Abstract
Metabolism of the teratogen thalidomide is proposed to be relevant to its toxicological action. We demonstrated formation of the glutathione (GSH) conjugate of (R)-5-hydroxythalidomide in vivo in chimeric NOD-scid IL2Rgnull mice with humanized liver (uPA-NOG mice). After an oral administration of racemic thalidomide (270 mg/kg), plasma concentrations of 5-hydroxythalidomide were significantly higher in humanized mice than in control mice. The GSH conjugate of 5-hydroxythalidomide was detected in the plasma. These results indicate that livers of humanized mice mediate thalidomide 5-hydroxylation and further oxidation leading to the GSH conjugate in vivo as well as in vitro and suggest that thalidomide activation occurs.
P4501 comprises a superfamily of enzymes (1) involved in the oxidation of a large number of endogenous and exogenous compounds, including the teratogenic drug thalidomide (2). Thalidomide was withdrawn from clinical use in the early 1960s because of its teratogenic effects in humans but has been approved for the treatment of refractory multiple myeloma since 2000 (3, 4). Significant roles for human P450 3A4 and 3A5 were indicated for thalidomide oxidation in our recent study (2), in addition to participation of human P450 2C19 (5). Human P450s 3A4 and 3A5 mediated thalidomide 5-hydroxylation and further oxidation of this product, leading to non-enzymatic GSH conjugation (Figure 1), which might be relevant to its pharmacological and toxicological actions. However, it is not known if these two-step oxidations of thalidomide and trapping by GSH occur in human in vivo situations, particularly in light of the low in vitro activity and high Km values for oxidation (2).
Figure 1.
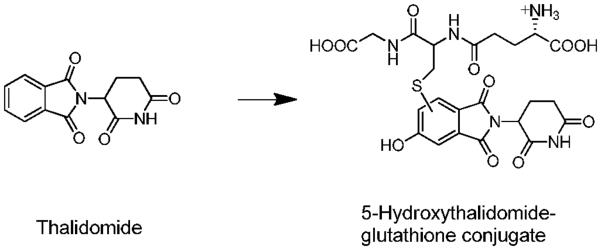
Chemical structures of thalidomide and a 5-hydroxythalidomide GSH conjugate (2).
“Humanized” mice, in which various kinds of human cells can be engrafted and retain the same functions as in humans, are extremely useful (6). With the newly combined immunodeficient non-obese diabetes (NOD)- severe combined immunodeficiency (scid) interleukin-2 receptor gamma chain-deficient (IL2Rgnull) NOD-scid IL2Rgnull (NOG) mouse model expressing transgenic urokinase-type plasminogen activator (uPA) in the liver (uPA-NOG) (7), it has become possible to expand applications by human hepatocyte transplantation. The uPA-NOG mouse model has several advantages over previous chimeric mouse models of human liver, including easier surgical manipulation and improved recipient survival (6, 7). The purpose of this study was to investigate the oxidative metabolism of thalidomide by human liver P450 enzymes in vivo and to better understand the activation of thalidomide in uPA-NOG mice utilizing human liver cells. Conjugation of the secondary oxidized product 5-hydroxythalidomide with GSH (2) in vivo is now reported, indicating that activation of thalidomide occurs to produce an electrophilic product in humanized mice.
The metabolism of thalidomide was first investigated in vitro, based on earlier work (2) (Table 1). (R)-Thalidomide was oxidized to 5'- and 5-hydroxythalidomide by recombinant P450s 2C19, 3A4, and 3A5. P450 3A5 was able to transform thalidomide to a reactive metabolite(s) that bounds to cellular macromolecule, e.g. GSH (shown in Figure 1), directly in the presence of GSH. Under these conditions, liver microsomes prepared from control uPA-NOG mice preferentially mediated 5'-hydroxythalidomide formation (Table 1). On the other hand, liver microsomes prepared from humanized uPA-NOG mice and pooled human liver microsomes catalyzed 5'- and 5-hydroxythalidomide formation to similar extents. Recombinant P450 enzymes used in the present study produced 5'-hydroxythalidomide in a similar way as liver microsomes (Table 1), in contrast to our previously reported lower activity (using a different expression system (2)). When (R)-5-hydroxythalidomide was used as a substrate, transformation of this primary metabolite of thalidomide to the GSH conjugate was observed in liver microsomes in the present study.
Table 1.
Metabolism of thalidomide and 5-hydroxythalidomide catalyzed by human or mouse liver microsomes and human P450s expressed in insect cells.
| substrate |
||||
|---|---|---|---|---|
| (R)-thalidomide |
(R)-5-OH-thalidomide |
|||
| enzyme source | 5-OH-thalidomide | 5'-OH-thalidomide | GSH adduct | GSH adduct |
| microsomes | pmol formed min−1 (mg protein)−1 | |||
| uPA-NOG mice | 1.6 | 48 | <0.1 | 2.0 |
| uPA-NOG mice with transplantation of human liver cells | 3.3 | 4.9 | <0.1 | 2.1 |
| pooled human liver (H150) | 0.4 | 1.1 | <0.1 | 3.2 |
| baculovirus expression system | pmol formed min−1 (nmol P450)−1 | |||
| P450 2C19 | 23 | 66 | <0.1 | 12 |
| P450 3A4 | 1.4 | 26 | <0.1 | 11 |
| P450 3A5 | 1.1 | 9.8 | 0.8 | 9.1 |
Thalidomide and 5-hydroxythalidomide (1000 μM) were incubated with liver microsomes (1.0 mg protein mL−1) or P450 2C19, 3A4, or 3A5 (100 nM P450, Supersomes®, BD Biosciences) in the presence of an NADPH-generating system and GSH (5 mM) at 37 °C for 60 min. The pooled human liver microsomes are from a set of 150 individuals (BD Biosciences).
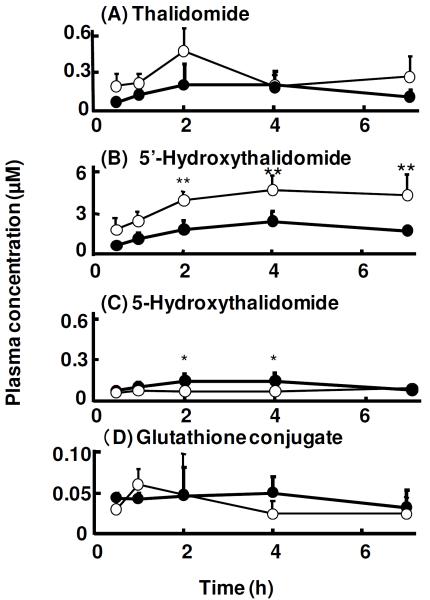
Thalidomide and its primary metabolite, 5-hydroxythalidomide, were detected by LC-MS/MS analysis in plasma obtained from mice following oral administration (270 mg/kg) (retention times of thalidomide and 5'- or 5-hydroxythalidomide were 6.6 and 6.9 min, respectively, under the present conditions, data not shown). Thalidomide was absorbed and detected 30 min after its oral administration (Figure 2A). Thalidomide concentrations in plasma were not different between control uPA-NOG mice and humanized uPA-NOG mice. Plasma concentrations of 5'-thalidomide were significantly higher in control uPA-NOG mice (3.9 and 4.7 μM at 2 and 4 h, respectively) than in humanized uPA-NOG mice (1.8 and 2.4 μM) (Figure 2B). In contrast, 5-hydroxythalidomide—a thalidomide metabolite known to be activated to reactive epoxide products (2)—was preferentially produced in humanized uPA-NOG mice (Figure 2C). The 5-hydroxythalidomide-GSH conjugate formed in vivo was measured using LC-MS/MS (using the m/z 580→451 transition) (Figure S1, Supporting Information) based on co-migration using in vitro samples in which the GSH conjugate had previously been characterized (2). 5-Hydroxythalidomide-GSH conjugates were detected in plasma samples from thalidomide administered to either control NOG mice or humanized uPA-NOG mice (Figure 2D).
Figure 2.
Plasma concentrations of thalidomide (A), 5'-hydroxythalidomide (B), 5-hydroxythalidomide (C), and GSH conjugate (D) in control uPA-NOG mice (◯) and chimeric uPA-NOG mice with humanized liver cells (●) after oral administration of thalidomide (270 mg/kg). Results are expressed as mean values (± SD) obtained with four mice (*p < 0.05 and **p < 0.01, two-way ANOVA with Bonferroni post tests).
Thalidomide has been the subject of numerous studies following the teratogenicity problems associated with its use 50 years ago (3, 8). However, relatively few studies have been done on the metabolism of thalidomide in vivo. With the renewed interest in this drug for other indications where teratology is not an issue (3, 4, 9), several studies have been reported. Earlier work had shown the presence of phenolic products produced in rabbits, a sensitive species, but not in rats (10, 11). Subsequent work has confirmed that the 4-and 5-hydroxylated and 5'-hydroxylated products are formed in various species including in humans (5, 10, 12–14). Our recent in vitro studies demonstrated that 5-hydroxythalidomide can be further oxidized by recombinant P450s 3A4 and 3A5 and finally forms (multiple) phenyl ring-based 5-hydroxythalidomide-GSH conjugates (Figure 1) via a highly reactive and unstable intermediate(s) (2).
Thalidomide, a mild sedative drug, was responsible for inducing congenital malformation of limbs in children whose mothers took the drug during pregnancy (15). Numerous hypotheses have been proposed to explain thalidomide teratogenesis, including oxidative stress, growth control of angiogenesis, and gene expression control for adhesion receptors (16–18). Although the pharmacological basis for the effects of thalidomide remains controversial, the net results of thalidomide exposure are decreased growth of limb-bud mesenchyme in primates and rabbits but not in rodents (16, 19). Differences in species susceptibility might result from differences in biotransformation of the compound by drug metabolizing enzymes. Schumacher et al. (11) reported that (phenyl ring) 4- and 5-hydroxylated metabolites of thalidomide were recovered from the urine of rabbits but not from rats, and more thalidomide metabolites were bound to liver macromolecules in the rabbit than in the rat. Limited information is available regarding thalidomide metabolism by P450s in vivo, especially in humans. In the present study, we analyzed thalidomide metabolism in vivo using humanized uPA-NOG mice in which the liver was replaced with transplanted human liver cells. Preferentially higher levels of 5-hydroxythalidomide (that would be metabolized by human P450 3A enzymes (2)) were observed in humanized mouse plasma samples (Figure 2). Furthermore, we demonstrated the presence of 5-hydroxythalidomide-GSH conjugates in humanized mice plasma after oral administration of thalidomide. GSH was attached on the phenyl ring of 5-hydroxythalidomide (2). The presence of phenolic derivatives of thalidomide suggests that the drug might undergo oxidative metabolism via an arene oxide intermediate (20), and our in vitro work showed that the conjugate could be explained by an epoxide intermediate but not a catechol (2). Thus, congenital malformation of the limb in children caused by the thalidomide may result from an arene oxide metabolite that covalently binds to critical structures in the developing fetus, as proposed by Gordon et al. (20).
Recently Ito et al. (21) reported (non-covalent) binding of thalidomide with the protein cereblon to form an E3 ubiquitin ligase complex and proposed teratogenic effects due to the inhibition of the associated ubiquitin ligase activity. Enzymatic deactivation of these reactive metabolite(s) is also a possibility with regard to species differences in thalidomide toxicity. In this context, the humanized uPA-NOG model may serve as a suitable in vivo tool for studying thalidomide metabolism and for the toxic effects of several intermediate metabolites against both pregnant and fetal animals/humans. It will be of interest to investigate the toxic effects of administration of 5-hydroxythialidomide to humanized mice in order to address the issue of reactive metabolite formation in vivo, following the in vitro studies (2) and those reported here. The possible production of reactive metabolites of thalidomide by intestinal mouse P450s has not yet been considered (when the drug is administered orally to chimeric mice with humanized liver). In conclusion, the present study suggests that human liver cells expressed in chimeric mice effectively mediate thalidomide 5-hydroxylation and further oxidation leading to a GSH conjugate in vivo, which may be relevant to its pharmacological and toxicological actions via adduct formation.
Supplementary Material
Acknowledgment
The authors thank Dr. Norie Murayama for her assistance.
Funding Support. This work was supported in part by the Ministry of Education, Culture, Sports, Science, and Technology of Japan (H.Y.) and United States Public Health Service grants R37 CA090426 and P30 ES000267 (F.P.G.).
Footnotes
Abbreviations: IL2Rgnull; interleukin-2 receptor gamma chain-deficient; NOD, non-obese diabetes; scid, severe combined immunnodeficiency; NOG, NOD-scid IL2Rgnull; uPA, urokinase-type plasminogen activator.
Supporting Information Available: Experimental Procedures. Representative ESI-LC-MS/MS chromatogram of the GSH conjugate of oxidized 5-hydroxythalidomide. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- (1).Guengerich FP. Cytochrome P450 and chemical toxicology. Chem. Res. Toxicol. 2008;21:70–83. doi: 10.1021/tx700079z. [DOI] [PubMed] [Google Scholar]
- (2).Chowdhury G, Murayama N, Okada Y, Uno Y, Shimizu M, Shibata N, Guengerich FP, Yamazaki H. Human liver microsomal cytochrome P450 3A enzymes involved in thalidomide 5-hydroxylation and formation of a glutathione conjugate. Chem. Res. Toxicol. 2010;23:1018–1024. doi: 10.1021/tx900367p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Calabrese L, Resztak K. Thalidomide revisited: pharmacology and clinical applications. Expert. Opin. Investig. Drugs. 1998;7:2043–2060. doi: 10.1517/13543784.7.12.2043. [DOI] [PubMed] [Google Scholar]
- (4).Sembongi K, Tanaka M, Sakurada K, Kobayashi M, Itagaki S, Hirano T, Iseki K. A new method for determination of both thalidomide enantiomers using HPLC systems. Biol. Pharm. Bull. 2008;31:497–500. doi: 10.1248/bpb.31.497. [DOI] [PubMed] [Google Scholar]
- (5).Ando Y, Fuse E, Figg WD. Thalidomide metabolism by the CYP2C subfamily. Clin. Cancer Res. 2002;8:1964–1973. [PubMed] [Google Scholar]
- (6).Ito M, Kobayashi K, Nakahata T. NOD/Shi-scid IL2rgamma(null) (NOG) mice more appropriate for humanized mouse models. Curr. Top. Microbiol. Immunol. 2008;324:53–76. doi: 10.1007/978-3-540-75647-7_3. [DOI] [PubMed] [Google Scholar]
- (7).Suemizu H, Hasegawa M, Kawai K, Taniguchi K, Monnai M, Wakui M, Suematsu M, Ito M, Peltz G, Nakamura M. Establishment of a humanized model of liver using NOD/Shi-scid IL2Rgnull mice. Biochem. Biophys. Res. Commun. 2008;377:248–252. doi: 10.1016/j.bbrc.2008.09.124. [DOI] [PubMed] [Google Scholar]
- (8).Bosch ME, Sanchez AJ, Rojas FS, Ojeda CB. Recent advances in analytical determination of thalidomide and its metabolites. J. Pharm. Biomedi. Anal. 2008;46:9–17. doi: 10.1016/j.jpba.2007.10.003. [DOI] [PubMed] [Google Scholar]
- (9).Okada Y, Murayama N, Yanagida C, Shimizu M, Guengerich FP, Yamazaki H. Drug interactions of thalidomide with midazolam and cyclosporine A: heterotropic cooperativity of human cytochrome P450 3A5. Drug Metab. Dispos. 2009;37:18–23. doi: 10.1124/dmd.108.024679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Eriksson T, Bjorkman S, Roth B, Bjork H, Hoglund P. Hydroxylated metabolites of thalidomide: formation in-vitro and in-vivo in man. J. Pharm. Pharmacol. 1998;50:1409–1416. doi: 10.1111/j.2042-7158.1998.tb03368.x. [DOI] [PubMed] [Google Scholar]
- (11).Schumacher H, Smith RL, Williams RT. The metabolism of thalidomide: the fate of thalidomide and some of its hydrolysis products in various species. Br. J. Pharmacol. Chemother. 1965;25:338–351. doi: 10.1111/j.1476-5381.1965.tb02054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Lu J, Helsby N, Palmer BD, Tingle M, Baguley BC, Kestell P, Ching LM. Metabolism of thalidomide in liver microsomes of mice, rabbits, and humans. J. Pharmacol. Exp. Ther. 2004;310:571–577. doi: 10.1124/jpet.104.067793. [DOI] [PubMed] [Google Scholar]
- (13).Ando Y, Price DK, Dahut WL, Cox MC, Reed E, Figg WD. Pharmacogenetic associations of CYP2C19 genotype with in vivo metabolisms and pharmacological effects of thalidomide. Cancer Biol. Ther. 2002;1:669–673. doi: 10.4161/cbt.318. [DOI] [PubMed] [Google Scholar]
- (14).Teo SK, Sabourin PJ, O'Brien K, Kook KA, Thomas SD. Metabolism of thalidomide in human microsomes, cloned human cytochrome P-450 isozymes, and Hansen's disease patients. J. Biochem. Mol. Toxicol. 2000;14:140–147. doi: 10.1002/(sici)1099-0461(2000)14:3<140::aid-jbt3>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- (15).Lenz W. A short history of thalidomide embryopathy. Teratology. 1988;38:203–215. doi: 10.1002/tera.1420380303. [DOI] [PubMed] [Google Scholar]
- (16).Parman T, Wiley MJ, Wells PG. Free radical-mediated oxidative DNA damage in the mechanism of thalidomide teratogenicity. Nat. Med. 1999;5:582–585. doi: 10.1038/8466. [DOI] [PubMed] [Google Scholar]
- (17).Therapontos C, Erskine L, Gardner ER, Figg WD, Vargesson N. Thalidomide induces limb defects by preventing angiogenic outgrowth during early limb formation. Proc. Natl. Acad. Sci. U. S. A. 2009;106:8573–8578. doi: 10.1073/pnas.0901505106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Neubert R, Hinz N, Thiel R, Neubert D. Down-regulation of adhesion receptors on cells of primate embryos as a probable mechanism of the teratogenic action of thalidomide. Life Sci. 1996;58:295–316. doi: 10.1016/0024-3205(95)02290-2. [DOI] [PubMed] [Google Scholar]
- (19).Stephens TD. Proposed mechanisms of action in thalidomide embryopathy. Teratology. 1988;38:229–239. doi: 10.1002/tera.1420380307. [DOI] [PubMed] [Google Scholar]
- (20).Gordon GB, Spielberg SP, Blake DA, Balasubramanian V. Thalidomide teratogenesis: evidence for a toxic arene oxide metabolite. Proc. Natl. Acad. Sci. U. S. A. 1981;78:2545–2548. doi: 10.1073/pnas.78.4.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Ito T, Ando H, Suzuki T, Ogura T, Hotta K, Imamura Y, Yamaguchi Y, Handa H. Identification of a primary target of thalidomide teratogenicity. Science. 2010;327:1345–1350. doi: 10.1126/science.1177319. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.