on the unaffected side, and the left knee is bent 90° with a slight external rotation. The clinician placed the left hand on the posterior fibular head, with the other hand on the anterior part of the lower end of the fibula. A rotatory thrust was then applied in the opposite direction (Fig 2).13 Following this, with the patient in a supine position, muscle energy techniques for the psoas and hamstring muscles and the posterior fibular head were applied.5 The total time required for the combined treatment techniques lasted approximately 10 minutes.
Fig 2.
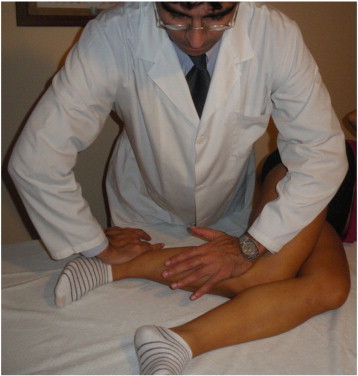
Fibular head manipulation.
Method of application of NM
The NM technique consisted of a sliding mobilization of the sciatic nerve. Neurodynamic mobilization treatment was commenced the day after the first examination and continued for 3 sessions in the week. At each session, the NM techniques were applied to the involved lower limb 3 times for a 4-minute period with a 1-minute pause between each application. The NM of the sciatic nerve consisted of the following movements: (1) proximal slider, a straight leg raise and application of plantar-flexion without producing symptoms (Fig 3A) and (2) distal slider; during the phase in which the leg is flexed, the foot is dorsiflexed (Fig 3B).14,15 These motions were alternated at a rate of approximately 2 seconds per cycle (1 second into extension and 1 second into flexion),as described by Butler16 in 2005.
Fig 3.
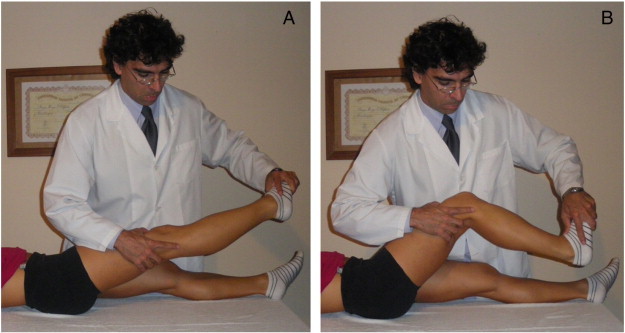
A, Proximal slider, a straight leg raise and application of plantar-flexion without producing symptoms. B, Distal slider; during the phase in which the leg is flexed, the foot is dorsiflexed.
Outcomes
The patient maintained full treatment compliance throughout the course of the study, with no reported adverse effects.
The treatment produced clinical improvements in patient pain and function, as measured at 1 week posttreatment. The NRS pain report decreased by 1 point during the treatment, a clinically significant effect,17 which was maintained at follow-up.
All affected lower limb muscles were retested at completion of treatment and registered 5/5 strength on manual muscle testing with the notable exception of the tibialis anterior (measured at 4/5 strength), and this improvement was maintained at 3-month follow-up.
Pressure pain threshold increased from 1.85 to 5.75 kg/cm2 at the completion of treatment, which was maintained at 3-month follow-up, with PPT levels measured at 5.9 kg/cm2.
The patient returned for follow-up at 3 months. Evaluations were repeated, and subjective questionnaire was completed. The patient reported that he had recovered full function and was pain free; and objective measurements of range of motion, strength, and sensation of his left foot indicated full motor and sensory recovery in the distribution of the previously affected left common peroneal nerve.
Outcomes are summarized in Table 1. The patient provided written consent for his health information to be published in this study.
Table 1.
Outcome measures
| Pretreatment right | Pretreatment left | Posttreatment left | Follow-up left | |
|---|---|---|---|---|
| HADS | ||||
| DS | 2 | 2 | 1 | |
| AS | 2 | 2 | 1 | |
| NPS | ||||
| 0 | 7 | 1 | 1 | |
| PPT (kg/cm2) | ||||
| Fibular head | 6.1 | 1.85 | 5.75 | 5.9 |
| Manual muscle testing | ||||
| Tibialis anterior | 5 | 1 | 4 | 4 |
| Extensor hallucis longus | 5 | 2 | 5 | 5 |
| Extensor digitorum longus | 5 | 2 | 5 | 5 |
| Peroneus muscles | 5 | 1 | 5 | 5 |
AS, anxiety; DS, depression; HADS, Hospital Anxiety and Depression Scale; NPS, numeric pain scale; PPT, pressure pain threshold.
Discussion
A cause of foot drop may be from a lumbar herniated disk that produces damage to the nerve that runs down the lower limb into the foot. This pathology may induce pain to nerves beyond the sciatic nerve, including the peroneal nerve. Thus, aiming treatment to the lumbar region using a very gentle side-posture manipulation may be effective for this type of condition.
Neural mobilization is a technique adopted to treat unfavorable neurodynamic conditions. This technique is theoretically aimed at reducing physical “pressure” on nerves.18 It is thought that NM assists with facilitation of relative motions between nerves and adjacent tissues, decreases nerve adherence, assists with diffusion of noxious liquids, and improves neural vascularity.19 Previous reports in literature show that this therapy may help in lumbar spine and lower limb conditions,19,20 and these results are confirmed in this work.
The positive results of this case report align with previous studies showing improvement in motor effects and reduction in pain produced by neural mobilization in the upper and lower limb; however, in this case, a more dramatic and immediate response may have been attributable to combined NM and manipulation.11,21-23
According to Nee and Butler (2006), cited in Boyd et al,24 the criteria for interpretation of neurodynamic testing include whether the test (a) reproduces the symptoms, (b) identifies a significant deviation from normal, or (c) produces alterations in the symptoms by adding in a distal joint movement, also called sensitizing movements.24 For example, muscle EMG activity, location of symptoms, and hip motions are altered when performing 2 sensitizing versions of the straight leg raise maneuver, assisting clinicians to detect meaningful clinical differences in relation to tension in the neural structures.24,25
It has been postulated that NM may produce its effects by increasing axoplasmic flow, improving intraneural swelling, and reducing pressure and inflammation in neural tissue.26,27 In addition, previous studies have demonstrated that neural provocation tests produce statistically significant increases of muscle activity compared to resting levels in the upper and lower limb.24,28
Neural mobilization is currently being used to reduce spasticity in individuals suffering from neurological disorders29; for example, NM can reduce EMG activity in the biceps brachii muscle in patients with stroke.30
The results of this case also align with previous studies showing improvement in motor effects via EMG activity and reduction in pain produced by neural mobilization in the upper limb.11,30
Examinations, especially the EMG, showed the presence of a severe left common peroneal neuropathy below the branch leading to the short head of the biceps femoris muscle, which predominantly affected the common peroneal nerve. This neuropathy, as mentioned, was associated to a medical history revealing a single episode of sciatic pain and MRI indicating a lumbar disk herniation.
To explain the mechanisms by which these effects are produced, it has been hypothesized that neural mobilization has an impact on motor performance due to improved axoplasmic flow, neural connective tissue elasticity, and reduced sensitivity of the nervous system.31
Limitations
The motor and analgesic benefits of combined NM and manipulation for PNP reported in this study cannot be generalized to other patients. Additional limitations of this study are the lack of numerical quantification of muscle strength and impairment of lower limb function pre- and postintervention. As well, no follow-up EMG or MRI was performed; so it is not certain that these findings would have been changed. Moreover, as with any case report, the findings may have been due to several factors, including the natural history of the condition, concurrent life events, and other treatment modalities used by the patient in the same period.
Future studies using larger sample sizes and blinded randomization are required to further enhance our understanding on the motor and sensory effects of NM and manipulation in PNP.
Conclusion
This case illustrates the potential benefits of manipulation combined with neural mobilization on muscle strength and pain for those with PNP.
Funding sources and potential conflicts of interest
No funding sources or conflicts of interest were reported for this study.
Contributor Information
Jorge Hugo Villafañe, Email: mail@villafane.ita.
Paolo Pillastrini, Email: Paolo.pillastrini@unibo.it.
Alberto Borboni, Email: alberto.borboni@ing.unibs.itd.
References
- 1.Tehli O., Celikmez R.C., Birgili B., Solmaz I., Celik E. Pure peroneal intraneural ganglion cyst ascending along the sciatic nerve. Turk Neurosurg. 2011;21(2):254–258. doi: 10.5137/1019-5149.JTN.2660-09.1. [DOI] [PubMed] [Google Scholar]
- 2.Masakado Y., Kawakami M., Suzuki K., Abe L., Ota T., Kimura A. Clinical neurophysiology in the diagnosis of peroneal nerve palsy. Keio J Med. 2008;57(2):84–89. doi: 10.2302/kjm.57.84. [DOI] [PubMed] [Google Scholar]
- 3.Oh S.J., Demirci M., Dajani B., Melo A.C., Claussen G.C. Distal sensory nerve conduction of the superficial peroneal nerve: new method and its clinical application. Muscle Nerve. 2001;24(5):689–694. doi: 10.1002/mus.1056. [DOI] [PubMed] [Google Scholar]
- 4.Stewart J.D. Foot drop: where, why and what to do? Pract Neurol. 2008;8(3):158–169. doi: 10.1136/jnnp.2008.149393. [DOI] [PubMed] [Google Scholar]
- 5.Lavelle J.M., McKeigue M.E. Musculoskeletal dysfunction and drop foot: diagnosis and management using osteopathic manipulative medicine. J Am Osteopath Assoc. 2009;109(12):648–650. [PubMed] [Google Scholar]
- 6.Kim Y.C., Jung T.D. Peroneal neuropathy after tibio-fibular fracture. Ann Rehabil Med. 2011;35(5):648–657. doi: 10.5535/arm.2011.35.5.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jensen M.P., Karoly P., Braver S. The measurement of clinical pain intensity: a comparison of six methods. Pain. 1986;27(1):117–126. doi: 10.1016/0304-3959(86)90228-9. [DOI] [PubMed] [Google Scholar]
- 8.Mykletun A., Stordal E., Dahl A.A. Hospital Anxiety and Depression (HAD) scale: factor structure, item analyses and internal consistency in a large population. Br J Psychiatry. 2001;179:540–544. doi: 10.1192/bjp.179.6.540. [DOI] [PubMed] [Google Scholar]
- 9.Snaith R.P. The hospital anxiety and depression scale. Health Qual Life Outcomes. 2003;1:29. doi: 10.1186/1477-7525-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischer A.A. Pressure algometry over normal muscles. Standard values, validity and reproducibility of pressure threshold. Pain. 1987;30(1):115–126. doi: 10.1016/0304-3959(87)90089-3. [DOI] [PubMed] [Google Scholar]
- 11.Villafañe J.H., Silva G.B. Short-term effects of neurodynamic mobilization in 15 patients with secondary thumb carpometacarpal osteoarthritis. J Manipulative Physiol Ther. 2011;34:449–456. doi: 10.1016/j.jmpt.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 12.Hislop H., Montgomery J. Daniels and Worthingham’s muscle testing: techniques of manual examination. 6th ed. WB Saunders; Philadelphia: 1995. [Google Scholar]
- 13.Ricard F., Sallé J.L. UTET; Turin, Italy: 2003. Trattamento di Osteopatia. [Google Scholar]
- 14.Butler D., Jones M. Ed. Churchill Livingstone; New York, USA: 1991. Mobilisation of the nervous system. [Google Scholar]
- 15.Shacklock M. Ed. Elsevier; Adelaide City, Australia: 2005. Clinical neurodynamics. [Google Scholar]
- 16.Butler D. Noigroup Publications; Adelaide City West, Australia: 2005. The neurodynamic techniques. [Google Scholar]
- 17.Salaffi F., Stancati A., Silvestri C.A., Ciapetti A., Grassi W. Minimal clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scale. Eur J Pain. 2004;8(4):283–291. doi: 10.1016/j.ejpain.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Shacklock M. Neural mobilization: a systematic review of randomized controlled trials with an analysis of therapeutic efficacy. J Man Manip Ther. 2008;16(1):23–24. doi: 10.1179/106698108790818602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scrimshaw S.V., Maher C.G. Randomized controlled trial of neural mobilization after spinal surgery. Spine (Phila Pa 1976) 2001;26(24):2647–2652. doi: 10.1097/00007632-200112150-00002. [DOI] [PubMed] [Google Scholar]
- 20.Cleland J.A., Childs J.D., Palmer J.A., Eberhart S. Slump stretching in the management of non-radicular low back pain: a pilot clinical trial. Man Ther. 2006 Nov;11(4):279–286. doi: 10.1016/j.math.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Villafañe J.H., Silva G.B. Radial nerve mobilization decreases pain sensitivity and improves motor performance in patients with thumb carpometacarpal osteoarthritis: a randomized controlled trial. Arch Phys Med Rehabil. 2012;93(3):396–403. doi: 10.1016/j.apmr.2011.08.045. [DOI] [PubMed] [Google Scholar]
- 22.Villafañe J.H., Silva G.B., Chiarotto A., Ragusa Orazio L.F. Botulinum toxin type a combined with neurodynamic mobilization for upper limb spasticity after stroke. J Chiropr Med. 2012 Sep;11(3):186–191. doi: 10.1016/j.jcm.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Villafañe JH. Botulinum toxin type A combined with neurodynamic mobilization for lower limb spasticity: a case report. J Chiropr Med. 2013. Mar;12(1):39-44. [DOI] [PMC free article] [PubMed]
- 24.Boyd B.S., Wanek L., Gray A.T., Topp K.S. Mechanosensitivity of the lower extremity nervous system during straight-leg raise neurodynamic testing in healthy individuals. J Orthop Sports Phys Ther. 2009 Nov;39(11):780–790. doi: 10.2519/jospt.2009.3002. [DOI] [PubMed] [Google Scholar]
- 25.Göeken L.N., Hof A.L. Instrumental straight-leg raising: a new approach to Lasègue's test. Arch Phys Med Rehabil. 1991 Nov;72(12):959–966. [PubMed] [Google Scholar]
- 26.Coppieters M.W., Butler D.S. Do ‘sliders’ slide and ‘tensioners’ tension? An analysis of neurodynamic techniques and considerations regarding their application. Man Ther. 2008;13(3):213–221. doi: 10.1016/j.math.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 27.Diniz K.T., Miranda R.M., Ribeiro C.D., Vasconcelos D.A., Cabral-Filho J.E. Neural mobilization effects in posterior myofascial chain flexibility and in head posture. Neurobiologia. 2010;73:53–57. [Google Scholar]
- 28.Balster S.M., Jull G.A. Upper trapezius muscle activity during the brachial plexus tension test in asymptomatic subjects. Man Ther. 1997;2(3):144–149. doi: 10.1054/math.1997.0294. [DOI] [PubMed] [Google Scholar]
- 29.Marinzeck, S. “Mobilizacao Neural-Aspectos Gerais” [cited 03/04/2010]. Available in: http://www.terapiamanual.com.br/br/artigos.php?vZ1&pgZartigos/mobilizacaoneural.htm.). 2010.
- 30.Castilho J., Ferreira L.A., Pereira W.M. Analysis of electromyographic activity in spastic biceps brachii muscle following neural mobilization. J Bodyw Mov Ther. 2012;16(3):364–368. doi: 10.1016/j.jbmt.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 31.Butler D. Australia. Noigroup Publications; Adelaide City West: 2000. The sensitive nervous system. [Google Scholar]


