Abstract
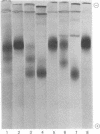
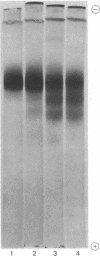
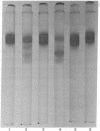
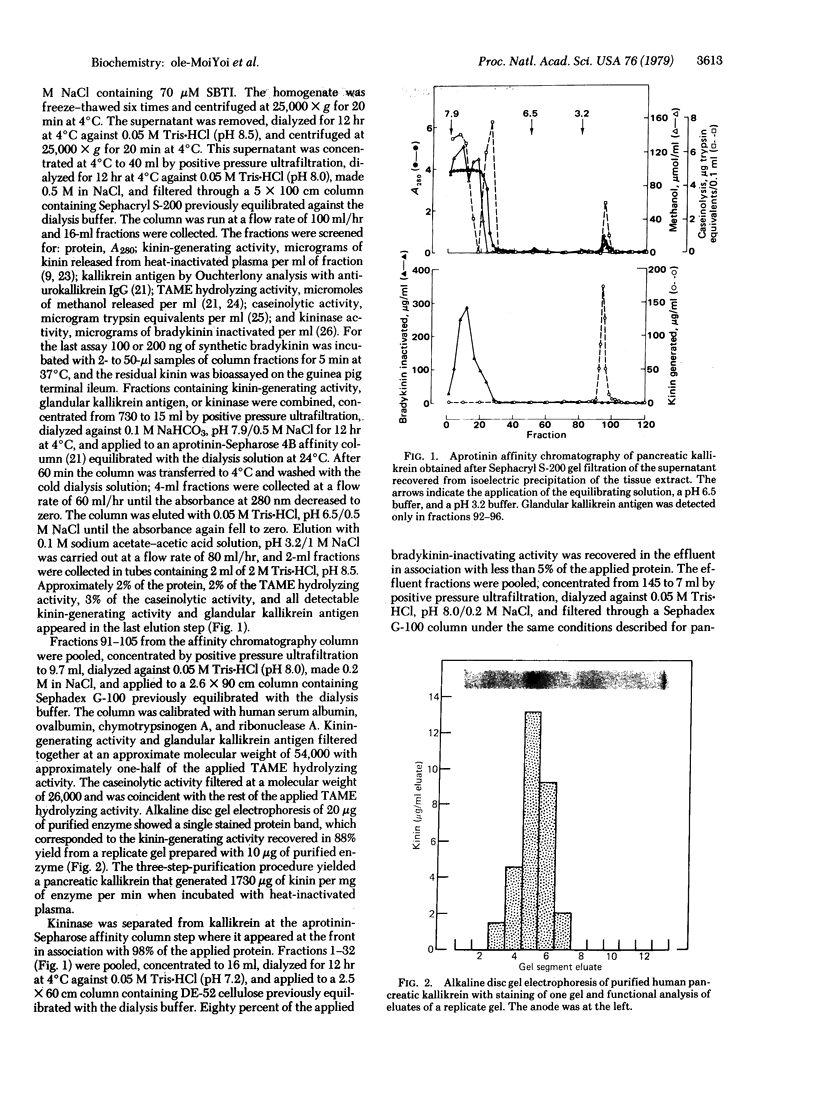
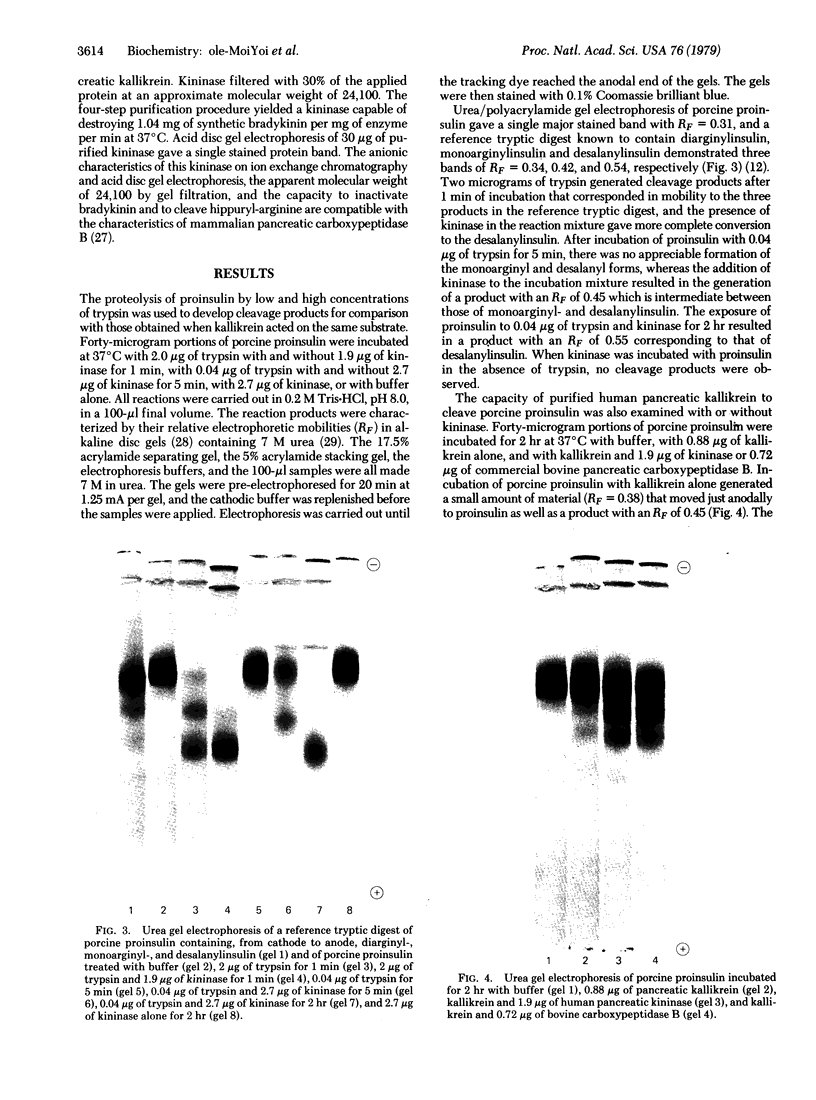
A pancreatic endopeptidase localized to the β-cells of the pancreas by immunohistochemical techniques has been purified to homogeneity by following its functional and antigenic characteristics as a glandular kallikrein (EC 3.4.21.8). The enzyme gave a single stained band on alkaline disc gel electrophoresis which corresponded in location with the kinin-generating activity eluted from a replicate gel, was of 54,000 molecular weight by gel filtration, was devoid of caseinolytic activity, elicited a monospecific antiserum in a rabbit, and gave a line of complete identity with a single constituent in pancreatic extract, crude urine, and purified urokallikrein when analyzed with monospecific antibody to urokallikrein. The pancreatic glandular kallikrein generated three cleavage products of increasing anodal mobility from bovine and porcine proinsulin, and the presence of pancreatic kininase or bovine carboxypeptidase B increased the quantity of these products. Although the conversion products did not correspond to diarginyl- and monoarginylinsulin, the product of intermediate mobility was also obtained when proinsulin was treated with a low concentration of trypsin in the presence of kininase. The most rapidly migrating product did correspond to desalanylinsulin in the reference standard. Kininase alone had no action on proinsulin, and aprotinin prevented cleavage by kallikrein alone or in combination with kininase. Although the chemical structure of the proinsulin cleavage products has not been established, human pancreatic kallikrein is considered a putative activator of proinsulin because of its location in the β-cell, its preferential action on proinsulin and kininogen as compared to azocasein, and its capacity to generate insulin intermediate products that are further modified by human pancreatic kininase or bovine carboxypeptidase B.
Keywords: β cell endopeptidase, aprotinin inhibition
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baugh R. J., Travis J. Human leukocyte granule elastase: rapid isolation and characterization. Biochemistry. 1976 Feb 24;15(4):836–841. doi: 10.1021/bi00649a017. [DOI] [PubMed] [Google Scholar]
- Czop J. K., Fearon D. T., Austen K. F. Opsonin-independent phagocytosis of activators of the alternative complement pathway by human monocytes. J Immunol. 1978 Apr;120(4):1132–1138. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Habal F. M., Movat H. Z., Burrowes C. E. Isolation of two functionally different kininogens from human plasma--separation from proteinase inhibitors and interaction with plasma kallikrein. Biochem Pharmacol. 1974 Aug 15;23(16):2291–2303. doi: 10.1016/0006-2952(74)90558-9. [DOI] [PubMed] [Google Scholar]
- Kemmler W., Peterson J. D., Rubenstein A. H., Steiner D. F. On the biosynthesis, intracellular transport and mechanism of conversion of proinsulin to insulin and C-peptide. Diabetes. 1972;21(2 Suppl):572–581. doi: 10.2337/diab.21.2.s572. [DOI] [PubMed] [Google Scholar]
- Kemmler W., Peterson J. D., Steiner D. F. Studies on the conversion of proinsulin to insulin. I. Conversion in vitro with trypsin and carboxypeptidase B. J Biol Chem. 1971 Nov 25;246(22):6786–6791. [PubMed] [Google Scholar]
- Kemmler W., Steiner D. F., Borg J. Studies on the conversion of proinsulin to insulin. 3. Studies in vitro with a crude secretion granule fraction isolated from rat islets of Langerhans. J Biol Chem. 1973 Jul 10;248(13):4544–4551. [PubMed] [Google Scholar]
- Orstavik T. B., Glenner G. G. Localization of kallikrein and its relation to other trypsin-like esterases in the rat pancreas. A comparison with the submandibular gland. Acta Physiol Scand. 1978 Aug;103(4):384–393. doi: 10.1111/j.1748-1716.1978.tb06232.x. [DOI] [PubMed] [Google Scholar]
- Reeck G. R., Walsh K. A., Hermodson M. A., Neurath H. New forms of bovine carboxypeptidase B and their homologous relationships to carboxypeptidase A. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1226–1230. doi: 10.1073/pnas.68.6.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIEGELMAN A. M., CARLSON A. S., ROBERTSON T. Investigation of serum trypsin and related substances. I. The quantitative demonstration of trypsinlike activity in human blood serum by a micromethod. Arch Biochem Biophys. 1962 Apr;97:159–163. doi: 10.1016/0003-9861(62)90058-9. [DOI] [PubMed] [Google Scholar]
- Sorenson R. L., Shank R. D., Lindall A. W. Effect of pH on conversion of proinsulin to insulin by a subcellular fraction of rat islets. Proc Soc Exp Biol Med. 1972 Feb;139(2):652–655. doi: 10.3181/00379727-139-36207. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Kemmler W., Tager H. S., Peterson J. D. Proteolytic processing in the biosynthesis of insulin and other proteins. Fed Proc. 1974 Oct;33(10):2105–2115. [PubMed] [Google Scholar]
- Sun A. M., Lin B. J., Haist R. E. Studies on the conversion of proinsulin to insulin in the isolated islets of Langerhans in the rat. Can J Physiol Pharmacol. 1973 Mar;51(3):175–182. doi: 10.1139/y73-025. [DOI] [PubMed] [Google Scholar]
- Tung A. K., Yip C. C. Biosynthesis of insulin in bovine fetal pancreatic slices: the incorporation of tritiated leucine into a single-chain proinsulin, a double-chain intermediate, and insulin in subcellular fractions. Proc Natl Acad Sci U S A. 1969 Jun;63(2):442–449. doi: 10.1073/pnas.63.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEBSTER M. E., PIERCE J. V. The nature of the kallidins released from human plasma by kallikreins and other enzymes. Ann N Y Acad Sci. 1963 Feb 4;104:91–107. doi: 10.1111/j.1749-6632.1963.tb17655.x. [DOI] [PubMed] [Google Scholar]
- Yang H. Y., Erdös E. G., Levin Y. A dipeptidyl carboxypeptidase that converts angiotensin I and inactivates bradykinin. Biochim Biophys Acta. 1970 Aug 21;214(2):374–376. doi: 10.1016/0005-2795(70)90017-6. [DOI] [PubMed] [Google Scholar]
- Yip C. C. A bovine pancreatic enzyme catalyzing the conversion of proinsulin to insulin. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1312–1315. doi: 10.1073/pnas.68.6.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ole-Moi Yoi O., Spragg J., Halbert S. P., Austen K. F. Immunologic reactivity of purified human urinary kallikrein (urokallikrein) with antiserum directed against human pancreas. J Immunol. 1977 Feb;118(2):667–672. [PubMed] [Google Scholar]
- ole-MoiYoi O. K., Spragg J., Austen K. F. Inhibition of human urinary kallikrein (urokallikrein) by anti-enzyme FAB. J Immunol. 1978 Jul;121(1):66–71. [PubMed] [Google Scholar]