Abstract
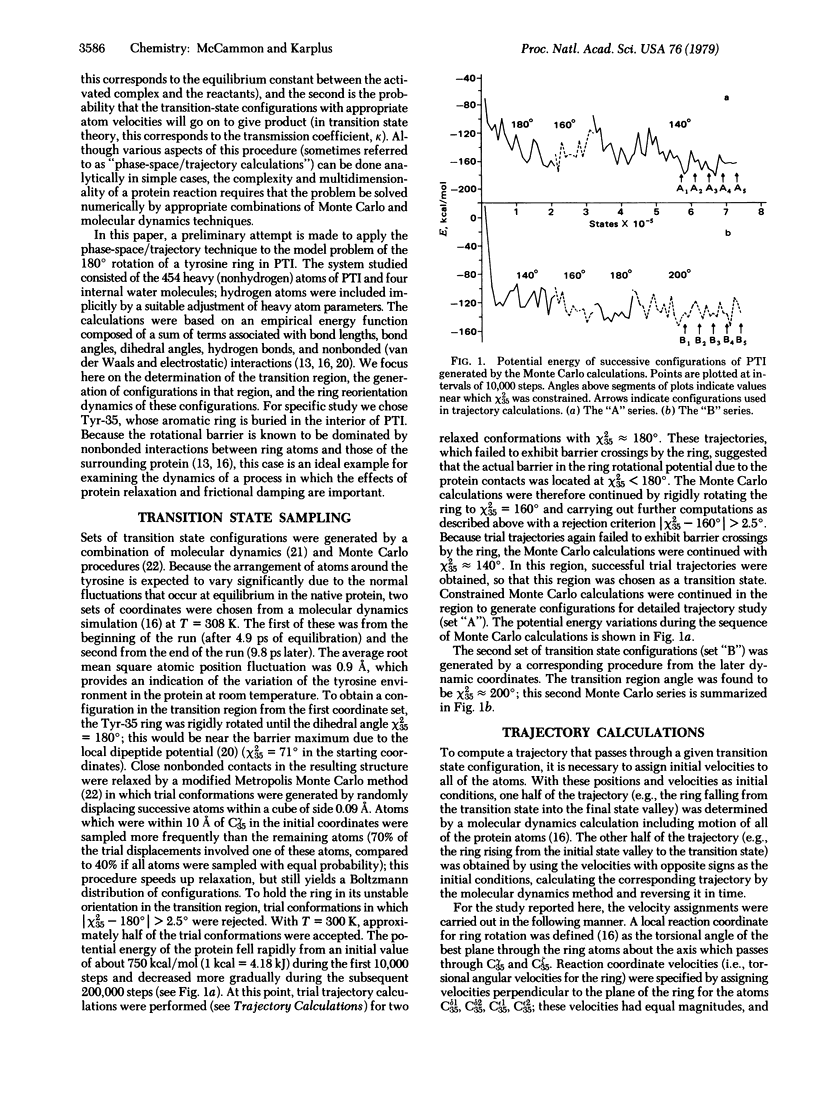
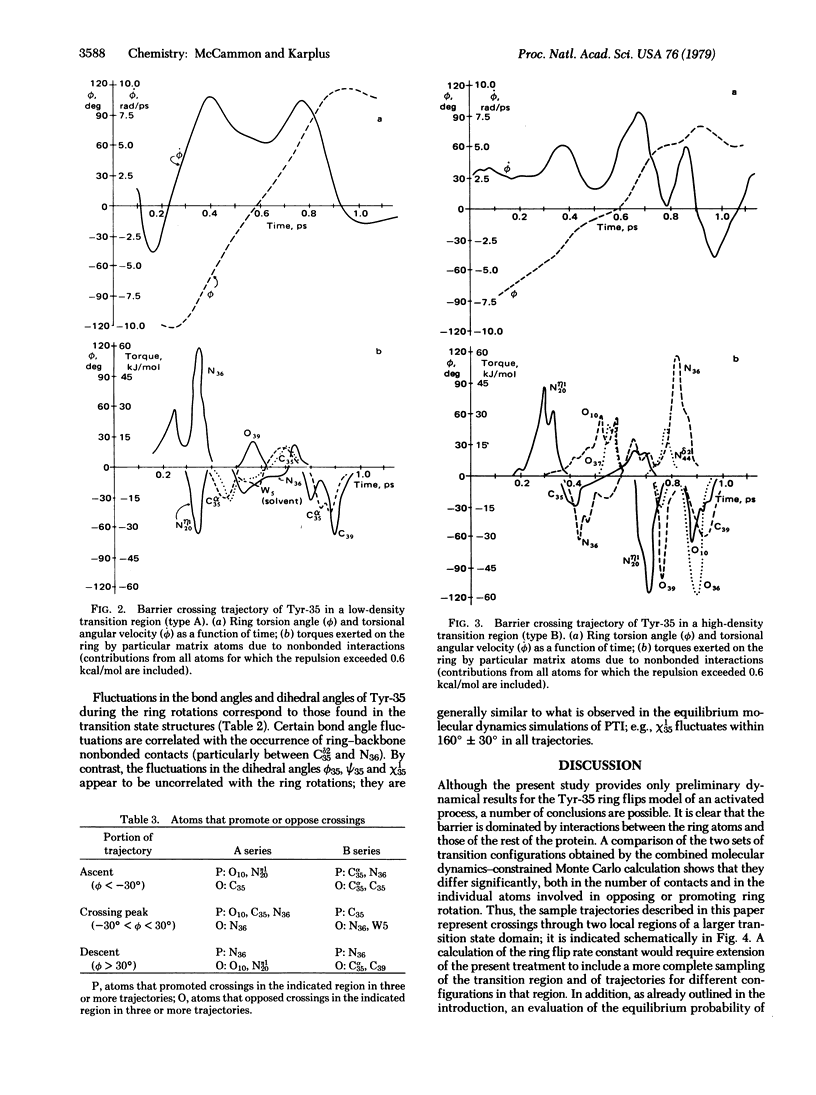
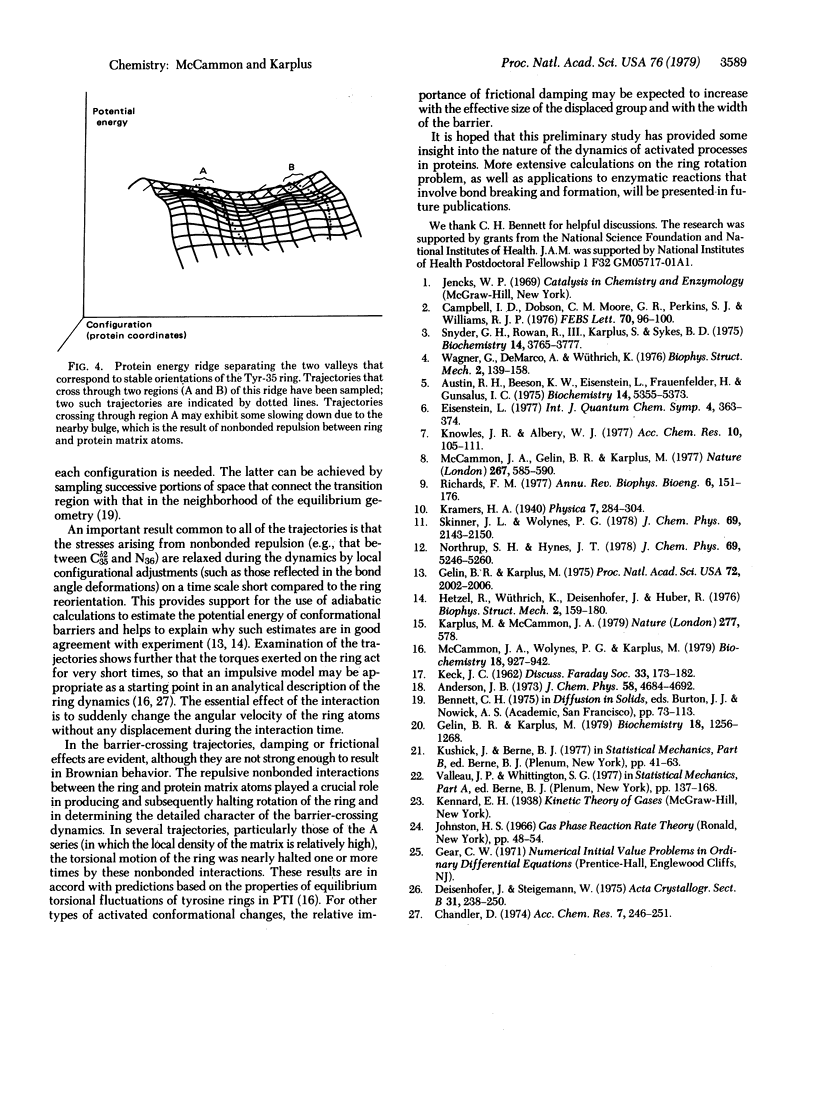
A procedure for the dynamical simulation of activated processes, such as ligand binding and enzymatic reactions, in a globular protein is outlined. Preliminary calculations of the transition state geometry and barrier crossing trajectories are presented for a model reaction, the rotation of an aromatic ring in the bovine pancreatic trypsin inhibitor. The results show that repulsive nonbonded interactions between the ring atoms and the atoms in the surrounding protein matrix determine the dynamical character of the reorientation process; the nonbonded interactions are the source of the rotational barrier and of the impulses that speed up or slow down the ring motion during the barrier crossings.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austin R. H., Beeson K. W., Eisenstein L., Frauenfelder H., Gunsalus I. C. Dynamics of ligand binding to myoglobin. Biochemistry. 1975 Dec 2;14(24):5355–5373. doi: 10.1021/bi00695a021. [DOI] [PubMed] [Google Scholar]
- Campbell I. D., Dobson C. M., Moore G. R., Perkins S. J., Williams R. J. Temperature dependent molecular motion of a tyrosine residue of ferrocytochrome C. FEBS Lett. 1976 Nov;70(1):96–100. doi: 10.1016/0014-5793(76)80734-x. [DOI] [PubMed] [Google Scholar]
- Gelin B. R., Karplus M. Side-chain torsional potentials: effect of dipeptide, protein, and solvent environment. Biochemistry. 1979 Apr 3;18(7):1256–1268. doi: 10.1021/bi00574a022. [DOI] [PubMed] [Google Scholar]
- Gelin B. R., Karplus M. Sidechain torsional potentials and motion of amino acids in porteins: bovine pancreatic trypsin inhibitor. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2002–2006. doi: 10.1073/pnas.72.6.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetzel R., Wüthrich K., Deisenhofer J., Huber R. Dynamics of the aromatic amino acid residues in the globular conformation of the basic pancreatic trypsin inhibitor (BPTI). II. Semi-empirical energy calculations. Biophys Struct Mech. 1976 Aug 23;2(2):159–180. doi: 10.1007/BF00863707. [DOI] [PubMed] [Google Scholar]
- Karplus M., McCammon J. A. Protein structural fluctuations during a period of 100 ps. Nature. 1979 Feb 15;277(5697):578–578. doi: 10.1038/277578a0. [DOI] [PubMed] [Google Scholar]
- McCammon J. A., Gelin B. R., Karplus M. Dynamics of folded proteins. Nature. 1977 Jun 16;267(5612):585–590. doi: 10.1038/267585a0. [DOI] [PubMed] [Google Scholar]
- McCammon J. A., Wolynes P. G., Karplus M. Picosecond dynamics of tyrosine side chains in proteins. Biochemistry. 1979 Mar 20;18(6):927–942. doi: 10.1021/bi00573a001. [DOI] [PubMed] [Google Scholar]
- Richards F. M. Areas, volumes, packing and protein structure. Annu Rev Biophys Bioeng. 1977;6:151–176. doi: 10.1146/annurev.bb.06.060177.001055. [DOI] [PubMed] [Google Scholar]
- Snyder G. H., Rowan R., 3rd, Karplus S., Sykes B. D. Complete tyrosine assignments in the high field 1H nuclear magnetic resonance spectrum of the bovine pancreatic trypsin inhibitor. Biochemistry. 1975 Aug 26;14(17):3765–3777. doi: 10.1021/bi00688a008. [DOI] [PubMed] [Google Scholar]
- Wagner G., DeMarco A., Wüthrich K. Dynamics of the aromatic amino acid residues in the globular conformation of the basic pancreatic trypsin inhibitor (BPTI). I. 1H NMR studies. Biophys Struct Mech. 1976 Aug 23;2(2):139–158. doi: 10.1007/BF00863706. [DOI] [PubMed] [Google Scholar]



