Summary
We characterize an inhibitory circuit motif in the Drosophila olfactory system, parallel inhibition, which differs from feed-forward or feedback inhibition. Excitatory and GABAergic inhibitory projection neurons (ePNs and iPNs) each receive input from antennal lobe glomeruli and send parallel output to the lateral horn, a higher center implicated in regulating innate olfactory behavior. Ca2+ imaging of specific lateral horn neurons as an olfactory readout revealed that iPNs selectively suppress food-related odorant responses, but spared signal transmission from pheromone channels. Co-applying food odorant did not affect pheromone signal transmission, suggesting that the differential effects likely result from connection specificity of iPNs, rather than a generalized inhibitory tone. Ca2+ responses in the ePN axon terminals show no detectable suppression by iPNs, arguing against presynaptic inhibition as a primary mechanism. The parallel inhibition motif may provide specificity in inhibition to funnel specific olfactory information, such as food and pheromone, into distinct downstream circuits.
Introduction
Inhibition occurs throughout the nervous system, impacting diverse processes like spinal cord reflexes (Sherrington, 1906), receptive field formation of the retinal ganglion cells (Kuffler, 1953), and cortical representations of sensory information (Isaacson and Scanziani, 2011). In many well-studied circuits, inhibition is local, carried out by GABAergic neurons that lie close to the brain areas on which they exert their functions. Long-range communication between different brain regions is instead often conveyed by excitatory neurons. There are also notable examples of long-distance projecting GABAergic neurons, such as cerebellar Purkinje cells and striatal spiny projection neurons. In both cases, GABAergic neurons constitute the sole output from the brain regions where their cell bodies reside. In this study, we analyze a paradigm in the fly olfactory system in which excitatory and GABAergic projection neurons each receive input from antennal lobe glomeruli and both send parallel output to overlapping regions in a higher order olfactory center, the lateral horn.
The Drosophila olfactory system (Figure 1A) is a well-established and genetically tractable model system for studying how sensory information is processed to produce internal representations of the outside world (reviewed in Liang and Luo, 2010; Olsen and Wilson, 2008a; Su et al., 2009; Vosshall and Stocker, 2007). Odorants are first recognized by a large repertoire of olfactory receptors, each of which is expressed in a specific class of olfactory receptor neurons (ORNs). ORNs expressing a given odorant receptor project their axons to one of ~50 stereotypic glomeruli in the antennal lobe, where their axons synapse with dendrites of the corresponding class of projection neurons (PNs). This organization creates ~50 parallel information-processing channels. An extensive network of local inhibitory neurons in the antennal lobe receive input from ORNs and PNs, and send output back to ORN axon terminals, PN dendrites and other local interneurons. The actions of these local interneurons contribute to the transformation of odor representations between ORNs and PNs (e.g. Bhandawat et al., 2007; Olsen et al., 2010). The mammalian olfactory system shares many of these properties and organizational principles, highlighting a common solution to odor representation in the brain (Bargmann, 2006).
Figure 1. Characterization of Mz699-GAL4+ Inhibitory Projection Neurons and vlpr Neurons.
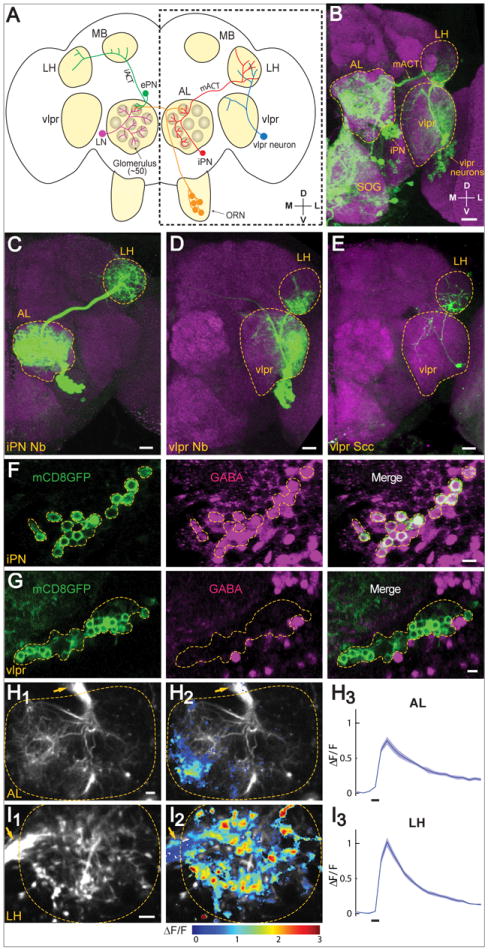
(A) Schematic diagram of the fly olfactory system. Odor is first detected by olfactory receptor neurons (ORNs, orange), which send axons to the antennal lobe (AL) in both hemispheres and synapse with dendrites of projection neurons (PNs) and local interneurons (LNs, purple). Excitatory PNs (ePNs, green) project their axons through the inner antennocerebral tract (iACT) to the mushroom body (MB) and lateral horn (LH). Inhibitory PNs (iPNs, red) send axons through the middle antennocerebral tract (mACT) to innervate the LH only. Also shown is a class of putative 3rd order neurons (blue) that connects the LH with the ventrolateral protocerebrum (vlpr). The area outlined by the dotted box is shown in Panels B-E. D, dorsal; V, ventral; M, medial; L, lateral.
(B-E) (B) Mz699-GAL4 is expressed in iPNs, vlpr neurons, and neurons that innervate the suboesophageal ganglion (SOG) as revealed by the UAS-mCD8GFP reporter. We occasionally observed Mz699+ neurons (0-2 per hemisphere) lateral to the lateral horn neuropil, which send processes into the dorsal lateral horn. Because of inconsistent and weak expression, we did not further consider these neurons in this study. (C-E) MARCM analysis allowed separate visualization of an iPN neuroblast clone (C), a vlpr neuroblast clone (D), or a vlpr single cell clone (E). Green: anti-GFP staining; magenta: mAb nc82 (B) or anti-N-Cadherin (C-E) staining of the general neuropil. Scale, 20 μm.
(F-G) The inhibitory neurotransmitter GABA is present in the majority of iPNs (F) but is absent in the vlpr neurons (G). Green: anti-mCD8 staining (driven from Mz699-GAL4); magenta: anti-GABA. Scale, 5 μm.
(H-I) Ca2+ imaging of Mz699-GAL4 driven GCaMP3 expressing antennal lobe (H) and lateral horn (I) (outlined), in response to 0.1% isoamyl acetate. Arrows point to the mACT. Scale, 5 μm. (H1, I1) Averaged basal fluorescence at the AL (H1) and LH (I1). (H2, I2) Normalized fluorescence changes ΔF/F (1 = 100%) are superimposed on the averaged basal fluorescence. (H3, I3) Time courses of ΔF/F over the region with positive Ca2+ signals. Odor durations (500 msec) are indicated as horizontal bars below. mean ± SEMs (3 repeats).
See Figure S1 for more MARCM analysis of Mz699+ neurons, and Figure S2 for more iPN response in the antennal lobe.
An outstanding question is how olfactory inputs direct innate and learned behavior. The axons from the excitatory PNs relay olfactory information to the mushroom body, a center for olfactory learning and memory (Davis, 2005; Heisenberg, 2003), and to the lateral horn, a less understood higher order center presumed to direct olfaction-mediated innate behavior (Heimbeck et al., 2001). Indeed, the terminal arborization patterns of PN axons within the lateral horn are highly stereotyped according to PN glomerular class, whereas their innervation patterns in the mushroom body are much more variable (Jefferis et al., 2007; Marin et al., 2002; Tanaka et al., 2004; Wong et al., 2002). Interestingly, presynaptic terminals for PNs that represent food odors and pheromones are spatially segregated in the lateral horn (Jefferis et al., 2007), but little is known about how olfactory inputs are transformed from PNs to higher order lateral horn neurons (Ruta et al., 2010).
Thus far, most physiological and behavioral studies of Drosophila PNs have focused on the uniglomerular excitatory PNs (ePNs), which reside dorsal and lateral to the antennal lobe and whose axons form the inner antenno-cerebral tract (iACT), innervating both the mushroom body and the lateral horn (Figure 1A). However, a separate group of PNs reside ventral to the antennal lobe. Individual ventral PNs send dendrites to either single or multiple glomeruli, and project their axons through the middle antenno-cerebral tract (mACT) to terminate only in the lateral horn, bypassing the mushroom body altogether (Jefferis et al., 2007; Lai et al., 2008; Okada et al., 2009; Stocker et al., 1990). In this study, we use the olfactory response of a specific set of higher order neurons to show that these ventral PNs provide GABAergic inhibition in the lateral horn to route selective inputs to specific higher order neurons.
Results
Mz699-GAL4 Allows Genetic Access to Ventral PNs (iPNs) and vlpr Lateral Horn Neurons
Ventral PNs of the antennal lobe have previously been characterized using two GAL4 lines. GH146-GAL4 labels ~6 ventral PNs (Jefferis et al., 2001), all of which are GABAergic (Jefferis et al., 2007) and all are uniglomerular except one that innervates all glomeruli (Marin et al., 2002). Mz699-GAL4 labels >45 ventral PNs that are mostly complementary to those labeled by GH146-GAL4 (Lai et al., 2008). Most Mz699-GAL4-positive (Mz699+ hereafter) ventral PNs project to multiple glomeruli (Lai et al., 2008) and more than 80% are GABAergic (Okada et al., 2009). Mz699-GAL4 also labels neurons in the ventrolateral protocerebrum (vlpr) that send processes into the lateral horn (Okada et al., 2009; Figure 1B).
To further characterize neurons labeled by Mz699-GAL4, we used MARCM-based clonal analysis (Lee and Luo, 1999). Consistent with a previous study (Lai et al., 2008), we found that Mz699+ ventral PNs were derived from a single neuroblast (Figure 1C; Figure S1A). Most single cell clones innervated a few glomeruli (Figure S1B; n=38/39), which collectively covered the majority of glomeruli. We also introduced Synaptotagmin-HA (Syt-HA) as a presynaptic vesicle marker in these MARCM clones, and found that Syt-HA was highly enriched in the lateral horn, but was largely absent from the antennal lobe in neuroblast and single cell clones (Figure S1A-C). This is consistent with a previous report based on labeling of all Mz699+ neurons (Okada et al., 2009). With single cell resolution, we observed that the majority of the vPN neural processes in the antennal lobe had fine terminal branches without Syt-HA signal (Figure S1B), whereas Syt-HA puncta, likely represent presynaptic terminals of en passant synapses, were distributed throughout the branches in the lateral horn (Figure S1C). These data suggest that Mz699+ ventral PNs deliver olfactory information from the antennal lobe to the lateral horn. Consistent with a previous report (Okada et al., 2009), we found that the vast majority of Mz699+ ventral PNs were GABAergic based on GABA staining (Figure 1F; 87.6±2.5% GABA-positive, from 8 antennal lobes with an average of 55 cells per lobe). As described later, the ventral PNs provide inhibition that requires GABA synthesis. Thus, we refer to them hereafter as inhibitory PNs (iPNs) to distinguish them from the excitatory PNs (ePNs) from the anterodorsal and lateral lineages.
We also examined Mz699+ vlpr neurons that also project to the lateral horn as putative higher order neurons in the olfactory pathway. Neuroblast and single cell clone analyses of vlpr neurons showed that a subset projected to the lateral horn as well as the vlpr neuropil (Figure 1D, 1E). Both processes were enriched for Syt-HA (Figure S1D-E), similar to several other lateral horn neurons including those that connect the lateral horn to the vlpr (Jefferis et al., 2007). Furthermore, in the single cell clone of vlpr neurons, Syt-HA+ puncta are distributed through the processes in the vlpr neuropil including most of the terminals. In the lateral horn, however, the neural processes end with fine branches without Syt-HA puncta (Figure S1E). This result suggests that Mz699+ vlpr neurons mostly send information from the lateral horn to the vlpr neuropil. Thus, these lateral-horn-projecting Mz699+ vlpr neurons represent a subset of putative third order neurons in the lateral horn. None of the Mz699+ vlpr neurons were GABA-positive (Figure 1G). Below, we used their odor-evoked response as a means to investigate the role of iPN function in olfactory signal processing.
iPNs Are Activated by Odors
To investigate the function of iPNs, we first examined their odorant responses utilizing two-photon Ca2+ imaging in alert flies labeled by Mz699-GAL4 driving UAS-GCaMP3 (Tian et al., 2009). When we applied to the antennae 500 msec pulses of 0.1% isoamyl acetate (IA), a major component of the banana odor, we observed a robust increase of Ca2+ signals in both the antennal lobe (Figure 1H, S2A) and the lateral horn (Figure 1I), including the mACT tract before entering the lateral horn neuropil (Figure 1I, arrow). 1% apple cider vinegar application gave similar results (data not shown; see below). We further tested Ca2+ response of iPNs to IA applied at different concentrations in the antennal lobe (Figure S2C, E). At low IA concentration, the response was sparse and weak. As the odorant concentration increased, more glomeruli were recruited with elevated Ca2+ signals, similar to the concentration-dependent odorant responses of ORN and ePN (Hallem and Carlson, 2006; Wang et al., 2003).
Because the neuronal elements that express GCaMP3 in the antennal lobe were derived exclusively from dendrites of Mz699+ iPNs, we conclude that iPNs are activated by odors, likely through ORN→iPN synapses in the antennal lobe glomeruli although it is possible that iPNs are instead or additionally activated by ePNs. At the same time, through their mACT axonal projection, iPNs effectively send olfactory signals to the lateral horn (also see below).
Acute mACT Transection Distinguishes the iPNs and vlpr Responses at the Lateral Horn
Since both iPNs and vlpr neurons send processes to the lateral horn, IA-elicited Ca2+ signals within the lateral horn (Figure 1I) could be contributed by either or both of these neuronal types. We next aimed to isolate putative postsynaptic signals of vlpr neurons from presynaptic signals in iPNs within the same lateral horn using a laser transection protocol outlined in Figure 2A. Specifically, we first obtained lateral horn odor responses from control and experimental hemispheres. We then used Mz699-labeled iPN axons as a guide and applied spatially confined laser pulses from the two-photon laser (Ruta et al., 2010) to transect the mACT prior to its entry to the lateral horn on the experimental hemisphere. Following the laser transection, we imaged lateral horn odor responses in both experimental and control hemispheres again.
Figure 2. Lateral Horn Response of vlpr Neurons to Isoamyl Acetate Is Inhibited by iPNs via the mACT Projection.
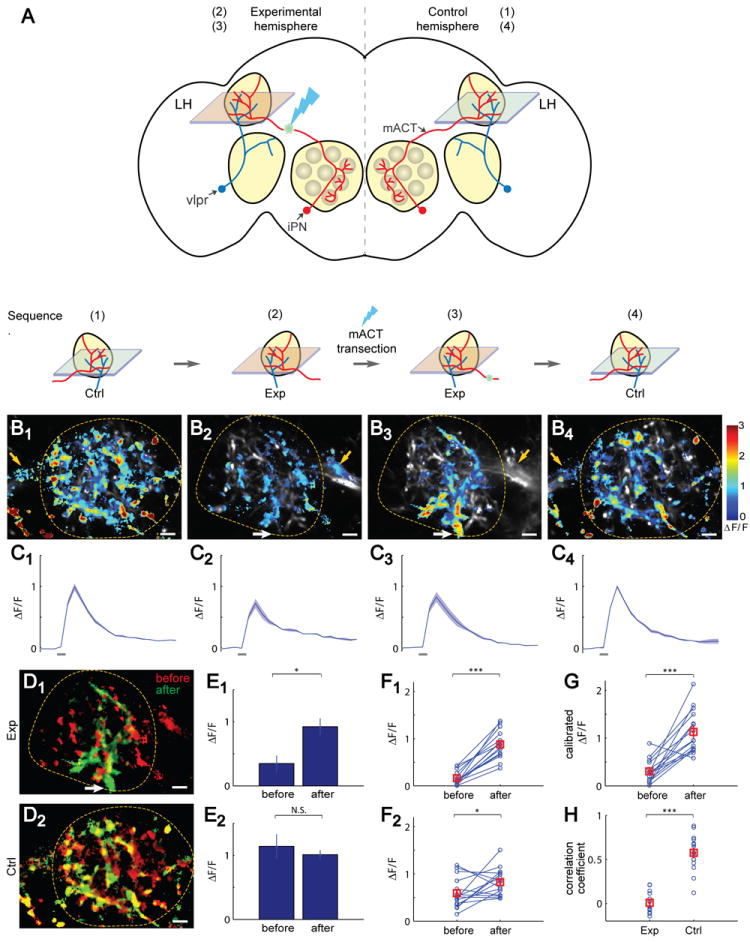
(A) Schematic diagram of the experimental procedures to dissect iPN and vlpr Ca2+ responses in the lateral horn (LH). Ctrl, control hemisphere; Exp, experimental hemisphere. The sequence of the experimental procedures is outlined at the bottom, which correspond to the images and time course in Panels B-C.
(B1-B4) Isoamyl acetate (IA)-induced Ca2+ signals corresponding to each step above from a representative fly. The lateral horn is outlined. Yellow arrows point to the mACT prior to its entry into the lateral horn. White arrows point to the entry site of vlpr dendrites into the lateral horn. After transection, Ca2+ signals disappeared from the mACT, but appeared at the vlpr dendrites (compare B3 with B2). The differences between B1 and B2 are due to images taken at different focal planes of the lateral horn in the control and experimental hemispheres.
(C1-C4) Time course of the corresponding ΔF/F in (B1-B4). Odor durations (500 msec) are indicated as horizontal bars below. mean ± SEMs (3 repeats).
(D) Overlay of the before- (red) and after-transection (green) Ca2+ signals for experimental (D1) and control (D2) hemispheres. The spatial patterns of green and red are distinct in D1 but overlap extensively in D2.
(E) Peak ΔF/F values before or after transection within the region of interest (ROI) as defined by the spatial pattern after transection (green in D1). The vlpr response exhibited a significant increase on the experimental side (3 repeats, p < 0.05; t-test) but not on the control side (3 repeats).
(F-G) Paired comparisons of ΔF/F before and after mACT transection across different flies (n=15). (F1) The experimental hemisphere shows a highly significant increase of vlpr responses after transection (paired t-test). (F2) The control hemisphere shows a slight but significant increase. With repeated measures two-way ANOVA, mACT transection, brain hemisphere (experimental vs. control), and their interaction are all statistically significant (p < 0.001; p < 0.01; p< 0.001 respectively). (G) Calibrated ΔF/F of vlpr responses (experimental hemisphere/control hemisphere for the same fly) exhibited a significant increase after transection (paired t-test).
(H) Each circle represents the correlation coefficient of spatial patterns before and after mACT transection for an individual fly. There is little correlation on the experimental hemisphere (mean = 0.067), but high correlation on the control hemisphere (mean = 0.516). The correlation coefficient between the two groups is significantly different (paired t-test).
For all statistical analyses in this and subsequent figures, error bars represent ±SEM. *: p < 0.05; **: p < 0.01; ***: p < 0.001; N.S. (not significant), p > 0.05.
See Figure S2 for validation of mACT transection, and Figure S6 for dependence of vlpr response to IA concentration.
Several lines of evidence suggested that our laser transection of mACT was complete and specific. First, we could observe a small cavitation bubble at the mACT from basal GCaMP3 fluorescence with our two-photon microscope immediately following the laser application (Figure S3A), a hallmark of laser transection (Vogel and Venugopalan, 2003). Second, retrospective immunostaining validated the complete transection of the mACT (Figure S3B, n=15) with no visible effect on the integrity of the nearby iACT that conveys signals from the ePNs (data not shown). Third, odor-evoked GCaMP3 signals in mACT near the lateral horn entry site (e.g. Figure 2B2, yellow arrow) were invariably abolished after laser transection of mACT (Figure 2B3, yellow arrow), validating that the responses observed in intact preparations were due to iPN contributions and were lost after mACT transection. Fourth, applying the same energy from the two-photon laser at locations away from mACT did not cause similar changes in lateral horn Ca2+ signals (data not shown). Finally, we did not detect changes of iPN responses in the antennal lobe before and after mACT transection (data not shown), suggesting that olfactory input still activates iPNs in the antennal lobe similarly after mACT transection. Thus, we could assume that olfactory response in the lateral horn neuropil after mACT transection is mostly contributed by the vlpr neurons.
mACT Transection Revealed that iPNs Normally Inhibit vlpr Responses to Isoamyl Acetate
How does iPN projection contribute to olfactory information processing at the lateral horn, and specifically, how are the responses of putative 3rd order vlpr neurons modulated by iPN input? To address these questions, we compared Ca2+ signals in response to isoamyl acetate application in the lateral horn (referred as IA response hereafter) before and after laser transection (Figure 2B, 2C). In all cases, IA responses in the lateral horn were robust (Figure 2C). However, a striking change occurred in the spatial patterns in the experimental hemisphere (compare Figure 2B3 and 2B2). Before transection, the IA response was scattered across the lateral horn (Figure 2B2). After transection, IA response appeared most intense in the ventral lateral horn near the lateral horn entry site of vlpr dendrites (Figure 2B3, white arrow). This change of spatial pattern was evident when we superimposed the IA response before and after transection on the same lateral horn (Figure 2D1). By contrast, the spatial patterns of IA response in the control hemisphere appeared similar before and after mACT transection (compare Figure 2B1 and 2B4; Figure 2D2).
We used two approaches to quantitatively analyze the changes of IA response before and after mACT transection. In the first approach, we defined a region of interest (ROI) based on the spatial pattern of the after-transection IA response for each imaging plane (see Supplemental Experimental Procedures). In the control hemisphere, this ROI encompasses the activated regions of both iPNs and vlpr neurons. In the experimental hemisphere, however, this ROI would correspond to activated regions of vlpr neurons only, since iPN input was eliminated after mACT transection. We then quantified ΔF/F signals within the ROI for the IA responses before and after transection. In the experimental hemisphere, the after-transection response was significantly increased compared to before transection (Figure 2E1), suggesting that most after-transection responses in the ROI (i.e. vlpr neuronal responses) were newly gained as a consequence of mACT transection. This difference was highly significant across individual flies (Figure 2F1).
To rule out the contribution of olfactory adaptation or potential non-specific deterioration of fly physiology during the imaging procedure, we used the lateral horn IA response in the control hemisphere from the same fly as an internal control. The magnitude of the IA response in the lateral horn remained unchanged in the example fly (Figure 2E2). Although across flies there was a slight increase in the control hemisphere after transection compared with before (Figure 2F2; see Supplemental Experimental Procedures for a likely cause), when we used calibrated responses (IA responses within ROI of the experimental hemisphere divided by that of the control hemisphere from the same fly), IA response increase was highly significant across individual flies after mACT transection (Figure 2G).
In the second approach, we analyzed the correlation of spatial patterns of IA response before and after mACT transection (see Supplemental Experimental Procedures). The control hemisphere showed a high correlation (Figure 2H, right column), consistent with the resemblance of their spatial activity patterns before and after transection. By contrast, the experimental hemisphere exhibited a significantly smaller correlation coefficient (Figure 2H, left column) compared to the control hemisphere. This smaller correlation coefficient likely reflected combined effects of a loss of iPN response and gain of vlpr response.
Taken together, these data indicate that while the iPN contribution to the lateral horn IA response was abolished as a result of mACT transection, there was an additional, highly significant gain of IA response in the vlpr neurons after mACT transection. This suggests that the vlpr response to IA stimulation is normally inhibited by iPN projections through the mACT.
Blocking GABA Synthesis in iPNs Suppresses iPN Inhibition of vlpr Neurons
To test whether GABA release mediates the observed inhibitory signals from the mACT onto the vlpr lateral horn neurons, we perturbed GABA synthesis from iPNs introducing UAS-Gad1-RNAi into our imaging flies (Mz699-GAL4, UAS-GCaMP3) to knock down Glutamic acid decarboxylase 1 (Gad1), the critical enzyme responsible for GABA biosynthesis (Kuppers et al., 2003). Immunostaining revealed no detectable GABA in 49/51 Mz699+ neurons under the experimental condition (Figure 3B; compared to control in Figure 3A). Although the Gad1 RNAi transgene was also expressed in Mz699+ vlpr neurons, these neurons should be unaffected since they were not GABAergic (Figure 1G).
Figure 3. Blocking GABA Synthesis in iPNs Suppresses iPN Inhibition of vlpr Neurons.
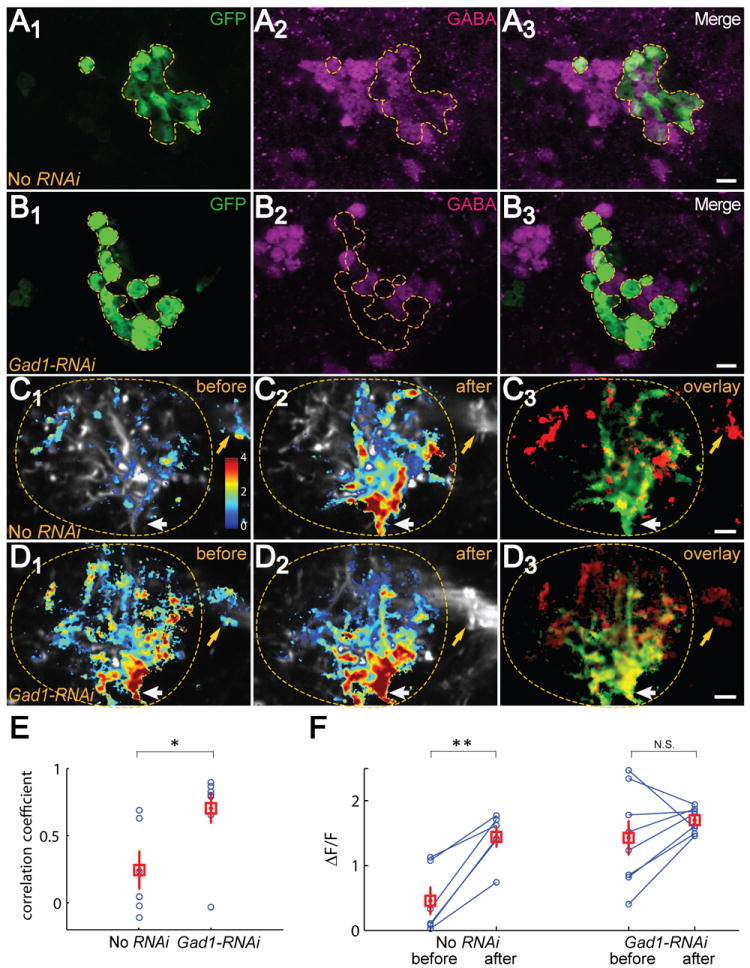
(A-B) Compared with No-RNAi control (A), Gad1-RNAi expression caused a loss of GABA staining in Mz699+ neurons (B). Magenta; anti-GABA; green: anti-GFP. The confocal images are from single z planes. Scale, 5 μm.
(C-D) Compared with No RNAi (C1), fly expressing Gad1-RNAi in Mz699+ neurons shows greatly enhanced IA response in the lateral horn (D1) before mACT transection. mACT transection induced robust changes in the intensity and spatial pattern of IA responses in control (compare C2 with C1), including the loss of mACT signal (yellow arrows) and gain of signals at the vlpr dendrite entry site (white arrows). By contrast, mACT transection did not cause robust increases in IA response (D2) nor change the spatial pattern in the Gad1-RNAi fly (compare D2 with D1), except the signal loss at the mACT (yellow arrows). ΔF/F of Ca2+ signals are superimposed on the averaged raw images. C3 and D3 are an overlay of Ca2+ signals before (red) and after (green) mACT transection for C1 and C2, and D1 and D2, respectively. Lateral horns are outlined. Scale, 5 μm.
(E) The spatial patterns of the ΔF/F in the lateral horn before and after laser transection are highly correlated in individual Gad1-RNAi flies, and as a group is significantly different from the No-RNAi group (t-test).
(F) Paired comparison of ΔF/F before and after transection across flies. After-transection patterns were used to define an ROI for quantification to isolate the vlpr response. With repeated measures two-way ANOVA, mACT transection, genotype, and their interaction are all statistically significant (p < 0.01; p < 0.05; p < 0.05 respectively). No-RNAi flies exhibited a significant increase after mACT transection compared with before, but no significant change was observed for Gad1-RNAi flies. Gad1-RNAi: n=8; No-RNAi: n=6.
Control flies exhibited general elevation and a spatial pattern change of IA response in the lateral horn after mACT transection (Figure 3C2) compared with before (Figure 3C1), as we have described (Figure 2). However, Gad1 knockdown in iPNs resulted in a robust lateral horn IA response in intact flies, with a spatial pattern that resembled IA response after mACT transection (Figure 3D1). Specifically, in intact Gad1 knockdown flies, IA robustly activated the ventral lateral horn near the vlpr dendrite entry site (Figure 3D1, white arrow), a region that normally exhibited robust IA response only after transection in control flies. mACT transection no longer resulted in significant spatial pattern changes, as seen by the representative images (Figure 3D2, D3), and by a higher correlation coefficient of spatial patterns before and after mACT transection compared with controls (Figure 3E). Using ROIs defined by after-transection patterns to isolate vlpr responses, we found a statistically significant interaction between the fly genotype and mACT transection. Separate statistical tests on the ablation effect showed no statistically significant change in Gad1 knockdown flies before and after mACT transection, in contrast to the increase of IA response in control animals after mACT transection (Figure 3F).
Together, these experiments indicate that GABAergic inhibition from the mACT is largely responsible for the suppression of IA responses of vlpr neurons under physiological conditions. The phenotypic similarity between mACT transection and Gad1 knockdown in Mz699+ neurons also suggests that Mz699+ neurons provide the major inhibitory input through the mACT to the lateral horn in our experimental context.
iPN Inhibition of vlpr Neurons Is Odorant-dependent
Our results thus far suggest that GABA release from iPNs inhibits IA responses of higher order vlpr neurons. Do iPNs inhibit all odorants similarly? To address this question, we used the same paradigm and analysis method (Figure 2) to examine the Ca2+ response of vlpr neurons to several other odorants.
We first examined apple cider vinegar, a natural attractant for flies that has been used for physiological and behavioral experiments (Semmelhack and Wang, 2009). We found similar results as IA, both qualitatively and quantitatively (Figure 4A, compared with Figure 2). Specifically, there was a marked increase of vinegar responses in new regions of the lateral horn after mACT transection (Figure 4A1-A3). The correlation coefficient for spatial patterns before and after mACT transection was significantly smaller in the experimental hemisphere compared to the control hemisphere (Figure 4A4). Using ROIs created from after-transection patterns to isolate the vlpr response, we found a significant increase of vlpr vinegar response after mACT transection in the experimental but not control hemisphere (Figure 4A5).
Figure 4. iPN Inhibition of vlpr Neurons Is Odorant-Specific.
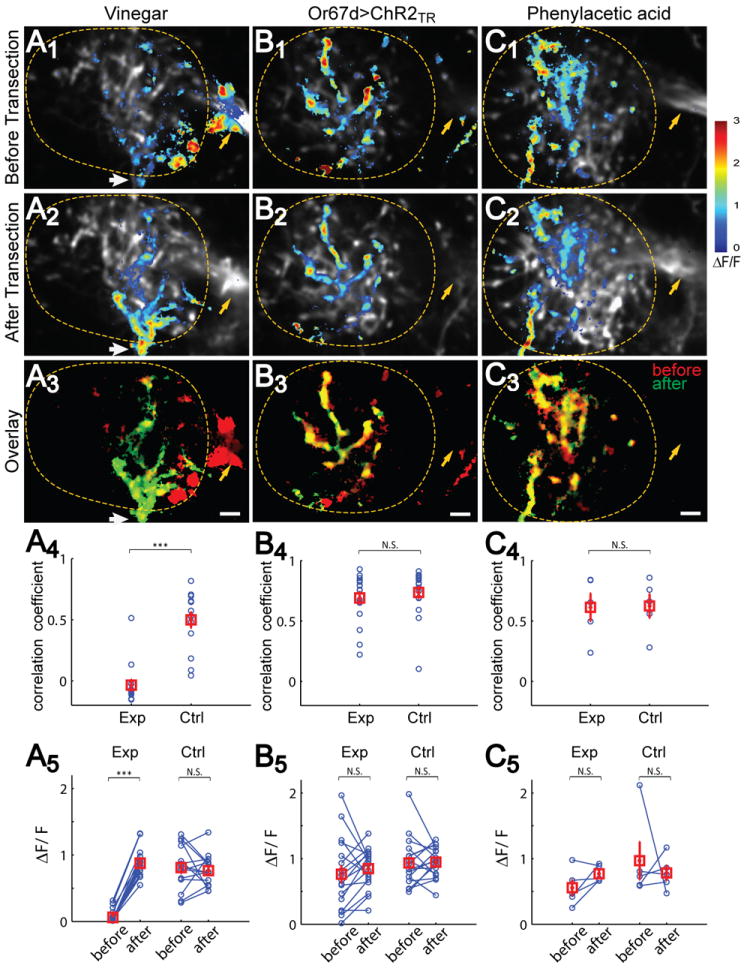
(A1-C3) Ca2+ responses to 1% apple cider vinegar (A), optogenetic activation of Or67d ORNs (B) and 1% phenylacetic acid (PAA) (C) are shown as representative images before (A1-C1) or after (A2-C2) mACT transection, and their overlay (A3-C3). Whereas the spatial patterns of vinegar response changes drastically after mACT transection, including signal loss in mACT (yellow arrows) and medial lateral horn and signal gain in vlpr dendrite entry site at the ventral lateral (white arrows), few changes are seen in Or67d and PAA responses before and after transection. Lateral horns are outlined. Scale, 5 μm.
(A4-C4) Correlation coefficients of spatial patterns of Ca2+ response for before- and after-transection. For vinegar, the average correlation before vs after is close to 0 and significantly smaller than that in the control hemisphere (paired t-test) (A4). For Or67d and PAA stimuli, the correlation coefficients in experimental hemispheres are comparable to the control hemispheres. (A5-C5) Quantification of ΔF/F across different animals for the three stimuli in ROI created by after-transection responses. mACT transection leads to a drastic increase in vlpr neuron response to vinegar stimulation (n=14, paired t-test), but not to OR67d>ChR2TR (n=17) or PAA (n=5) stimulation. In all cases, Ca2+ signals in the control hemisphere are not significantly changed. With repeated measures two-way ANOVA, mACT transection, brain hemisphere (experiment vs. control) and their interaction are all statistically significant (p < 0.001) to vinegar stimulation but none in the case of Or67d>ChR2TR or PAA stimulation.
See Figure S2 for Or67d>ChR2TR characterization in the antennal lobe, Figure S4 for characterization of in mammalian neurons, Figure S5 for characterization of Or67d-QF and QUAS-ChR2TR transgenes, and Figure S6 for dependence of vlpr response to Or67d>ChR2TR stimulation intensity.
Next, we examined the lateral horn responses triggered by optogenetic activation of Or 67d ORNs, which are activated by a well-characterized pheromone, 11-cis-vaccenyl acetate (cVA) (Ejima et al., 2007; Kurtovic et al., 2007; van der Goes van Naters and Carlson, 2007). Activating these neurons largely recapitulates behavioral responses to cVA (Kurtovic et al., 2007). To optimize light responses in expressing neurons, we used a new channelrhodopsin variant that contained both the H134R mutation that increases photocurrent sizes (Nagel et al., 2005), and the C128T mutation that slows the channel photocycle (Berndt et al., 2009). The resulting ChR2TR channels showed robust photocurrents in cultured mammalian neurons and triggered spiking with high light sensitivity in vivo (Figure S4). To genetically access two neuronal populations independently for optogenetic stimulation and Ca2+ imaging, we utilized the Q system (Potter et al., 2010) to express ChR2TR in Or67d neurons (Figure S5A, B). Blue light stimulation induced a robust and specific Ca2+ response of ePNs in the DA1 glomerulus, the target of Or67d ORN axons (Figure S5C), supporting the potency and specificity of optogenetic activation. We also characterized iPN antennal lobe responses to different levels of optogenetic activation of Or67d ORNs. iPN signals are restricted to the DA1 glomerulus, and increased with increasing laser power from 0.012 to 0.12 mW/mm2 (Figure S2B, D, F).
We chose the 0.06 mW/mm2 as the laser power to activate Or67d ORNs, and examined the lateral horn Ca2+ response (Or67d responses in short hereafter). We found a robust Or67d response (Figure 4B1), which is dependent on the presence of the ChR2TR transgene. In contrast to the marked gain of new regions for IA or vinegar responses after mACT transection (Figure 2, Figure 4A), the spatial patterns of Or67d responses appeared similar before and after mACT transection (Figure 4B). This can be seen by the minimal changes from superimposition of the spatial patterns (Figure 4B3, compared with Figure 4A3 and Figure 2D1), similar correlation coefficient in the experimental and control hemispheres (Figure 4B4), and similar Ca2+ response magnitudes before and after transection using an ROI defined by the after-transection response (Figure 4B5). Together, these data suggest that Or67d activation led to robust vlpr neuronal response in intact flies, and that this response was not significantly inhibited by iPNs.
To test whether other olfactory processing channels behave similarly to Or67d, we tested phenylacetic acid (PAA), which is derived from food but enhances male courtship (Grosjean et al., 2011). PAA activates mostly Ir84a-expressing ORNs that project to the VL2a glomerulus (Grosjean et al., 2011; Silbering et al., 2011). The axonal projections of VL2a PNs in the lateral horn exhibit more similarities to pheromone-representing rather than food-representing PNs, consistent with its function in promoting mating behavior (Grosjean et al., 2011). We found that the lateral horn responses of Mz699-GAL4, UAS-GCaMP3 flies to PAA resembled those of Or67d activation: the responses exhibited strong similarity before and after mACT transection (Figure 4C), suggesting that PAA normally activates vlpr neurons, and this activation is minimally inhibited by iPNs. Thus, using olfactory response of vplr neurons as the readout, our data suggest a difference in iPN inhibition of food- versus pheromone-related odor processing channels, although we cannot rule out the possibility that the difference is due to simultaneously activating multiple glomeruli in the case of IA or vinegar, and stimulating single glomeruli in the case of Or67d or PAA.
To examine whether the odor-selective iPN inhibition is affected by stimulus intensity, we performed additional experiments with varying stimulus strengths. We found that lateral horn responses to higher or lower concentrations of IA than our original concentration (10-3) were both elevated after mACT transection, although higher concentration of IA (3×10-3) evoked Ca2+ response of vlpr neurons in some intact animals (Figure S6A). By contrast, mACT transection did not affect the dose-response curve of Or67d stimulation (Figure S6B). These experiments suggest that the differential inhibition is dependent on the nature of the odorants, rather than is a consequence of different levels of excitation by these different odorants.
The Or67d→vlpr Processing Channel Is Insulated from iPN Inhibition
Of the above four stimuli we have examined, IA and vinegar responses of vlpr neurons were robustly inhibited by iPNs, whereas Or67d neurons and PAA stimulations were not. We envisioned two contrasting models that could account for these differences. In the first model, which we termed “bulk inhibition” (Figure 5A), iPN inhibition is non-selective and is proportional to the number of iPNs that are excited by the odor. Since IA or vinegar each activate many glomeruli (Semmelhack and Wang, 2009; Wang et al., 2003), they should also activate a large number of iPNs (as most iPNs are multiglomerular), and therefore send a strong bulk inhibitory signal to the lateral horn (Figure 5A1). By contrast, Or67d neurons or PAA stimulation each activates a single glomerulus, and therefore engage a smaller number of iPNs, with limited inhibitory tone in the lateral horn (Figure 5A2). In the alternative model, which we termed “selective inhibition” (Figure 5B), the Or67d or PAA processing channel is insulated from iPN inhibition that applies to the IA and vinegar processing channels.
Figure 5. vlpr Response to Or67d Activation Is Not Inhibited by Co-application of IA.
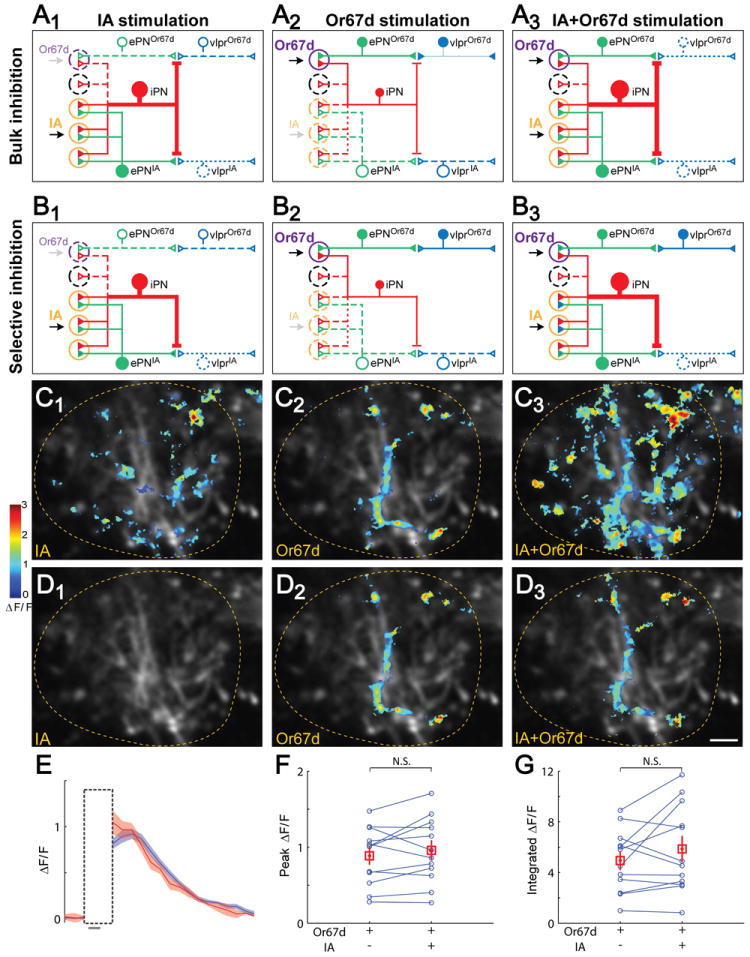
(A) In the bulk inhibition model, iPNs as a whole (represented by a single red neuron) inhibit all vlpr responses non-discriminately, but the strength of inhibition (represented by the size of the iPN) is proportional to the number of activated glomeruli. IA stimulation (A1) causes stronger inhibition than Or67d stimulation (A2) because IA activates more glomeruli (orange circle) than Or67d (purple circle). IA+Or67d co-stimulation is predicted to decrease vlpr response to Or67d (A3). In the schematic shown here and in Panel B, solid neurons represent active neurons; dotted neurons are either not activated and/or are inhibited.
(B) In the selective inhibition model, iPN inhibition is processing channel-selective. Specifically, iPNs inhibit vlpr responses to IA (B1) but not to Or67d (B2) stimulation. Hence, IA+Or67d co-stimulation is predicted not to affect vlpr response to Or67d (B3).
(C) Ca2+ responses of the same fly to IA (C1), OR67d>ChR2TR(C2), and IA + OR67d>ChR2TR (C3). Each image is the average of three raw images from three iterations of first IA, followed by alternating Or67d and IA+Or67d stimulations.
(D) Ca2+ responses as in C, shown only for regions within an ROI that isolates vlpr response to Or67d stimulation. This ROI was determined by after-transection Or67d response subtracting its small overlap with the before-transection IA response, to ensure that only the vlpr response to Or67d stimulation was quantified. Lateral horns are outlined. Scale, 5 μm.
(E) Averaged ΔF/F over time for the response to OR67d>ChR2TR (blue) and to co-application with IA (red) (mean ± SEM, 3 repeats). Gray bar at the bottom indicates the 500 msec optogenetic and/or odor stimulation duration. The vertical box represents the time when imaging was turned off to avoid sensing the light from the ChR2TR -activating blue laser.
(F-G) Quantification of the peak (F) and integrated (G) ΔF/F response. In both cases, Or67d response was not affected by IA co-stimulation (n=12).
These two models have different predictions if we were to co-stimulate Or67d neurons with IA. If the bulk inhibition model were correct, the lateral horn Or67d response (mostly contributed by vlpr neurons) would be diminished with IA co-application in intact animals, as IA application would activate many iPNs and send a strong inhibitory signal to the lateral horn (Figure 5A3). Alternatively, if the selective inhibition model were true, the Or67d response would not change with IA co-application (Figure 5B3).
We thus compared the lateral horn responses to IA, Or67d, and IA+Or67d in the same fly. Activating Or67d neurons by optogenetic means simplified the experimental paradigm and circumvented possible peripheral odor-odor interactions (Su et al., 2011) or cross-contamination of residual odors during odor delivery. We measured lateral horn odor response to IA, Or67d neuronal activation, and co-stimulation in intact animals for 3-6 iterations (Figure 5C). To test whether Or67d neuronal responses would be inhibited by IA co-application, we isolated the ROI of vlpr response to Or67d stimulation by performing mACT transection (Figure 5D). Within the ROI, we found that co-stimulation of IA did not cause a detectable change of Or67d response magnitude in intact flies (Figure 5E-G), despite the fact that IA clearly activated lateral horn responses outside the ROI (Figure 5C1, C3). This experiment provided strong support to the selective inhibition model, at least for the cVA processing channel.
Evidence against Presynaptic Inhibition of ePNs as the Primary Mechanism of iPNs Action
The lateral horn neuropil is composed of axon terminals from ePNs and iPNs, and dendrites of putative third order neurons including the vlpr neurons. In principle, iPN inhibition of vlpr response could be caused by a direct inhibition of vlpr neurons, presynaptic inhibition of ePNs, or a combination of both. Ca2+ imaging does not have sufficient temporal resolution to discern whether the vlpr neurons receive direct iPN input. However, we could examine the contribution of presynaptic inhibition of ePNs by comparing Ca2+ imaging of ePN terminals before and after mACT transection. If there were presynaptic inhibition on ePN terminals, and the inhibition occurred at a step at or before presynaptic Ca2+ entry that triggers neurotransmitter release as most GABA-mediated inhibition does, we would expect an elevated Ca2+ response to the same olfactory stimulation after mACT transection.
To test if ePN presynaptic Ca2+ signals are normally inhibited by iPNs, we used GH146-GAL4 to drive GCaMP3 expression, since this GAL4 labels the majority of ePNs that allowed us to image Ca2+ response of IA at their axon terminals, and a few iPNs that allowed us to target mACT for transection (Figure 6A). We found that the Ca2+ responses in the lateral horn were similar before and after mACT transection (compared Figure 6B1 and 6B2) both in their spatial patterns (Figure 6C, 6D) and in response magnitude (Figure 6E). The lack of elevation of Ca2+ signal in response to mACT transection was not due to saturation of GCaMP3 sensors in the ePN axon terminal, as this response was elevated by stimulation with a higher IA concentration (Figure 6B3). These data argue against the presynaptic inhibition mediated by reduction of Ca2+ influx as a primary mechanism for iPN inhibition.
Figure 6. Presynaptic Ca2+ signals of ePNs are not affected by mACT transection.
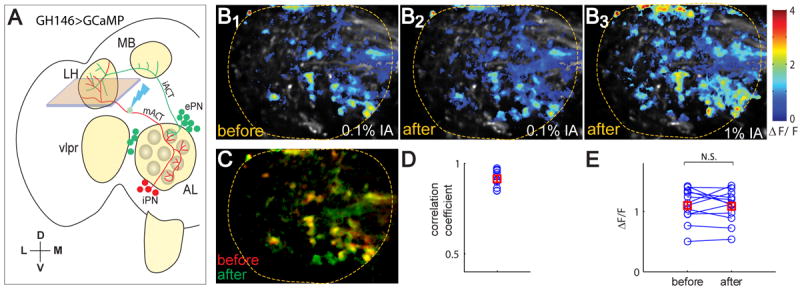
(A) In GH146-GAL4, UAS-GCaMP3 flies, lateral horn Ca2+ signal in intact flies is a sum of ~90 ePNs and 6 iPNs in intact flies. After mACT transection (guided by iPN axons), lateral horn Ca2+ signal is contributed by ePNs only, which should be elevated to intact flies if iPNs act by presynaptic inhibition, but should remain mostly unchanged if iPNs do not inhibit ePNs presynaptically (the 5 of the 6 iPNs are uniglomerular iPNs that innervating glomeruli not activated by IA; see Jefferis et al., 2007).
(B) In a representative fly, 0.1% IA evoked robust response in the axon terminals of PNs in intact fly (B1). There was no apparent change of response pattern after the mACT transection (B2). Delivery of 1% IA at the end of the experiment elevated ePN response (B3), indicating that 0.1% IA did not saturate the PN response.
(C) Overlay of the Ca2+ signals before and after mACT transection in the example fly.
(D) The spatial patterns of before and after laser transection showed high correlation.
(E) The peak ePN response to 0.1% IA was not changed after mACT transection across flies (n=12), in an ROI defined by the spatial pattern after laser transection.
Discussion
Two general circuit motifs involving inhibitory neurons are widely used in vertebrate and invertebrate nervous systems. In feedback inhibition (Figure 7A), inhibitory neurons are locally activated by excitatory neurons; they in turn inhibit a broad array of excitatory neurons including those that excite them. In feed-forward inhibition (Figure 7B), excitatory input activates both excitatory and inhibitory target neurons, and the activated inhibitory target neurons further inhibit the excitatory target neurons. The mammalian olfactory bulb, for instance, provides examples of both motifs. As an example of feedback inhibition, granule cells are activated by mitral cells in response to odor stimuli; they in turn inhibit the same and neighboring mitral cells. As an example of feed-forward inhibition, ORN axons excite periglomerular cells and mitral cells in parallel; some periglomerular cells inhibit mitral cells in the same and adjacent glomeruli. Both granule cells and periglomerular cells contribute to the lateral inhibition and sharpening of the olfactory signals that mitral cells deliver to the olfactory cortex (Shepherd et al., 2004). Similarly, the fly antennal lobe, the equivalent of mammalian olfactory bulb, has a diversity of GABAergic local interneurons (LNs) (Chou et al., 2010). Some LNs are excited by ORNs, and subsequently provide feedback inhibition onto ORN axon terminals for gain control (Olsen and Wilson, 2008b; Root et al., 2008). Other LNs may act on PN dendrites for feed-forward inhibition. Here we describe an inhibitory circuit motif that differs from classic feed-forward and feedback inhibition, which we term parallel inhibition (Figure 7C), wherein excitatory and inhibitory projection neurons receive parallel input and send parallel output to a common target region (the lateral horn; Figure 7D).
Figure 7. Inhibitory Circuit Motifs and Schematic Summary.
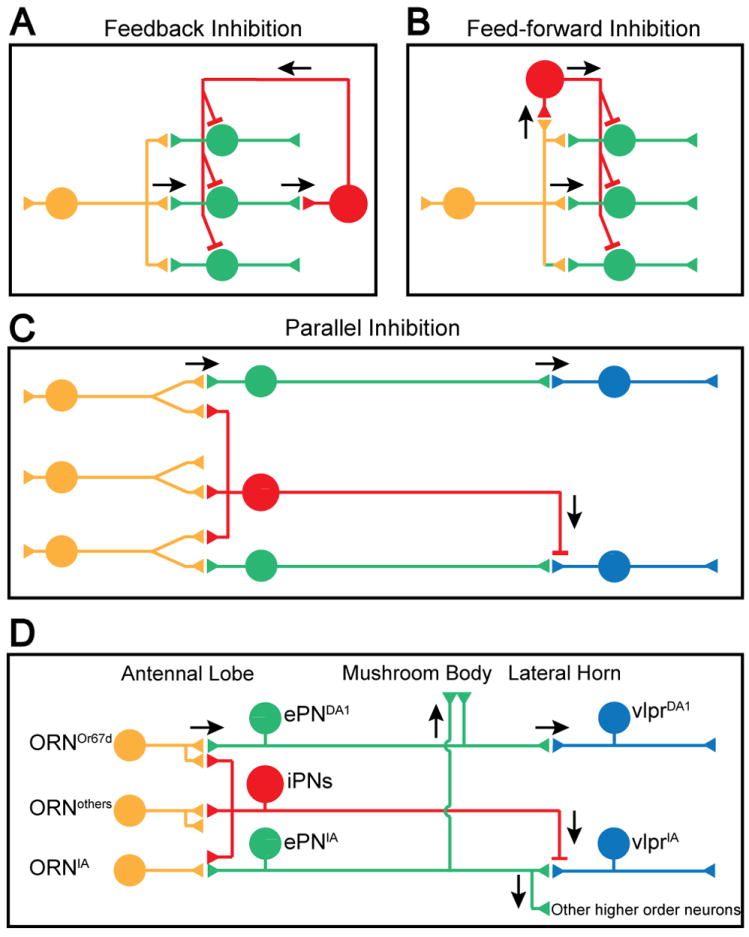
(A-C) Schematic for feedback inhibition (A), feed-forward inhibiton (B), and parallel inhibition (C). Arrows indicate the direction of signal flow. See text for details.
(D) Schematic summary of the current study. From the perspective of the Mz699+ vlpr olfactory response, iPNs function via a parallel inhibition strategy to suppress vlpr activities elicited by food odors but not pheromones. ePNIA presumably also activate other higher order neurons to elicit foraging behavior (bottom right). Likewise, ePNDA1 also activate other higher order neurons (not shown; see Ruta et al., 2010). In contrast to ePNs, which send collaterals to the mushroom body, iPNs project axons only to the lateral horn.
What are the possible roles of iPNs and what advantages might the parallel inhibition motif confer? By monitoring olfactory responses of a subset of putative 3rd order lateral horn neurons (the vlpr neurons), and by laser transecting the ascending mACT input from iPNs while sparing ePNs, we showed that iPNs selectively route olfactory input to vlpr neurons. Specifically, the vlpr responses to the food odorants are inhibited by the iPNs but the response to the cVA pheromone processing channel is not subjected to this inhibition (Figure 7D). Previous anatomical studies revealed highly stereotyped branching and terminal arborization patterns for uniglomerular ePNs and iPNs (Jefferis et al., 2007; Lai et al., 2008). Results in this study provide functional demonstration that GABAergic iPNs regulate olfactory inputs to the lateral horn neurons. Indeed, the fact that removing iPN inhibition allows IA and vinegar signals to activate vlpr neurons suggests that anatomical segregation of PN axon terminals representing food and pheromone (Jefferis et al., 2007) alone is not sufficient to prevent food odors to activate vlpr neurons, at least some of which are normally activated by pheromones. iPN inhibition provides another level of specificity of the higher order neuronal responses to olfactory input.
This specificity of inhibition provides a special feature of parallel inhibition (Figure 7C-D) in comparison with feed-forward and feedback inhibition (Figure 7A-B). Feed-forward and feedback inhibitions tend to be non-specific with respect to their target population within the same neuronal type, which is optimal for certain functions these motifs serve, such as lateral inhibition and gain control (Isaacson and Scanziani, 2011). In the Drosophila antennal lobe, for example, while exhibiting a large variety of arborization patterns, most LNs innervate many to all glomeruli, where they both receive input and send output (Chou et al., 2010). By contrast, the specific dendritic glomerular innervation of individual iPNs in the antennal lobe, as well as their stereotyped axonal arborization patterns in the lateral horn, enable iPNs to selectively inhibit some olfactory processing channels but not others (Figure 7D). We speculate that food odors should activate other lateral horn higher order neurons relevant to foraging, and that such activation is not strongly inhibited by iPNs perhaps also due to inhibition specificity (Figure 7D, bottom right).
Another interesting feature of parallel inhibition is the timing of inhibition. Inhibition from feed-forward and certainly feedback motifs arrive later than excitation due to transmission through an extra synapse, which is used to confine the magnitude and/or duration of excitation (Buzsaki, 1984; Isaacson and Scanziani, 2011). The parallel inhibition motif in principle allows for simultaneous arrival of excitation and inhibition at the postsynaptic neurons, potentially enabling inhibition to completely suppress excitation, and is ideally suited for information gating. We provided evidence that the primary action of iPNs is unlikely through presynaptic inhibition of ePNs, as ePN presynaptic Ca2+ signals in response to olfactory stimuli were not elevated by mACT transection. A caveat of this interpretation is that some forms of presynaptic inhibition can bypass Ca2+ entry, for instance through Gβγ action on the release machinery (Gerachshenko et al., 2005); however, we are not aware of GABAergic inhibition that acts in this manner. Thus, we favor the idea that iPNs act directly on postsynaptic 3rd order neurons under our experimental conditions. Due to the limited temporal resolution of Ca2+ imaging, we have not explored the temporal property of parallel inhibition in this study. It will be interesting for future research to measure the arrival time of both excitatory and inhibitory input directly with more sensitive and temporally precise electrophysiological methods.
To our knowledge, this is the first description of the use of the parallel inhibition motif in sensory systems. However, long-distance GABAergic projections are prevalent in the mammalian brain (see Introduction). Specifically, some GABAergic neurons in the hippocampus and cortex have recently been identified that send long-distance projections, sometimes to the same area as the glutamatergic projection neurons (Higo et al., 2009; Jinno et al., 2007; Melzer et al., 2012). Thus, parallel inhibition can potentially be a widely used mechanism in the nervous system.
We identified a new class of higher order neurons that respond to Or67d (and presumably cVA) activation. Or67d ORNs and their postsynaptic partner DA1 excitatory PNs express FruM, a male-specific transcription factor that is a key regulator of sexual behavior (Manoli et al., 2005; Stockinger et al., 2005). A previous study identified a number of Fru+ higher order cVA-responsive neurons whose cell bodies reside dorsal and lateral to the lateral horn (Ruta et al., 2010). Indeed, the analyses of Fru+ neurons have so far provided many examples where Fru+ neurons are connected with each other to regulate different aspects of sexual behavior (Dickson, 2008; Yu et al., 2010). However, lateral-horn projecting Mz699+ vlpr neurons do not appear to express FruM (data not shown), despite their robust activation by Fru+ Or67d ORNs. This may reflect a broad function of cVA as a pheromone that regulates not only mating but also aggression (Wang and Anderson, 2010) and social aggregation (Bartelt et al., 1985).
Our study revealed a difference between food- and pheromone-processing channels in their susceptibility to inhibition by iPNs, and suggests that pheromone channels may be insulated from general inhibition by iPNs. It is almost certain that iPNs play additional functions than reported here, as we only examined iPN function from the perspective of their effect on the olfactory response of a specific subset of higher order neurons. Indeed, in a companion manuscript, Parnas et al. showed that iPNs play an instrumental role in facilitating the discrimination of mostly food odorants as assayed by quantitative behavioral experiments. Taken together, these studies uncovered two distinct aspects of iPN function: increased discrimination of diverse food odorants and information gating between qualitatively different olfactory stimuli.
Finally, it is noteworthy that of the two major ePN targets, iPN axons only project to the lateral horn but spare the mushroom body (Figure 7D). The mushroom body is a well-documented center for olfactory learning and memory, whereas PN projections to the lateral horn are implicated in regulating innate olfactory behavior (Heimbeck et al., 2001) (see also Parnas et al., companion manuscript). ePN axons exhibit striking stereotypy in their terminal arborization patterns in the lateral horn but not in the mushroom body (Caron et al., 2013; Jefferis et al., 2007; Marin et al., 2002; Wong et al., 2002). Recent anatomical tracing in mice also revealed differential input organization in distinct olfactory cortical areas (Miyamichi et al., 2011; Sosulski et al., 2011), suggesting a common principle in olfactory systems of insects and mammals. The selective innervation by iPNs of targeting neurons in the lateral horn suggests that regulation of innate olfactory behavior engages an additional level of specific inhibition to ensure that olfactory information carrying different biological values, such as food and pheromone, is funneled into distinct downstream circuits, resulting in the activation of distinct behavioral outputs.
Experimental Procedures
Ca2+ Imaging of Odor Response
Two-photon GCaMP imaging experiments were performed with either a LSM 510 confocal/two-photon laser-scanning microscope (Zeiss) with a 40x NA 0.8 water-immersion objective (Zeiss) and modelocked Ti:Sapphire laser (Coherent) tuned to 920 nm, or a customized two-photon microscope (Prairie Technologies) with a 40x NA 1.0 water-immersion objective (Zeiss) and laser tuned to 927 nm at 73~75 °F. The excitation power at the specimen was ~10 mW and the pixel dwell time was 2.0 μs. All lateral horn images were acquired at 2.488 Hz frame rate with 460 × 300 pixels per frame. Each imaging cycle was 45 sec; 500 msec odor stimuli (as determined by the solenoid valves) were always delivered at 5 sec. To minimize bleaching, images were only taken from the first 16 sec (40 frames) of each cycle. In most experiments, the same odor was applied every other cycle for three repeats while different odors were usually applied in an alternate manner to minimize potential olfactory adaptation. Image acquisition was suspended during the 500 msec optogenetic stimulation period to protect the PMTs. On average, each imaging session lasted ~1.5 hours with most of the flies appeared healthy at the end of the experiments; they could still move their legs at a regular pace. For some experiments, the fly brains were dissected and fixed for post hoc staining.
Time lapse imaging series of GCaMP3 from a single z plane was usually recorded in the control hemisphere once before mACT transection and once after transection. On the experimental hemisphere, three different z planes were recorded for both before and after transection in most experiments, to maximize the likelihood to capture vlpr processes in the imaging plane. The z plane with the largest area of vlpr responses after laser transection was used for image analysis.
Optogenetic Stimulation
Flies of the genotype Or67d-QF/y; UAS-GCaMP3, QUAS-ChR2TR; Mz699-Gal4/+ were used for optogenetic stimulation of Or67d ORNs. Adult male flies were collected 0-2 days after eclosion and transferred to new vials containing 10 g instant fly food (Carolina Biological, formula 4-24) dissolved in 500 ml 5 mM all trans-Retinal (Sigma, R2500) and kept in a dark humidified container at room temperature. Flies were transferred to a new vial of such food every 2 days for 8~10 days before imaging. ChR2TR was activated by a 473 nm diode pumped blue solid-state laser (CrystaLaser 60mW). The blue laser was coupled to an optical fiber for light delivery. The other end of the fiber was screwed into a connector mounted on the fly chamber so as to deliver the same light power for each experiment. The same TriggerSync plugin (Prairie Technology) was used to synchronize the laser activation and image acquisition. For each imaging cycle, optogenetic stimulation was on between 5 sec and 5.5 sec.
Laser Transection
Guided by the GCaMP3 basal level fluorescence at 927 nm, we first defined a transection window (~4 μm × 3 μm, at zoom ×10 and in a single focal plane) centered on the mACT about 10 μm before its entry site into the lateral horn. A ~80mW laser pulse (measured after the objective) at 800 nm was then applied onto this window. The pulse contains 16 repetitions of continuous frame scanning with pixel dwell time of 8 μsec and a total estimated energy of 0.04 J. Successful transection usually resulted in a small cavitation bubble formed in the mACT as reported before (Vogel and Venugopalan, 2003).
Supplemental Experimental Procedures contain addition sections on Genetics & Molecular Biology, MARCM Analysis & Immunostaining, Preparing Flies for Ca2+ Imaging, and Data Analysis.
Supplementary Material
Highlights.
Excitatory and inhibitory PNs send parallel output to a higher olfactory center
Inhibitory PNs (iPNs) suppress food-related odor responses of higher-order neurons
Pheromone channels are insulated from iPN inhibition
Parallel inhibition can route specific olfactory information to distinct circuits
Acknowledgments
We thank Y. Chou, B. Dickson, V. Jayaraman, T. Lee, L. Looger, K. Wehner, X. Yu, the Bloomington Stock Center, the Vienna Drosophila RNAi Center, the Developmental Studies Hybridoma Bank, the BACPAC Resources Center, for fly stocks, reagents and antibodies; S. Sinha, D. Profitt and D. Luginbuhl for technical assistance; K. Beier, X. Chen, T. Clandinin, X. Gao, N. Makki, A. Mizrahi, T. Mosca and R. Wilson for providing critiques of the manuscript; M. Schnitzer for support; G. Miesenböck for communicating unpublished data. This work is supported by an NIH grant (R01-DC005982 to L. Luo). L. Liang has been supported by a Stanford Graduate Fellowship and a Lubert Stryer Stanford Interdisciplinary Graduate Fellowship. L. Luo is an investigator of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bargmann CI. Comparative chemosensation from receptors to ecology. Nature. 2006;444:295–301. doi: 10.1038/nature05402. [DOI] [PubMed] [Google Scholar]
- Bartelt RJ, Schaner AM, Jackson LL. cis-Vaccenyl acetate as an aggregation pheromone in Drosophila melanogaster. J Chem Ecology. 1985;11:1747–1756. doi: 10.1007/BF01012124. [DOI] [PubMed] [Google Scholar]
- Berndt A, Yizhar O, Gunaydin LA, Hegemann P, Deisseroth K. Bi-stable neural state switches. Nat Neurosci. 2009;12:229–234. doi: 10.1038/nn.2247. [DOI] [PubMed] [Google Scholar]
- Bhandawat V, Olsen SR, Gouwens NW, Schlief ML, Wilson RI. Sensory processing in the Drosophila antennal lobe increases reliability and separability of ensemble odor representations. Nat Neurosci. 2007;10:1474–1482. doi: 10.1038/nn1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G. Feed-forward inhibition in the hippocampal formation. Progress in neurobiology. 1984;22:131–153. doi: 10.1016/0301-0082(84)90023-6. [DOI] [PubMed] [Google Scholar]
- Caron SJ, Ruta V, Abbott LF, Axel R. Random convergence of olfactory inputs in the Drosophila mushroom body. Nature. 2013;497:113–117. doi: 10.1038/nature12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou YH, Spletter ML, Yaksi E, Leong JC, Wilson RI, Luo L. Diversity and wiring variability of olfactory local interneurons in the Drosophila antennal lobe. Nat Neurosci. 2010;13:439–449. doi: 10.1038/nn.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RL. Olfactory memory formation in Drosophila: from molecular to systems neuroscience. Annu Rev Neurosci. 2005;28:275–302. doi: 10.1146/annurev.neuro.28.061604.135651. [DOI] [PubMed] [Google Scholar]
- Dickson BJ. Wired for sex: the neurobiology of Drosophila mating decisions. Science. 2008;322:904–909. doi: 10.1126/science.1159276. [DOI] [PubMed] [Google Scholar]
- Ejima A, Smith BP, Lucas C, van der Goes van Naters W, Miller CJ, Carlson JR, Levine JD, Griffith LC. Generalization of courtship learning in Drosophila is mediated by cis-vaccenyl acetate. Curr Biol. 2007;17:599–605. doi: 10.1016/j.cub.2007.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerachshenko T, Blackmer T, Yoon EJ, Bartleson C, Hamm HE, Alford S. Gbetagamma acts at the C terminus of SNAP-25 to mediate presynaptic inhibition. Nat Neurosci. 2005;8:597–605. doi: 10.1038/nn1439. [DOI] [PubMed] [Google Scholar]
- Grosjean Y, Rytz R, Farine JP, Abuin L, Cortot J, Jefferis GS, Benton R. An olfactory receptor for food-derived odours promotes male courtship in Drosophila. Nature. 2011;478:236–240. doi: 10.1038/nature10428. [DOI] [PubMed] [Google Scholar]
- Hallem EA, Carlson JR. Coding of odors by a receptor repertoire. Cell. 2006;125:143–160. doi: 10.1016/j.cell.2006.01.050. [DOI] [PubMed] [Google Scholar]
- Heimbeck G, Bugnon V, Gendre N, Keller A, Stocker RF. A central neural circuit for experience-independent olfactory and courtship behavior in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2001;98:15336–15341. doi: 10.1073/pnas.011314898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisenberg M. Mushroom body memoir: from maps to models. Nat Rev Neurosci. 2003;4:266–275. doi: 10.1038/nrn1074. [DOI] [PubMed] [Google Scholar]
- Higo S, Akashi K, Sakimura K, Tamamaki N. Subtypes of GABAergic neurons project axons in the neocortex. Frontiers in neuroanatomy. 2009;3:25. doi: 10.3389/neuro.05.025.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson JS, Scanziani M. How inhibition shapes cortical activity. Neuron. 2011;72:231–243. doi: 10.1016/j.neuron.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferis GS, Potter CJ, Chan AM, Marin EC, Rohlfing T, Maurer CR, Jr, Luo L. Comprehensive maps of Drosophila higher olfactory centers: spatially segregated fruit and pheromone representation. Cell. 2007;128:1187–1203. doi: 10.1016/j.cell.2007.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferis GSXE, Marin EC, Stocker RF, Luo L. Target neuron prespecification in the olfactory map of Drosophila. Nature. 2001;414:204–208. doi: 10.1038/35102574. [DOI] [PubMed] [Google Scholar]
- Jinno S, Klausberger T, Marton LF, Dalezios Y, Roberts JD, Fuentealba P, Bushong EA, Henze D, Buzsaki G, Somogyi P. Neuronal diversity in GABAergic long-range projections from the hippocampus. J Neurosci. 2007;27:8790–8804. doi: 10.1523/JNEUROSCI.1847-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffler SW. Discharge patterns and functional organization of mammalian retina. J Neurophysiol. 1953;16:37–68. doi: 10.1152/jn.1953.16.1.37. [DOI] [PubMed] [Google Scholar]
- Kuppers B, Sanchez-Soriano N, Letzkus J, Technau GM, Prokop A. In developing Drosophila neurones the production of gamma-amino butyric acid is tightly regulated downstream of glutamate decarboxylase translation and can be influenced by calcium. Journal of neurochemistry. 2003;84:939–951. doi: 10.1046/j.1471-4159.2003.01554.x. [DOI] [PubMed] [Google Scholar]
- Kurtovic A, Widmer A, Dickson BJ. A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature. 2007;446:542–546. doi: 10.1038/nature05672. [DOI] [PubMed] [Google Scholar]
- Lai SL, Awasaki T, Ito K, Lee T. Clonal analysis of Drosophila antennal lobe neurons: diverse neuronal architectures in the lateral neuroblast lineage. Development. 2008;135:2883–2893. doi: 10.1242/dev.024380. [DOI] [PubMed] [Google Scholar]
- Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- Liang L, Luo L. The olfactory circuit of the fruit fly Drosophila melanogaster. Science China Life sciences. 2010;53:472–484. doi: 10.1007/s11427-010-0099-z. [DOI] [PubMed] [Google Scholar]
- Manoli DS, Foss M, Villella A, Taylor BJ, Hall JC, Baker BS. Male-specific fruitless specifies the neural substrates of Drosophila courtship behaviour. Nature. 2005;436:395–400. doi: 10.1038/nature03859. [DOI] [PubMed] [Google Scholar]
- Marin EC, Jefferis GSXE, Komiyama T, Zhu H, Luo L. Representation of the glomerular olfactory map in the Drosophila brain. Cell. 2002;109:243–255. doi: 10.1016/s0092-8674(02)00700-6. [DOI] [PubMed] [Google Scholar]
- Melzer S, Michael M, Caputi A, Eliava M, Fuchs EC, Whittington MA, Monyer H. Long-range-projecting GABAergic neurons modulate inhibition in hippocampus and entorhinal cortex. Science. 2012;335:1506–1510. doi: 10.1126/science.1217139. [DOI] [PubMed] [Google Scholar]
- Miyamichi K, Amat F, Moussavi F, Wang C, Wickersham I, Wall NR, Taniguchi H, Tasic B, Huang ZJ, He Z, et al. Cortical representations of olfactory input by trans-synaptic tracing. Nature. 2011;472:191–196. doi: 10.1038/nature09714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel G, Brauner M, Liewald JF, Adeishvili N, Bamberg E, Gottschalk A. Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Curr Biol. 2005;15:2279–2284. doi: 10.1016/j.cub.2005.11.032. [DOI] [PubMed] [Google Scholar]
- Okada R, Awasaki T, Ito K. Gamma-aminobuyric acid (GABA)-mediated neural connections in the Drosophila antennal lobe. J Comp Neurol. 2009;514:74–91. doi: 10.1002/cne.21971. [DOI] [PubMed] [Google Scholar]
- Olsen SR, Bhandawat V, Wilson RI. Divisive normalization in olfactory population codes. Neuron. 2010;66:287–299. doi: 10.1016/j.neuron.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen SR, Wilson RI. Cracking neural circuits in a tiny brain: new approaches for understanding the neural circuitry of Drosophila. Trends Neurosci. 2008a;31:512–520. doi: 10.1016/j.tins.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen SR, Wilson RI. Lateral presynaptic inhibition mediates gain control in an olfactory circuit. Nature. 2008b;452:956–960. doi: 10.1038/nature06864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter CJ, Tasic B, Russler EV, Liang L, Luo L. The Q system: a repressible binary system for transgene expression, lineage tracing, and mosaic analysis. Cell. 2010;141:536–548. doi: 10.1016/j.cell.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root CM, Masuyama K, Green DS, Enell LE, Nassel DR, Lee CH, Wang JW. A presynaptic gain control mechanism fine-tunes olfactory behavior. Neuron. 2008;59:311–321. doi: 10.1016/j.neuron.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruta V, Datta SR, Vasconcelos ML, Freeland J, Looger LL, Axel R. A dimorphic pheromone circuit in Drosophila from sensory input to descending output. Nature. 2010;468:686–690. doi: 10.1038/nature09554. [DOI] [PubMed] [Google Scholar]
- Semmelhack JL, Wang JW. Select Drosophila glomeruli mediate innate olfactory attraction and aversion. Nature. 2009;459:218–223. doi: 10.1038/nature07983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd GM, Chen WR, Greer CA. Olfactory Bulb. In: Shepherd GM, editor. The synaptic organization of the brain. Oxford: Oxford University Press; 2004. [Google Scholar]
- Sherrington CS. The Integrative Action of the Nervous System. Yale University Press; 1906. Reprint. [Google Scholar]
- Silbering AF, Rytz R, Grosjean Y, Abuin L, Ramdya P, Jefferis GS, Benton R. Complementary function and integrated wiring of the evolutionarily distinct Drosophila olfactory subsystems. J Neurosci. 2011;31:13357–13375. doi: 10.1523/JNEUROSCI.2360-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosulski DL, Bloom ML, Cutforth T, Axel R, Datta SR. Distinct representations of olfactory information in different cortical centres. Nature. 2011;472:213–216. doi: 10.1038/nature09868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker RF, Lienhard MC, Borst A, Fischbach KF. Neuronal architecture of the antennal lobe in Drosophila melanogaster. Cell and tissue research. 1990;262:9–34. doi: 10.1007/BF00327741. [DOI] [PubMed] [Google Scholar]
- Stockinger P, Kvitsiani D, Rotkopf S, Tirian L, Dickson BJ. Neural circuitry that governs Drosophila male courtship behavior. Cell. 2005;121:795–807. doi: 10.1016/j.cell.2005.04.026. [DOI] [PubMed] [Google Scholar]
- Su CY, Martelli C, Emonet T, Carlson JR. Temporal coding of odor mixtures in an olfactory receptor neuron. Proc Natl Acad Sci U S A. 2011;108:5075–5080. doi: 10.1073/pnas.1100369108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su CY, Menuz K, Carlson JR. Olfactory perception: receptors, cells, and circuits. Cell. 2009;139:45–59. doi: 10.1016/j.cell.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka NK, Awasaki T, Shimada T, Ito K. Integration of chemosensory pathways in the Drosophila second-order olfactory centers. Curr Biol. 2004;14:449–457. doi: 10.1016/j.cub.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Tian L, Hires SA, Mao T, Huber D, Chiappe ME, Chalasani SH, Petreanu L, Akerboom J, McKinney SA, Schreiter ER, et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods. 2009;6:875–881. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Goes van Naters W, Carlson JR. Receptors and neurons for fly odors in Drosophila. Curr Biol. 2007;17:606–612. doi: 10.1016/j.cub.2007.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel A, Venugopalan V. Mechanisms of pulsed laser ablation of biological tissues. Chemical reviews. 2003;103:577–644. doi: 10.1021/cr010379n. [DOI] [PubMed] [Google Scholar]
- Vosshall LB, Stocker RF. Molecular architecture of smell and taste in Drosophila. Annu Rev Neurosci. 2007;30:505–533. doi: 10.1146/annurev.neuro.30.051606.094306. [DOI] [PubMed] [Google Scholar]
- Wang JW, Wong AM, Flores J, Vosshall LB, Axel R. Two-photon calcium imaging reveals an odor-evoked map of activity in the fly brain. Cell. 2003;112:271–282. doi: 10.1016/s0092-8674(03)00004-7. [DOI] [PubMed] [Google Scholar]
- Wang L, Anderson DJ. Identification of an aggression-promoting pheromone and its receptor neurons in Drosophila. Nature. 2010;463:227–231. doi: 10.1038/nature08678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AM, Wang JW, Axel R. Spatial representation of the glomerular map in the Drosophila protocerebrum. Cell. 2002;109:229–241. doi: 10.1016/s0092-8674(02)00707-9. [DOI] [PubMed] [Google Scholar]
- Yu JY, Kanai MI, Demir E, Jefferis GS, Dickson BJ. Cellular organization of the neural circuit that drives Drosophila courtship behavior. Curr Biol. 2010;20:1602–1614. doi: 10.1016/j.cub.2010.08.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


