Abstract
When an antipsychotic drug is given repeatedly and intermittently, there is often a long-term increase in its behavioral efficacy, termed antipsychotic sensitization. With the passage of time, the magnitude of antipsychotic sensitization may increase or decrease depending on the principle of Time-Dependent Sensitization (TDS) or memory decay, respectively. In the present study, we examined the time-dependent feature and possible dopamine D2 receptor mechanism of sensitization induced by the antipsychotics risperidone and asenapine in the conditioned avoidance response test. Well-trained male adult Sprague-Dawley rats were first repeatedly treated with risperidone (1.0 mg/kg) or asenapine (0.2 mg/kg) and tested for avoidance response daily for 5 consecutive days. Eight, 18 or 38 days after the 5th drug treatment, all rats were retested drug-free to assess the residual impact of prior risperidone or asenapine treatment. Drug-pretreated rats had significantly lower avoidance than vehicle-pretreated ones on this test, and the group differences increased with the passage of time. In the subsequent drug challenge test at 10, 20 or 40 days after the 5th drug treatment, all rats were injected with a low dose of risperidone (0.3 mg/kg) or asenapine (0.1 mg/kg). Drug-pretreated rats again made significantly less avoidances than controls, confirming the drug-induced sensitization effect. Finally, in the quinpirole (a D2/3 receptor agonist, 1.0 mg/kg, sc)-induced hyperlocomotion test, risperidone-pretreated rats exhibited a significantly higher level of motor activity than the vehicle-pretreated ones. These findings suggest that risperidone and asenapine sensitization is long-lasting, follows the TDS principle, and is likely mediated by D2 receptor supersensitivity.
Keywords: Time-Dependent Sensitization (TDS), Asenapine, Risperidone, Conditioned avoidance response, Quinpirole, Locomotor activity
1. Introduction
It is well documented that repeated and intermittent administrations of most antipsychotic drugs often cause an increase in the behavioral responsiveness to these drugs. This phenomenon is termed antipsychotic sensitization. Supersensitivity psychosis, tardive dyskinesia, and time-dependent increase in antipsychotic response are several well-known clinical examples of antipsychotic sensitization [1-3]. In preclinical studies, antipsychotic sensitization has been demonstrated in several different behavioral models [4-7]. It is often measured using two different methods[8] similar to those for assessing psychostimulant-induced behavioral sensitization[9]. The first index of antipsychotic sensitization is revealed through a within-subjects comparison, in which the behavioral effect of a drug treatment is stronger on the last treatment day than the first day (i.e., a comparison between days 1 and 5). The second index of antipsychotic sensitization is provided by a between-subjects comparison, in which the behavioral response of drug-pretreated animals to a challenge dose of an antipsychotic drug is compared to the response of vehicle-pretreated control animals. Here, antipsychotic sensitization is demonstrated by an increased sensitivity to the drug challenge in drug-pretreated animals relative to those pretreated with vehicle.
The conditioned avoidance response (CAR) model is an aversion motivated instrumental conditioning model, which is traditionally used in in the preclinical study of antipsychotic drugs (APDs) [10, 11]. In this model, animals can be trained to prevent the occurrence of an aversive stimulation (e.g. electric footshock) by performing a specific response to a conditioned stimulus (e.g. tone). This response is thought to reflect a persecutory delusion [12]. The treatment of antipsychotic drugs selectively disrupts avoidance responding without altering unconditioned escape response [13, 14], and thus this test has high predictive validity for antipsychotic efficacy [15]. This feature has been effectively used to identify potential antipsychotic drugs, to differentiate antipsychotic drugs from other classes of psychotropic drugs, and to predict the clinical potency of antipsychotic drugs [11, 14, 16-18]. Our work focuses on behavioral characteristics and neurobiological mechanisms of antipsychotic sensitization in the conditioned avoidance response (CAR) and phencyclidine (PCP)-induced hyperlocomotion models, two animal behavioral tests sensitive to antipsychotic activity [8, 19-23]. We have shown that repeated administration of haloperidol, olanzapine, asenapine or risperidone daily for 5-7 days tends to cause a progressively increased inhibition of avoidance responding and PCP-induced hyperlocomotion over days (a within-subjects sign of sensitization). A few days later, when all rats are given a challenge dose of these drugs, they often make significantly fewer avoidance responses and exhibit lower PCP-induced hyperlocomotion than those that are treated with these drugs for the first time (a between-subjects sign of sensitization). In addition, our previous studies also indicate that repeated administration of haloperidol and olanzapine causes a sensitization effect that can last up to 17 days [8], and are likely mediated by dopamine D2 and 5-HT2A receptor-related neural plasticity [24]. Recently, we further show that olanzapine sensitization can be induced in adolescent rats and this effect can last up to 45 days and persist into adulthood [21].
Antipsychotic sensitization likely reflects a composite impact from two sources. One is the relatively specific pharmacological actions of a given antipsychotic drug. As mentioned before, this is likely mediated by a drug’s actions on its immediate neuroreceptor targets (e.g. D2 and 5-HT2A receptors) [24] and should follow the basic principles of learning and memory, as antipsychotic sensitization represents a non-associative form of learning and memory. Under this principle, the magnitude of sensitization should decrease with the passage of time due to a memory trace decay process (similar to forgetting). Another source is the ubiquitous adaptive response to the foreign aspect of the drug (any drug is an exogenous agent to an organism), which tends to follow the “Time-dependent Sensitization (TDS)” principle. This principle, first articulated by Antelman and his colleagues [25], suggests that a drug effect often grows (i.e. sensitized or strengthened) with the passage of time upon acute exposure to the drug. In Antelman’s earlier work, he found that a single exposure to a clinically low dose of antipsychotic produced changes that were shown up to 8 weeks later [6]. In our previous study on the time course of antipsychotic sensitization [8], we assessed the magnitude of haloperidol and olanzapine sensitization in the conditioned avoidance response test at 4, 10, or 17 days after the last drug treatment. We did not find that haloperidol or olanzapine sensitization changed its magnitude over time, but maintained at a high level throughout the post-injection period. One limitation of the study was shorter time courses tested. It is possible that with a prolonged period of testing, the time-dependent feature of antipsychotic sensitization could be revealed.
The present study addressed this limitation by examining the potential time-dependent feature of antipsychotic sensitization induced by risperidone and asenapine, which represent the earliest and latest atypical antipsychotic drugs in a broader time frame. Both drugs are medically approved for the treatment of schizophrenia and bipolar disorders, with a multiple receptor binding profile targeting dopamine D2 and D1 receptors, serotonin receptors (5-HT2A, 5-HT2C, 5-HT7), adrenergic α1 and α2 receptors, histamine H1 and H2 receptors, etc. [26, 27]. Both drugs have shown to give rise to a sensitization effect in animal tests of antipsychotic activity, including conditioned avoidance test and PCP-induced hyperlocomotion. We also investigated the involvement of dopamine D2 receptor as a biological mechanism underlying antipsychotic sensitization by comparing the quinpirole-induced increase in motor activity in drug-pretreated animals to vehicle-pretreated animals. Because quinpirole is a selective D2/3 receptor agonist, a higher level of motor activity under quinpirole challenge presumably reflects an upregulation of D2 receptor function [28, 29]. Quinpirole-induced hyperlocomotion is also thought to be mediated through an increase in the efficacy of the post-synaptic D2/3 transductional mechanisms [30, 31], and has been widely used to assess drug or non-drug induced changes in D2/3 functions [32, 33].
2. Materials and methods
2.1. Animals
Adult male Sprague-Dawley rats (226-250g upon arrival, Charles River, Portage, MI) were housed two per cage, in 48.3 cm × 26.7 cm × 20.3 cm transparent polycarbonate cages under 12-h light/dark conditions (light on between 6:30am and 6:30pm). Room temperature was maintained at 22 ± 1°C with a relative humidity of 45-60%. Food and water was available ad libitum. Animals were allowed at least 5 days of habituation to the animal facility before being used in experiments. All behavioral tests took place between 9 am and 5 pm in the light cycle. All experimental treatment and procedures were approved by the Institutional Animal Care and Use Committee at the University of Nebraska-Lincoln.
2.2. Drugs and choice of doses
Risperidone (RIS) (a gift from the NIMH drug supply program) was dissolved in distilled sterile water with 1.0% glacial acetic acid. Asenapine Maleate (ASE) (a gift from the NIMH drug supply program) and quinpirole hydrochloride (Tocris Bioscience, Bristol, UK) were dissolved in 0.9% saline. All drugs were administered subcutaneously in a volume of 1.0 ml/kg body weight. Doses of RIS (1.0 and 0.3 mg/kg, for drug test and challenge respectively) and ASE (0.2 and 0.1 mg/kg, for drug test and challenge respectively) were chosen on the basis of literature review showing that these doses produce a reliable disruption of avoidance responding and cause a sensitization effect [23, 34-39]. The chosen quinpirole dose (1.0 mg/kg) targets post-synaptic D2 receptors and causes an increase in motor activity [29, 40-42].
2.3. Two-way avoidance conditioning apparatus
Ten identical two-way shuttle boxes custom designed and manufactured by Med Associates (St. Albans, VT) were used. Each box was housed in a ventilated, sound-insulated isolation cubicle (96.52 cm W × 35.56 cm D × 63.5 cm H). Each box was 64 cm long, 30 cm high (from grid floor), and 24 cm wide, and was divided into two equal-sized compartments by a partition with an arch style doorway (15 cm high × 9 cm wide at base). A barrier (4 cm high) was placed between the two compartments, so the rats had to jump from one compartment to the other. The grid floor consisted of 40 stainless-steel rods with a diameter of 0.48 cm, spaced 1.6 cm apart center to center, through which a scrambled footshock (as an unconditioned stimulus, US; 0.8mA, maximum duration: 5 s) was delivered by a constant current shock generator (Model ENV-410B) and scrambler (Model ENV-412). The rat location and crossings between compartments were monitored by a set of 16 photobeams (ENV-256-8P) affixed at the bottom of the box (3.5 cm above the grid floor). Illumination was provided by two houselights mounted at the top of each compartment. The conditioned stimulus (CS, i.e. 76 dB white noise) was produced by a speaker (ENV 224 AMX) mounted on the ceiling of the cubicle, centered above the shuttle box. Background noise (approximately 74 dB) was provided by a ventilation fan affixed at the top corner of each isolation cubicle. All training and testing procedures were controlled by Med Associates programs running on a computer.
2.4. Locomotor activity monitoring apparatus
Sixteen activity boxes were housed in a quiet room. The boxes were 48.3 cm × 26.7 cm × 20.3 cm transparent polycarbonate cages, which were similar to the home cages but were each equipped with a row of 6 photocell beams (7.8 cm between two adjacent photobeams) placed 3.2 cm above the floor of the cage. A computer with recording software (Aero Apparatus Sixbeam Locomotor System v1.4, Toronto, Canada) was used to detect the disruption of the photocell beams and recorded the number of beam breaks. All experiments were run during the light cycle.
2.5. Experiment 1: Effect of time interval between initial drug exposure and subsequent drug challenge on the strength of risperidone sensitization
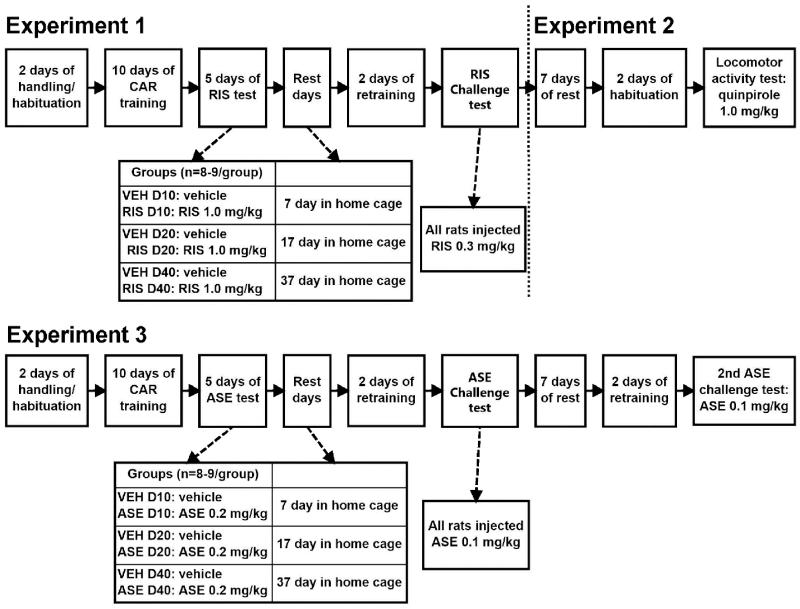
This experiment examined the time course of the risperidone sensitization effect in the CAR model. The experiment was comprised of the following three phases: Avoidance training, five days of repeated risperidone testing and risperidone challenge test. Figure 1 depicts the experimental procedure and groups at different phases of this experiment.
Figure 1.
A schematic depiction of the experimental procedures and groups in Experiment 1, 2 and 3. CAR: conditioned avoidance response; VEH: vehicle; RIS: risperidone; ASE: asenapine.
Avoidance training
Sixty-four rats were first habituated to the CAR boxes for 2 days (20 min/day). They were then trained for conditioned avoidance responding for 10 sessions (1 session/day). Each session consisted of 30 trials. Every trial started by presenting a white noise (CS) for 10 s, followed by a continuous scrambled foot shock (0.8 mA, US, maximum duration = 5 s) on the grid floor. An avoidance response was recorded if a subject moved from one compartment into the other within the 10 s of CS presentation (before the shock). An escape response was recorded if the rat remained in the same compartment for more than 10 s and made a crossing upon receiving the footshock. If the rat did not respond during the entire 5 s presentation of the shock, the trial was terminated and the intertrial intervals started. The total number of avoidance responses was recorded for each session. Inter-trial intervals varied randomly between 30 and 60s.
Five days of repeated risperidone testing
At the end of the training session, 51 rats acquired a robust avoidance responding (≥ 70% avoidance in each of the last 2 sessions). They were matched on the level of avoidance and then randomly assigned into 6 groups (n = 8-9/group): 2 ten-day groups (VEH D10 and RIS D10), 2 twenty-day groups (VEH D20 and RIS D20) and 2 forty-day groups (VEH D40 and RIS D40). They were then tested daily under the CS-only (no shock, 30 trials/session) condition for 5 consecutive days, following the same procedure as employed before [8, 19, 22, 43]. On each test day, rats were first injected with vehicle (VEH; sterile water with 1.0% glacial acetic acid) or RIS (1.0 mg/kg) sc and tested in the CAR boxes 1 h later.
Risperidone challenge test
After the last (5th) drug test session, rats were undisturbed in their home cages for either 7 (for the VEH D10 and RIS D10 groups), 17 (for the VEH D20 and RIS D20 groups) or 37 (for the VEH D40 and RIS D40 groups) days. They were then given 2 drug-free avoidance retraining sessions (one CS-only session and one CS-US session) to bring their avoidance responses back to the pre-drug level before the challenge test. These retraining sessions also served as a test for the residual impact of prior RIS treatment on avoidance responding. One day later, rats in each group pair (e.g. VEH D10 and RIS D10) were injected with RIS at 0.3 mg/kg and tested for avoidance performance in the CS-only condition (30 trials) 1 h later.
2.6. Experiment 2: Effect of prior risperidone treatment on quinpirole-induced hyperlocomotion
Forty-eight rats randomly selected from 51 used in Experiment 1 were used in this experiment. They included 8 rats from each of the 6 groups: RIS D10, VEH D10, RIS D20, VEH D20, RIS D40, and VEH D40 (n = 8/group). As shown in Figure 1, 48 days after the last (5th) RIS test, all rats were first habituated to the locomotor activity apparatus for 2 days (30 min/day), on the second day, all rats were injected with saline before putting into the test chambers. One day later, they were injected with quinpirole (1.0 mg/kg, sc) and immediately placed in the test apparatus for 120 min. Locomotor activity (number of photobeam breaks) was measured in 10-min blocks throughout the entire 120-min test period.
2.7. Experiment 3: Effect of time interval between initial drug exposure and subsequent drug challenge on the strength of asenapine sensitization
This experiment examined the time course of asenapine sensitization using an identical procedure as that in Experiment 1. Fifty-three rats that had met the training criterion (out of an initial 60 rats that were trained) were matched and assigned into 6 groups (n = 8-9/group): VEH D10, ASE D10, VEH D20, ASE D20, VEH D40, and ASE D40. All rats were first repeatedly tested for avoidance performance 30 min after VEH (saline) or ASE (0.2 mg/kg) injection for 5 consecutive days. After 7, 17 or 37 days of home cage rest, the rats were given 2 retraining sessions and 1 challenge test in 3 consecutive days. On the challenge day, the rats were injected with ASE (0.1 mg/kg) and tested for avoidance response in a 30-trial CS-only session 30 min later. One additional ASE challenge test was conducted in all rats at 50 days after the last (5th) ASE treatment to evaluate possible group differences under the same drug challenge condition. This test was conducted following 2 drug-free avoidance retraining sessions (see Figure 1 for the experimental procedure and groups in details).
2.8. Statistical Analysis
Data (mean + SEM) from the 5 drug test sessions (e.g. avoidance response) were analyzed using a split-plot repeated measures analysis of variance (ANOVA) with the between-subjects factor being drug group and the within-subjects factor being drug-test session. Differences between groups on the specific drug test days were analyzed using one-way ANOVAs followed by post hoc Tukey’s HSD tests. Repeated measures ANOVA was also used for analyzing locomotor activity test, with drug group and time-block as between- or within-subject factor respectively. Data from the challenge tests were analyzed using a two-way ANOVA, with treatment (RIS and VEH) and interval (10-, 20-, and 40-day) as two between-subjects factors, followed by post hoc Tukey’s HSD tests. Significance was predetermined at p < 0.05. All data was analyzed using SPSS Version 21.
3. Results
3.1. Experiment 1: Effect of time interval between initial drug exposure and subsequent drug challenge on the strength of risperidone sensitization
Avoidance response during the repeated RIS test period
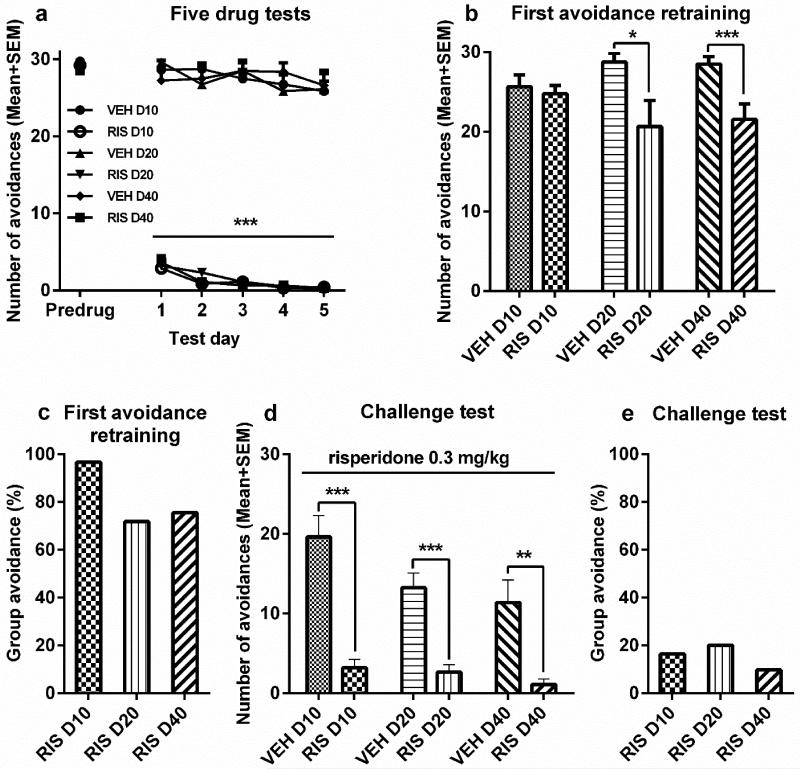
No significant group differences were found on the predrug day [F(5,45) = 0.549, p = 0.738]. Throughout the 5 days of RIS testing, avoidance response was suppressed by repeated RIS treatment. Repeated measures ANOVA revealed a significant main effect of group [F(5,45) = 391.892, p = 0.000], session [F(4,180) = 7.778, p = 0.000], but no significant interaction between the two [F(20,180) = 0.855, p = 0.644] (Figure 2a).
Figure 2.
The induction of risperidone (RIS) sensitization and its expression assessed at 3 time intervals. Number of avoidance responses made by the rats on the final training (predrug) day, five drug test days (a), and the first drug-free retraining day (b). Rats were injected with RIS (1.0 mg/kg, sc, −60 min) or vehicle (VEH) and tested for avoidance responses for 5 daily sessions. D10, D20, or D40 groups (n = 8-9/group) received either 7, 17, or 37 days of resting, and were then retrained in the conditioned avoidance response boxes for two sessions. (c) The mean group avoidance percentage calculated by dividing the mean group number of avoidance in the RIS group by that in its corresponding VEH group. (d, e) RIS sensitization tested at 10-, 20- and 40-day intervals. All groups were injected with RIS (0.3 mg/kg, sc) and avoidance responses were measured 60 min later. All data are expressed as mean + SEM. ***p < 0.001, **p < 0.01, *p < 0.05 for comparison to the respective VEH group.
Avoidance response on the retraining sessions
On the 1st retraining day (30 trials of CS-only session), RIS-pretreated rats in the 20-day and 40-day groups, but not 10-day group, had lower avoidances than their respective control groups. Independent samples t tests showed that the RIS D20 differed significantly from VEH D20 [t(15) = 2.221, p = 0.042], and RIS D40 from VEH D40 [t(15) = 3.084, p = 0.008] (Figure 2b). The difference between the 2 10-day groups was not significant [t(15) = 0.473, p = 0.643]. To further illustrate this point, we calculated the group percentage of avoidance response by dividing the group mean number of avoidance in the RIS-pretreated group by the mean number of avoidance in its corresponding vehicle group (Figure 2c). Using this calculation, a lower percentage of avoidance response indicates a stronger persistent impact of prior RIS treatment. As Figure 2c shows, the group mean avoidance percentages were lower at 40 and 20 days than at 10 days, suggesting that the residual impact of prior RIS treatment increased with the passage of time. No significant group difference was found on the 2nd retraining day (ps > 0.083).
Avoidance response on the challenge test
On the 3 challenge tests at the 10-, 20- and 40-day intervals, the 3 RIS groups all had lower avoidances than their VEH control groups under the same RIS challenge [10-day groups, t(15) = 5.946, p = 0.002; 20-day groups, t(15) = 5.319, p = 0.000; 40-day groups, t(15) = 3.723, p = 0.002; Figure 2d]. Two-way ANOVA revealed a main effect of group [F(1, 45) = 72.392, p = 0.000] and interval [F(2,45) = 4.360, p = 0.019], but no interaction between the two [F(2,45) = 1.869, p = 0.166]. Post hoc Tukey’s HSD tests comparing the interval differences found a significantly lower avoidance at the 40-day interval than at the 10-day interval (p = 0.006). The RIS sensitization effect appeared to be increased with the passage of time, as the relative group avoidance percentage was lower in the RIS D40 group than other groups (Figure 2e).
3.2. Experiment 2: Effect of prior risperidone treatment on quinpirole-induced hyperlocomotion
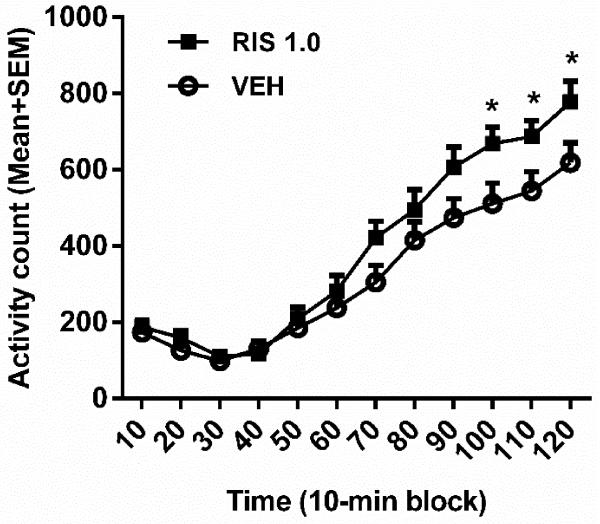
To test whether the D2 receptor system was involved in RIS sensitization, we tested quinpirole-induced locomotor activity in rats that were pretreated with RIS (3 RIS groups) or vehicle (3 VEH groups). This test was conducted at 50 days after the last (5th) RIS or vehicle treatment. As shown in Figure 3, quinpirole at 1.0 mg/kg increased motor activity during the 120-min test period, with an even higher motor activity found the RIS pretreated rats than the VEH pretreated ones. Repeated measures ANOVA revealed a significant main effect of time-block [F(11,506) = 114.795, p = 0.000], a significant block × group interaction [F(11,506) = 2.473, p = 0.005], but no main effect of group [F(1,46) = 3.798, p = 0.057]. Independent samples t test at each block showed that the RIS group had significantly higher motor activity than the VEH group on the last three 10-min blocks [100 min, t(46) = −2.279, p = 0.027; 110 min, t(46) = −2.176, p = 0.035; 120 min, t(46) = −2.135, p = 0.038]. These data suggest that prior RIS treatment induced a long-lasting supersensitivity of D2/3 receptors, which may serve as a mechanism underlying RIS sensitization.
Figure 3.
Quinpirole-induced locomotor activity in rats that were pretreated with risperidone (RIS, 1.0 mg/kg) for 5 days. The test was conducted 50 days after the last RIS (1.0 mg/kg) treatment. All rats were injected with quinpirole (1.0 mg/kg, sc) and then measured for motor activity for 120 min. All data are expressed as mean + SEM in 12 10-min blocks. *p < 0.05 for comparison to the respective VEH group.
3.3. Experiment 3: Effect of time interval between initial drug exposure and subsequent drug challenge on the strength of asenapine sensitization
Avoidance response during the repeated ASE test period
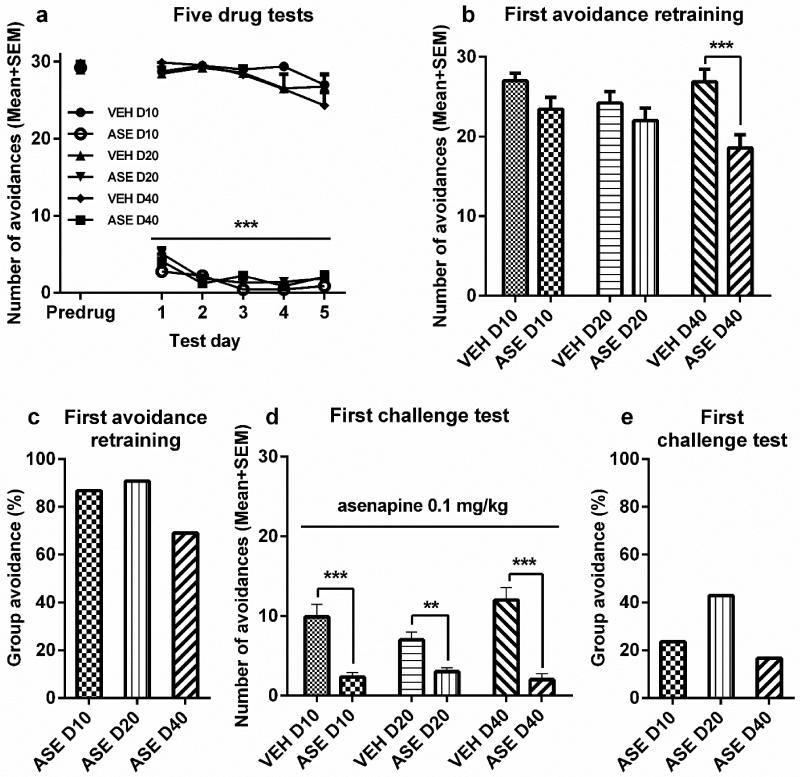
During the initial 5 days drug testing, repeated ASE treatment suppressed avoidance response. Repeated-measures ANOVA revealed a main effect of group [F(5,47) = 546.703, p = 0.000], session [F(4,188) = 13.914, p = 0.000] and a significant interaction between the two [F(20,188) = 2.383, p = 0.001]. Inspection of Figure 4a reveals that the suppressive effect of ASE increased across the 5 test sessions.
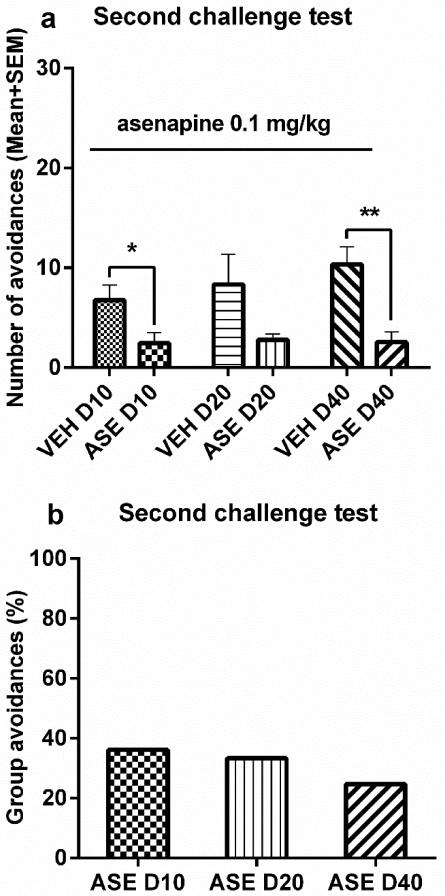
Figure 4.
The induction of Asenapine (ASE) sensitization and its expression assessed at 3 time intervals. Number of avoidance responses made by the rats on the final training (predrug) day, five drug test days (a), and the first drug-free retraining day (b). Rats were injected ASE (0.2 mg/kg, sc, −30 min) or vehicle (VEH) and tested for avoidance responses for 5 daily sessions. D10, D20, or D40 groups (n = 8-9/group) received either 7, 17, or 37 days of resting, and were then retrained in the conditioned avoidance response boxes for two sessions. (c) The mean group avoidance percentage calculated by dividing the mean group number of avoidance in the ASE group by that in its corresponding VEH group. (d, e) ASE sensitization tested at 10-, 20- and 40-day intervals. All groups were injected with ASE (0.1 mg/kg, sc) and avoidance responses were measured 30 min later. All data are expressed as mean + SEM. ***p < 0.001, **p < 0.01 for comparison to the respective VEH group.
Avoidance response on the retraining sessions
On the 1st retraining day (30 trials of CS-only session), only ASE-pretreated rats in the 40-day group had lower avoidance than their vehicle controls [t(16) = 3.613, p = 0.002, Figure 4b]. The differences between the two 10-day [t(15) = 1.964, p = 0.068] and 20-day groups [t(16) = 1.042, p = 0.313] were not significant. The mean group avoidance percentage also showed that the residual impact of prior ASE treatment on avoidance response was greater at the 40-day interval than at other intervals (Figure 4c). No significant group difference was found on the 2nd retraining day (ps > 0.095).
Avoidance response on the challenge test
On the challenge days, all 3 ASE groups had lower avoidances than their respective VEH groups. Two-way ANOVA showed a main effect of group [Figure 4d, F(1, 47) = 67.486, p = 0.000] and a significant interaction between group and interval [F(2,47) = 4.050, p = 0.024], suggesting that the RIS sensitization effect varied across the 3 test intervals (Figure 4e). Independent samples t-test confirmed that the 3 ASE groups all had significantly lower avoidances than their VEH control groups [10-day groups, t(15) = 4.681, p = 0.000; 20-day groups, t(16) = 3.660, p = 0.002; 40-day groups, t(16) = 5.669, p = 0.000; Figure 4d].
Avoidance response on the final challenge test
To evaluate possible group differences under the same condition, all 6 groups of rats were challenged with ASE at 0.1 mg/kg on the 50th day after the last ASE treatment. Before the challenge, there was no group difference (> 70% avoidances, data not shown). On the challenge day, ASE-pretreated rats had lower avoidance than the vehicle-pretreated ones (Figure 5). There was a main effect of group [F(1, 47) = 18.094, p = 0.000], but no main effect of interval [F(2,47) = 0.589, p = 0.559], nor a group × interval interaction [F(2,47) = 0.538, p = 0.588]. Independent samples t tests found a significant group difference between the ASE D10 and VEH D10 groups [t(15) = 2.358, p = 0.032] and between the ASE D40 and VEH D40 groups [t(16) = 3.848, p = 0.001], but not between the ASE D20 and VEH D20 [t(16) = 1.806, p = 0.090].
Figure 5.
The expression of Asenapine (ASE) sensitization assessed after a 50-day interval. Number of avoidance responses (a) and the mean group avoidance percentage (b) made by the rats on the challenge day. After retraining in the conditioned avoidance response boxes for two sessions, all groups were injected with ASE (0.1 mg/kg, sc) and avoidance responses were measured 30 min later on 50th day after the last drug test (ASE 0.2 mg/kg). All data are expressed as mean + SEM. **p < 0.01, *p < 0.05 for comparison to the respective VEH group.
4. Discussion
Although antipsychotic sensitization effects have been demonstrated in the CAR model [8, 22, 24, 38] and other behavioral models [4, 5, 44-47], little research is devoted to documenting the temporal feature of its development and related behavioral and receptor mechanisms, especially those induced by newer atypical antipsychotic drugs (e.g. asenapine, aripiprazole). The present study investigated the time-dependent feature of antipsychotic sensitization in the CAR model and found: 1) both risperidone (1.0 mg/kg) and asenapine (0.2 mg/kg) induced a long-lasting sensitization effect, detectable even 40 or 50 days after the initial drug exposure; 2) the time-dependence of risperidone and asenapine sensitization manifested mainly in the drug-free retraining session, less obvious under the drug challenge test session due to the persistently lower avoidance responding in the 3 drug pre-treated groups at the 3 test intervals; 3) risperidone-pretreated rats showed enhanced motor activity under quinpirole challenge, suggesting that repeated risperidone treatment (and possibly asenapine, too) may induce a relatively permanent D2/3 receptor supersensitivity that supports the long-lasting sensitization. Because suppression of avoidance response is thought to be a behavioral measure of antipsychotic activity, the time-dependent feature of enhanced efficacy due to past drug history may have clinical implications regarding the therapeutic management of schizophrenia.
In many previous antipsychotic sensitization studies, the magnitude of sensitization is often assessed at one single time point, several days or weeks after the initial drug treatment. Thus, these studies were unable to provide much information regarding the changes in sensitization with the passage of time. As mentioned in the Introduction, two basic principles (TDS and forgetting) could govern the time-dependence of antipsychotic sensitization, which give rise to two different predictions. According to the forgetting principle, antipsychotic sensitization, as a basic form of non-associative learning and memory, will decrease when the interval between the initial drug exposure (the induction/learning phase) and the challenge test (the expression/memory phase) is prolonged. In contrast, sensitization will increase with the passage of time on the basis of the TDS principle due to the body’s adaptive and compensatory responses to a foreign agent. The ultimate intensity of antipsychotic sensitization at any given time point likely reflects the consequence of a joint action from these two forces. In our previous CAR study of antipsychotic sensitization, we assessed haloperidol sensitization and olanzapine sensitization at 3 time intervals (i.e., 4, 10, or 17 days), but did not find much change across the 3 time points [8]. The present study extended the test time intervals up to 50 days after the last drug treatment. We not only replicated risperidone[23, 38] and asenapine sensitization [48], but also found their time-dependent feature. This feature was mainly observed in the drug-free CS-only retraining/retesting sessions and was less obvious under the drug challenge test sessions, consistent with our previous findings [8]. The risperidone and asenapine sensitization effects, indexed by a suppression of avoidances, on the drug-free retraining days increased with the passage of time. The D40 drug groups had significantly fewer avoidance responses than the D40 vehicle groups on the 1st retraining day, whereas the D10 groups did not differ from its vehicle controls (Fig. 2b and 4b). This increase in antipsychotic sensitization was also observed in the group avoidance percentage measure (Fig. 2c and 4c). Thus, it appears that the current test conditions were in favor of the manifestation of TDS as opposed to forgetting. We do not know why we failed to demonstrate the time-dependence of risperidone and asenapine sensitization in the drug challenge tests. One possibility is that the initial tested dose and challenge dose of risperidone and asenapine were still too high, causing a floor effect (i.e. a high dose causes a large disruption of avoidance responding, leaving no room to show the residual impact of prior risperidone or asenapine treatment), especially in the drug pretreated groups. As can be seen in Figures 2a and 4a, risperidone and asenapine at the chosen doses suppressed avoidance responding to the near bottom level even on the 1st test day. Similarly, on the challenge tests (Figure 2d and 4d), the drug pretreated groups at the 3 test intervals all displayed rather low levels of avoidance responding, causing difficulties in detecting their potential differences. One important future study is to further manipulate experimental conditions, including the utilization of different challenge doses, to delineate the exact experimental conditions that would allow TDS to mediate time-dependent alterations of antipsychotic sensitization.
The phenomenon of antipsychotic sensitization supports the notion that once an organism is exposed to an antipsychotic drug, this drug experience stays with it for a long period of time and may undergo a time-dependent change. Behaviorally, an antipsychotic drug may act as an occasion-setter [49], which sets the condition in which rats behave on the basis of their previous drug experience in the avoidance testing context. Neurobiologically, the antipsychotic drug may imprint the brain to create a drug “memory trace” of avoidance responding under drug treatment by altering the functions of the neuroreceptors it targets. Risperidone has a relatively high affinity for dopamine D2, serotonin 5-HT2A, 5-HT7, and adrenergic α1 receptors [27] and asenapine has a high affinity for 5-HT2A, 5-HT2C, 5-HT6, 5-HT7, adrenergic α1 and α2, and histamine H1 and H2 receptors, and a relatively low action on D2 and D1 receptors [26]. It is conceivable that 5 days of repeated risperidone and asenapine treatment altered the functions of these receptors. Indeed, substantial evidence suggests that chronic antipsychotic treatment does modify these neurochemical systems and induces various forms of neuroplasticity [50-53]. For example, it has been shown that the density and sensitivity of dopamine D2 receptors is upregulated after chronic antipsychotic treatment, including risperidone and asenapine in both animals [54, 55] and humans [56]. In the present study, using a behavioral assay of D2-mediated neurotransmission, we also obtained evidence of a functional upregulation of D2 receptors caused by prior risperidone exposure, as risperidone-pretreated rats had significantly higher motor activity than the corresponding vehicle rats under quinpirole challenge. We hypothesized that D2 receptor supersensitivity is one critical mechanism underlying antipsychotic sensitization (including, olanzapine, risperidone and asenapine sensitization), and this conjecture is strengthened by the following three observations. First, all antipsychotic drugs antagonize D2 receptors and their chronic use causes a long-lasting change in this receptor system [57]. Second, olanzapine sensitization in the CAR model also relies on D2 receptors. Li et al. (2010) showed that pretreatment of quinpirole during the induction phase attenuated the expression of olanzapine sensitization in the challenge test. Third, there exists a cross-sensitization between risperidone and olanzapine [38] as well as asenapine and olanzapine [48], as rats previously treated with risperidone or asenapine show enhanced responsiveness to olanzapine in the suppression of avoidance responding. This hypothesis may warrant further investigation. Because risperidone and asenapine also have relatively high 5-HT2A receptor occupancies[58], and chronic risperidone and asenapine treatments decrease 5-HT2A receptor binding in the medial prefrontal cortex [55, 59, 60], 5HT2A’s involvement in risperidone and asenapine sensitization also needs to be examined. Other receptors such as 5-HT1A, D4, NMDA, and adrenergic α1 and α2 receptors could also play a role in antipsychotic sensitization [26, 61-63].
Antelman et al. (2000) has argued that TDS is a useful principle for the explanation of clinical improvement which grows with the passage of time [64]. One direct implication is that “instead of managing disorders such as depression by multiple daily drug treatments, it may be possible to accomplish the same ends by treating once every few weeks.” (page 354). In the case of clinical treatment of schizophrenia, psychotic symptoms do improve exponentially with the passage of time and with the increase of treatment duration [3, 65], but the relative contributions from each factor (i.e. time vs. treatment duration) on symptom improvement has not been investigated. Currently, the most common practice in the clinic is to treat schizophrenic patients with antipsychotic drugs daily to achieve approximately 60% to 80% of dopamine D2 receptor occupancy [66]. If we do not need to maintain a daily treatment schedule, it would avoid many side effects, including extrapyramidal symptoms (EPS) and excess weight gain. Recent studies showing that dosing every 2-3 days is sufficient to maintain antipsychotic efficacy in schizophrenic patients is in support of this practice and TDS principle [67, 68]. This finding suggests that upon initial exposure, physiological events initiated by a drug enhance the antipsychotic’s effects beyond its presence at the receptor, thereby inducing efficacy without requiring constant receptor binding. This idea is strongly supported by our current findings that risperidone and asenapine sensitization persisted and even increased to some degree with the passage of time. Previous studies in the literature generally suggest that continuous antipsychotic treatment is more effective than interment antipsychotic regiment in preventing relapse in people with schizophrenia [67, 68]. One problem with the previous intermittent approach is that it allowed for lengthy “time off” and re-administration based on the re-appearance of symptoms. The current effective approach differs from the previous ones in which it requires an intermittent but regular dosing regardless of symptom status [68]. Our findings provide a preclinical support for this approach. Of course more preclinical and clinical research is needed to address the potential benefits or harms of these two different drug treatment regimens.
Repeated risperidone and asenapine treatment enhanced acute effect of these drugs in the conditioned avoidance model;
Risperidone and asenapine sensitization lasted up to 40 days;
Risperidone and asenapine sensitization increased with the passage of time;
Repeated risperidone treatment caused a functional upregulation of dopamine D2/3 receptors.
Acknowledgements
This study was supported by the National Institute of Mental Health of the National Institutes of Health under award number R01MH085635 to Professor Ming Li.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: None
References
- [1].Fallon P, Dursun SM. A naturalistic controlled study of relapsing schizophrenic patients with tardive dyskinesia and supersensitivity psychosis. J Psychopharmacol. 2011;25:755–62. doi: 10.1177/0269881109359097. [DOI] [PubMed] [Google Scholar]
- [2].Agid O, Kapur S, Arenovich T, Zipursky RB. Delayed-onset hypothesis of antipsychotic action: a hypothesis tested and rejected. Arch Gen Psychiatry. 2003;60:1228–35. doi: 10.1001/archpsyc.60.12.1228. [DOI] [PubMed] [Google Scholar]
- [3].Kapur S, Agid O, Mizrahi R, Li M. How antipsychotics work-from receptors to reality. NeuroRx. 2006;3:10–21. doi: 10.1016/j.nurx.2005.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lanis A, Schmidt WJ. NMDA receptor antagonists do not block the development of sensitization of catalepsy, but make its expression state-dependent. Behav Pharmacol. 2001;12:143–9. doi: 10.1097/00008877-200104000-00008. [DOI] [PubMed] [Google Scholar]
- [5].Amtage J, Schmidt WJ. Context-dependent catalepsy intensification is due to classical conditioning and sensitization. Behav Pharmacol. 2003;14:563–7. doi: 10.1097/00008877-200311000-00009. [DOI] [PubMed] [Google Scholar]
- [6].Antelman SM, Kocan D, Edwards DJ, Knopf S, Perel JM, Stiller R. Behavioral effects of a single neuroleptic treatment grow with the passage of time. Brain Res. 1986;385:58–67. doi: 10.1016/0006-8993(86)91547-7. [DOI] [PubMed] [Google Scholar]
- [7].Wolgin DL, Moore J. Sensitization to haloperidol-induced suppression of milk intake: effect of interdose interval. Psychopharmacology (Berl) 1992;107:290–6. doi: 10.1007/BF02245150. [DOI] [PubMed] [Google Scholar]
- [8].Swalve N, Li M. Parametric studies of antipsychotic-induced sensitization in the conditioned avoidance response model: roles of number of drug exposure, drug dose, and test-retest interval. Behav Pharmacol. 2012;23:380–91. doi: 10.1097/FBP.0b013e32835651ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Browman KE, Badiani A, Robinson TE. Modulatory effect of environmental stimuli on the susceptibility to amphetamine sensitization: a dose-effect study in rats. J Pharmacol Exp Ther. 1998;287:1007–14. [PubMed] [Google Scholar]
- [10].Levis DJ, Brewer KE. The neurotic paradox: Attempts by two-factor fear theory and alternative avoidance models to resolve the issues associated with sustained avoidance responding in extinction. In: Mowrer RR, Klein SB, editors. Handbook of contemporary learning theories. Lawrence Erlbaum Associates; Mahwah, NJ: 2001. pp. 561–97. [Google Scholar]
- [11].Wadenberg ML, Hicks PB. The conditioned avoidance response test re-evaluated: is it a sensitive test for the detection of potentially atypical antipsychotics? Neurosci Biobehav Rev. 1999;23:851–62. doi: 10.1016/s0149-7634(99)00037-8. [DOI] [PubMed] [Google Scholar]
- [12].Moutoussis M, Williams J, Dayan P, Bentall RP. Persecutory delusions and the conditioned avoidance paradigm: towards an integration of the psychology and biology of paranoia. Cogn Neuropsychiatry. 2007;12:495–510. doi: 10.1080/13546800701566686. [DOI] [PubMed] [Google Scholar]
- [13].Wadenberg ML, Soliman A, VanderSpek SC, Kapur S. Dopamine D(2) receptor occupancy is a common mechanism underlying animal models of antipsychotics and their clinical effects. Neuropsychopharmacology. 2001;25:633–41. doi: 10.1016/S0893-133X(01)00261-5. [DOI] [PubMed] [Google Scholar]
- [14].Arnt J. Pharmacological specificity of conditioned avoidance response inhibition in rats: inhibition by neuroleptics and correlation to dopamine receptor blockade. Acta Pharmacol Toxicol (Copenh) 1982;51:321–9. doi: 10.1111/j.1600-0773.1982.tb01032.x. [DOI] [PubMed] [Google Scholar]
- [15].Wadenberg ML. Conditioned avoidance response in the development of new antipsychotics. Curr Pharm Des. 2010;16:358–70. doi: 10.2174/138161210790170085. [DOI] [PubMed] [Google Scholar]
- [16].Bignami G. Effects of neuroleptics, ethanol, hypnotic-sedatives, tranquilizers, narcotics, and minor stimulants in aversive paradigms. In: Anisman H, Bignami G, editors. Psychopharmacology of aversively motivated behavior. Plenum Press; New York and London: 1978. pp. 385–453. [Google Scholar]
- [17].van der Heyden JA, Bradford LD. A rapidly acquired one-way conditioned avoidance procedure in rats as a primary screening test for antipsychotics: influence of shock intensity on avoidance performance. Behav Brain Res. 1988;31:61–7. doi: 10.1016/0166-4328(88)90158-1. [DOI] [PubMed] [Google Scholar]
- [18].Shannon HE, Hart JC, Bymaster FP, Calligaro DO, DeLapp NW, Mitch CH, et al. Muscarinic receptor agonists, like dopamine receptor antagonist antipsychotics, inhibit conditioned avoidance response in rats. J Pharmacol Exp Ther. 1999;290:901–7. [PubMed] [Google Scholar]
- [19].Feng M, Sui N, Li M. Environmental and behavioral controls of the expression of clozapine tolerance: Evidence from a novel across-model transfer paradigm. Behav Brain Res. 2013;238:178–87. doi: 10.1016/j.bbr.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Li M, Sun T, Mead A. Clozapine, but not olanzapine, disrupts conditioned avoidance response in rats by antagonizing 5-HT2A/2C receptors. Journal of neural transmission. 2012;119:497–505. doi: 10.1007/s00702-011-0722-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Qiao J, Li H, Li M. Olanzapine sensitization and clozapine tolerance: from adolescence to adulthood in the conditioned avoidance response model. Neuropsychopharmacology. 2013;38:513–24. doi: 10.1038/npp.2012.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zhang C, Li M. Contextual and behavioral control of antipsychotic sensitization induced by haloperidol and olanzapine. Behav Pharmacol. 2012;23:66–79. doi: 10.1097/FBP.0b013e32834ecac4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mead A, Li M. Avoidance-suppressing effect of antipsychotic drugs is progressively potentiated after repeated administration: an interoceptive drug state mechanism. J Psychopharmacol. 2010;24:1045–53. doi: 10.1177/0269881109102546. [DOI] [PubMed] [Google Scholar]
- [24].Li M, Sun T, Zhang C, Hu G. Distinct neural mechanisms underlying acute and repeated administration of antipsychotic drugs in rat avoidance conditioning. Psychopharmacology (Berl) 2010;212:45–57. doi: 10.1007/s00213-010-1925-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Antelman SM, Chiodo LA. Repeated antidepressant treatments induce a long-lasting dopamine autoreceptor subsensitivity: is daily treatment necessary for clinical efficacy? [proceedings] Psychopharmacol Bull. 1981;17:92–4. [PubMed] [Google Scholar]
- [26].Shahid M, Walker GB, Zorn SH, Wong EH. Asenapine: a novel psychopharmacologic agent with a unique human receptor signature. J Psychopharmacol. 2009;23:65–73. doi: 10.1177/0269881107082944. [DOI] [PubMed] [Google Scholar]
- [27].Miyamoto S, Duncan GE, Marx CE, Lieberman JA. Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry. 2005;10:79–104. doi: 10.1038/sj.mp.4001556. [DOI] [PubMed] [Google Scholar]
- [28].Moreno M, Lopez-Moreno JA, Rodriguez de Fonseca F, Navarro M. Behavioural effects of quinpirole following withdrawal of chronic treatment with the CB1 agonist, HU-210, in rats. Behavioural pharmacology. 2005;16:441–6. doi: 10.1097/00008877-200509000-00017. [DOI] [PubMed] [Google Scholar]
- [29].Luque-Rojas MJ, Galeano P, Suarez J, Araos P, Santin LJ, de Fonseca FR, et al. Hyperactivity induced by the dopamine D2/D3 receptor agonist quinpirole is attenuated by inhibitors of endocannabinoid degradation in mice. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2013;16:661–76. doi: 10.1017/S1461145712000569. [DOI] [PubMed] [Google Scholar]
- [30].Szumlinski KK, Goodwill AM, Szechtman H. Locomotor sensitization to quinpirole in rats: effects of drug abstinence and sex. Psychopharmacology. 2000;152:304–11. doi: 10.1007/s002130000538. [DOI] [PubMed] [Google Scholar]
- [31].Szumlinski KK, Allan M, Talangbayan H, Tracey A, Szechtman H. Locomotor sensitization to quinpirole: environment-modulated increase in efficacy and context-dependent increase in potency. Psychopharmacology. 1997;134:193–200. doi: 10.1007/s002130050442. [DOI] [PubMed] [Google Scholar]
- [32].Tenk CM, Foley KA, Kavaliers M, Ossenkopp KP. Neonatal immune system activation with lipopolysaccharide enhances behavioural sensitization to the dopamine agonist, quinpirole, in adult female but not male rats. Brain, behavior, and immunity. 2007;21:935–45. doi: 10.1016/j.bbi.2007.03.001. [DOI] [PubMed] [Google Scholar]
- [33].Vorhees CV, Johnson HL, Burns LN, Williams MT. Developmental treatment with the dopamine D2/3 agonist quinpirole selectively impairs spatial learning in the Morris water maze. Neurotoxicol Teratol. 2009;31:1–10. doi: 10.1016/j.ntt.2008.09.003. [DOI] [PubMed] [Google Scholar]
- [34].Franberg O, Wiker C, Marcus MM, Konradsson A, Jardemark K, Schilstrom B, et al. Asenapine, a novel psychopharmacologic agent: preclinical evidence for clinical effects in schizophrenia. Psychopharmacology (Berl) 2008;196:417–29. doi: 10.1007/s00213-007-0973-y. [DOI] [PubMed] [Google Scholar]
- [35].Marston HM, Young JW, Martin FD, Serpa KA, Moore CL, Wong EH, et al. Asenapine effects in animal models of psychosis and cognitive function. Psychopharmacology (Berl) 2009;206:699–714. doi: 10.1007/s00213-009-1570-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Li M, Fletcher PJ, Kapur S. Time course of the antipsychotic effect and the underlying behavioral mechanisms. Neuropsychopharmacology. 2007;32:263–72. doi: 10.1038/sj.npp.1301110. [DOI] [PubMed] [Google Scholar]
- [37].Li M, He W, Mead A. Olanzapine and risperidone disrupt conditioned avoidance responding in phencyclidine-pretreated or amphetamine-pretreated rats by selectively weakening motivational salience of conditioned stimulus. Behav Pharmacol. 2009;20:84–98. doi: 10.1097/FBP.0b013e3283243008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zhang C, Fang Y, Li M. Olanzapine and risperidone disrupt conditioned avoidance responding by selectively weakening motivational salience of conditioned stimulus: further evidence. Pharmacol Biochem Behav. 2011;98:155–60. doi: 10.1016/j.pbb.2010.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Aguilar MA, Rodriguez-Arias M, Mari-Sanmillan MI, Minarro J. Effects of risperidone on conditioned avoidance responding in male mice. Behav Pharmacol. 1997;8:669–76. doi: 10.1097/00008877-199712000-00001. [DOI] [PubMed] [Google Scholar]
- [40].Nakamura S, Yue JL, Goshima Y, Miyamae T, Ueda H, Misu Y. Non-effective dose of exogenously applied L-dopa itself stereoselectively potentiates postsynaptic D2 receptor-mediated locomotor activities of conscious rats. Neuroscience letters. 1994;170:22–6. doi: 10.1016/0304-3940(94)90229-1. [DOI] [PubMed] [Google Scholar]
- [41].Prosser ES, Pruthi R, Csernansky JG. Differences in the time course of dopaminergic supersensitivity following chronic administration of haloperidol, molindone, or sulpiride. Psychopharmacology (Berl) 1989;99:109–16. doi: 10.1007/BF00634463. [DOI] [PubMed] [Google Scholar]
- [42].Koller W, Herbster G, Anderson D, Wack R, Gordon J. Quinpirole hydrochloride, a potential anti-parkinsonism drug. Neuropharmacology. 1987;26:1031–6. doi: 10.1016/0028-3908(87)90245-0. [DOI] [PubMed] [Google Scholar]
- [43].Feng M, Sui N, Li M. Avoidance disruptive effect of clozapine and olanzapine is potentiated by increasing the test trials: further test of the motivational salience hypothesis. Pharmacology, biochemistry, and behavior. 2013;103:467–73. doi: 10.1016/j.pbb.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Klein A, Schmidt WJ. Catalepsy intensifies context-dependently irrespective of whether it is induced by intermittent or chronic dopamine deficiency. Behav Pharmacol. 2003;14:49–53. doi: 10.1097/00008877-200302000-00005. [DOI] [PubMed] [Google Scholar]
- [45].Li M, He E, Volf N. Time course of the attenuation effect of repeated antipsychotic treatment on prepulse inhibition disruption induced by repeated phencyclidine treatment. Pharmacol Biochem Behav. 2011;98:559–69. doi: 10.1016/j.pbb.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Sun T, Hu G, Li M. Repeated antipsychotic treatment progressively potentiates inhibition on phencyclidine-induced hyperlocomotion, but attenuates inhibition on amphetamine-induced hyperlocomotion: relevance to animal models of antipsychotic drugs. Eur J Pharmacol. 2009;602:334–42. doi: 10.1016/j.ejphar.2008.11.036. [DOI] [PubMed] [Google Scholar]
- [47].Varvel SA, Vann RE, Wise LE, Philibin SD, Porter JH. Effects of antipsychotic drugs on operant responding after acute and repeated administration. Psychopharmacology (Berl) 2002;160:182–91. doi: 10.1007/s00213-001-0969-y. [DOI] [PubMed] [Google Scholar]
- [48].Qin R, Chen Y, Li M. Repeated asenapine treatment produces a sensitization effect in two preclinical tests of antipsychotic activity. Neuropharmacology. 2013;75C:356–64. doi: 10.1016/j.neuropharm.2013.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Maes JH, Vossen JM. Conditional control by midazolam and amphetamine in a rapid appetitive discrimination procedure. Eur J Pharmacol. 1997;319:5–11. doi: 10.1016/s0014-2999(96)00953-3. [DOI] [PubMed] [Google Scholar]
- [50].Lieberman JA, Bymaster FP, Meltzer HY, Deutch AY, Duncan GE, Marx CE, et al. Antipsychotic drugs: comparison in animal models of efficacy, neurotransmitter regulation, and neuroprotection. Pharmacol Rev. 2008;60:358–403. doi: 10.1124/pr.107.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Meltzer HY, Matsubara S, Lee JC. The ratios of serotonin2 and dopamine2 affinities differentiate atypical and typical antipsychotic drugs. Psychopharmacol Bull. 1989;25:390–2. [PubMed] [Google Scholar]
- [52].Allen JA, Yost JM, Setola V, Chen X, Sassano MF, Chen M, et al. Discovery of beta-arrestin-biased dopamine D2 ligands for probing signal transduction pathways essential for antipsychotic efficacy. Proc Natl Acad Sci U S A. 2011;108:18488–93. doi: 10.1073/pnas.1104807108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Konradi C, Heckers S. Antipsychotic drugs and neuroplasticity: insights into the treatment and neurobiology of schizophrenia. Biol Psychiatry. 2001;50:729–42. doi: 10.1016/s0006-3223(01)01267-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Tarazi FI, Zhang K, Baldessarini RJ. Long-term effects of olanzapine, risperidone, and quetiapine on dopamine receptor types in regions of rat brain: implications for antipsychotic drug treatment. J Pharmacol Exp Ther. 2001;297:711–7. [PubMed] [Google Scholar]
- [55].Tarazi FI, Moran-Gates T, Wong EH, Henry B, Shahid M. Differential regional and dose-related effects of asenapine on dopamine receptor subtypes. Psychopharmacology (Berl) 2008;198:103–11. doi: 10.1007/s00213-008-1098-7. [DOI] [PubMed] [Google Scholar]
- [56].Silvestri S, Seeman MV, Negrete JC, Houle S, Shammi CM, Remington GJ, et al. Increased dopamine D2 receptor binding after long-term treatment with antipsychotics in humans: a clinical PET study. Psychopharmacology (Berl) 2000;152:174–80. doi: 10.1007/s002130000532. [DOI] [PubMed] [Google Scholar]
- [57].Seeman P. Targeting the dopamine D2 receptor in schizophrenia. Expert Opin Ther Targets. 2006;10:515–31. doi: 10.1517/14728222.10.4.515. [DOI] [PubMed] [Google Scholar]
- [58].Meltzer HY, Dritselis A, Yasothan U, Kirkpatrick P. Asenapine. Nat Rev Drug Discov. 2009;8:843–4. doi: 10.1038/nrd3027. [DOI] [PubMed] [Google Scholar]
- [59].Tarazi FI, Moran-Gates T, Wong EH, Henry B, Shahid M. Asenapine induces differential regional effects on serotonin receptor subtypes. J Psychopharmacol. 2010;24:341–8. doi: 10.1177/0269881108095704. [DOI] [PubMed] [Google Scholar]
- [60].Choi YK, Moran-Gates T, Gardner MP, Tarazi FI. Effects of repeated risperidone exposure on serotonin receptor subtypes in developing rats. Eur Neuropsychopharmacol. 2010;20:187–94. doi: 10.1016/j.euroneuro.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Franberg O, Marcus MM, Svensson TH. Involvement of 5-HT(2A) receptor and alpha(2)-adrenoceptor blockade in the asenapine-induced elevation of prefrontal cortical monoamine outflow. Synapse. 2012;66:650–60. doi: 10.1002/syn.21551. [DOI] [PubMed] [Google Scholar]
- [62].Choi YK, Wong EH, Henry B, Shahid M, Tarazi FI. Repeated effects of asenapine on adrenergic and cholinergic muscarinic receptors. Int J Neuropsychopharmacol. 2010;13:405–10. doi: 10.1017/S1461145709990824. [DOI] [PubMed] [Google Scholar]
- [63].Tarazi FI, Neill JC. The preclinical profile of asenapine: clinical relevance for the treatment of schizophrenia and bipolar mania. Expert Opin Drug Discov. 2012 doi: 10.1517/17460441.2013.738193. [DOI] [PubMed] [Google Scholar]
- [64].Antelman SM, Levine J, Gershon S. Time-dependent sensitization: the odyssey of a scientific heresy from the laboratory to the door of the clinic. Mol Psychiatry. 2000;5:350–6. doi: 10.1038/sj.mp.4000721. [DOI] [PubMed] [Google Scholar]
- [65].Agid O, Seeman P, Kapur S. The “delayed onset” of antipsychotic action--an idea whose time has come and gone. J Psychiatry Neurosci. 2006;31:93–100. [PMC free article] [PubMed] [Google Scholar]
- [66].Kapur S. A new framework for investigating antipsychotic action in humans: lessons from PET imaging. Mol Psychiatry. 1998;3:135–40. doi: 10.1038/sj.mp.4000327. [DOI] [PubMed] [Google Scholar]
- [67].Remington G, Seeman P, Shammi C, Mann S, Kapur S. “Extended” antipsychotic dosing: rationale and pilot data. J Clin Psychopharmacol. 2005;25:611–3. doi: 10.1097/01.jcp.0000185341.55096.65. [DOI] [PubMed] [Google Scholar]
- [68].Remington G, Seeman P, Feingold A, Mann S, Shammi C, Kapur S. “Extended” antipsychotic dosing in the maintenance treatment of schizophrenia: a double-blind, placebo-controlled trial. J Clin Psychiatry. 2011;72:1042–8. doi: 10.4088/JCP.09m05866yel. [DOI] [PubMed] [Google Scholar]