Abstract
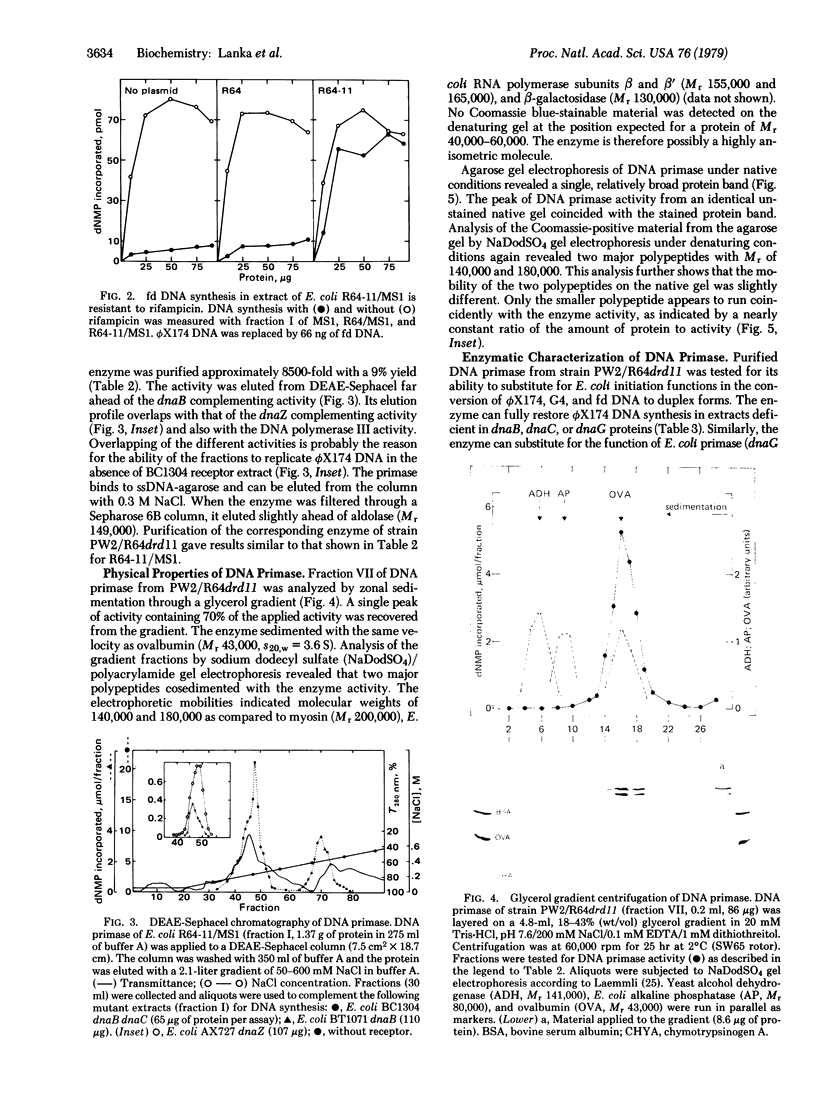
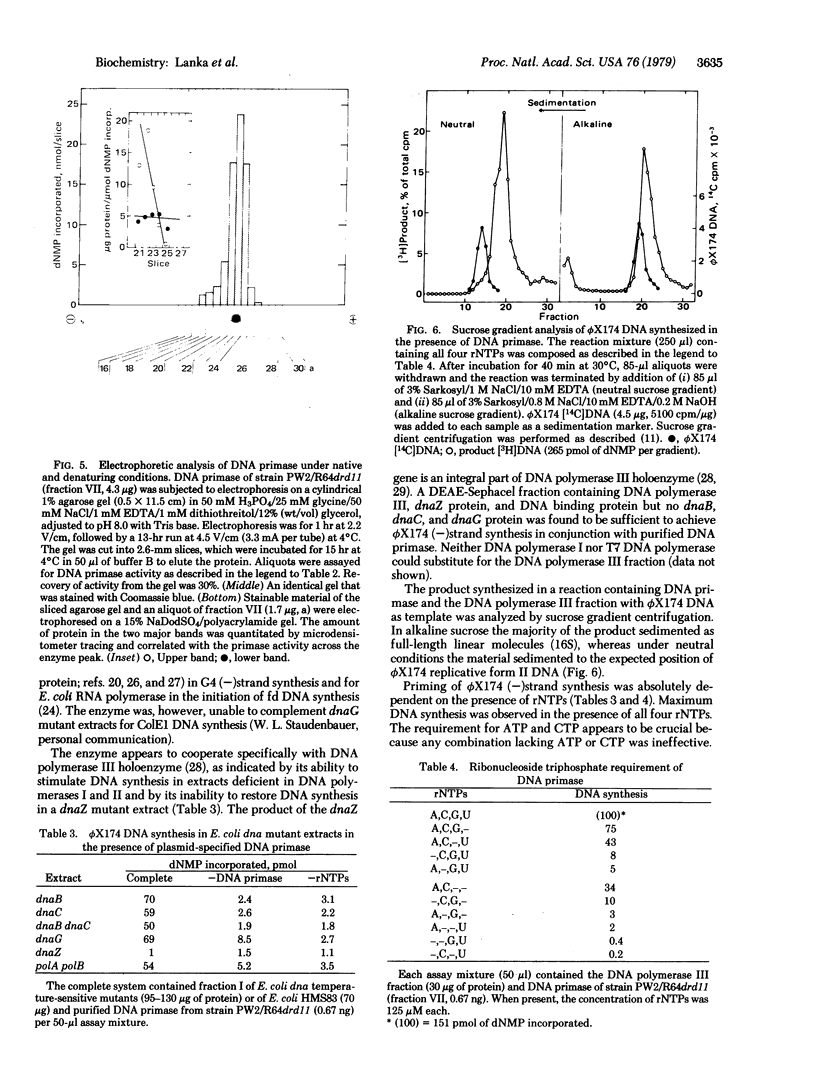
An enzyme has been isolated from Escherichia coli strains harboring the I-like plasmid R64drd11, which is capable of initiating DNA synthesis on the circular, single-stranded DNA of phages phi X174, fd, and G4. In the conversion of these templates to duplex forms in vitro, the enzyme can substitute for the functions of E. coli dna B-dnaB-dnaC-dnaG proteins, E. coli RNA polymerase, and E. coli dnaG protein, respectively. The enzyme requires all four ribonucleoside triphosphates for optimal activity, although a combination of ATP, CTP, and GTP can almost completely satisfy the rNTP requirement. The enzyme appears to cooperate specifically with DNA polymerases III because single-stranded DNA-dependent synthesis takes place in extracts deficient in DNA polymerases I and II but not in extracts from a dnaZ mutant. Highly purified enzyme preparations consist mostly of two major polypeptides, Mr 140,000 and 180,000, when analyzed by sodium dodecyl sulfate gel electrophoresis. These polypeptides cosediment with the enzyme activity through a glycerol gradient with a sedimentation coefficient of 3.6 S. DNA priming activity in extracts of E. coli strains harboring the mutant plasmids R64drd11 or ColIdrd1, which are derepressed in functions of conjugational DNA transfer, severalfold higher than the activity from strains carrying the corresponding wild-type plasmid. This correlation suggests that the enzyme may play a role in conjugational DNA synthesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campbell J. L., Soll L., Richardson C. C. Isolation and partial characterization of a mutant of Escherichia coli deficient in DNA polymerase II. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2090–2094. doi: 10.1073/pnas.69.8.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelbluth C., Lanka E., von der Hude W., Mikolajczyk M., Schuster H. Association of the prophage P1ban protein with the dnaB protein of Escherichia coli. Overproduction of ban protein by a P1bac crr mutant. Eur J Biochem. 1979 Mar;94(2):427–435. doi: 10.1111/j.1432-1033.1979.tb12910.x. [DOI] [PubMed] [Google Scholar]
- Edwards S., Meynell G. G. General method for isolating de-repressed bacterial sex factors. Nature. 1968 Aug 24;219(5156):869–870. doi: 10.1038/219869a0. [DOI] [PubMed] [Google Scholar]
- Fenwick R. G., Jr, Curtiss R., 3rd Conjugal deoxyribonucleic acid replication by Escherichia coli K-12: stimulation in dnaB(ts) donors by minicells. J Bacteriol. 1973 Dec;116(3):1212–1223. doi: 10.1128/jb.116.3.1212-1223.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gefter M. L., Hirota Y., Kornberg T., Wechsler J. A., Barnoux C. Analysis of DNA polymerases II and 3 in mutants of Escherichia coli thermosensitive for DNA synthesis. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3150–3153. doi: 10.1073/pnas.68.12.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geider K., Kornberg A. Conversion of the M13 viral single strand to the double-stranded replicative forms by purified proteins. J Biol Chem. 1974 Jul 10;249(13):3999–4005. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lanka E., Mikolajczyk M., Schlicht M., Schuster H. Association of the prophage P1ban protein with the dnaB protein of Escherichia coli. J Biol Chem. 1978 Jul 10;253(13):4746–4753. [PubMed] [Google Scholar]
- McHenry C., Kornberg A. DNA polymerase III holoenzyme of Escherichia coli. Purification and resolution into subunits. J Biol Chem. 1977 Sep 25;252(18):6478–6484. [PubMed] [Google Scholar]
- McMacken R., Ueda K., Kornberg A. Migration of Escherichia coli dnaB protein on the template DNA strand as a mechanism in initiating DNA replication. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4190–4194. doi: 10.1073/pnas.74.10.4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meynell E., Datta N. Mutant drug resistant factors of high transmissibility. Nature. 1967 May 27;214(5091):885–887. doi: 10.1038/214885a0. [DOI] [PubMed] [Google Scholar]
- Rowen L., Kornberg A. Primase, the dnaG protein of Escherichia coli. An enzyme which starts DNA chains. J Biol Chem. 1978 Feb 10;253(3):758–764. [PubMed] [Google Scholar]
- Schaller H., Nüsslein C., Bonhoeffer F. J., Kurz C., Nietzschmann I. Affinity chromatography of DNA-binding enzymes on single-stranded DNA-agarose columns. Eur J Biochem. 1972 Apr 24;26(4):474–481. doi: 10.1111/j.1432-1033.1972.tb01789.x. [DOI] [PubMed] [Google Scholar]
- Schekman R., Wickner W., Westergaard O., Brutlag D., Geider K., Bertsch L. L., Kornberg A. Initiation of DNA synthesis: synthesis of phiX174 replicative form requires RNA synthesis resistant to rifampicin. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2691–2695. doi: 10.1073/pnas.69.9.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster H., Mikolajczyk M., Rohrschneider J., Geschke B. phiX174 DNA-dependent DNA synthesis in vitro: requirement for P1 ban protein in dnaB mutant extracts of Escherichia coli. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3907–3911. doi: 10.1073/pnas.72.10.3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster H., Schlicht M., Lanka E., Mikolajczyk M., Edelbluth C. DNA synthesis in an Escherichia coli dna B dnaC mutant. Mol Gen Genet. 1977 Feb 28;151(1):11–16. doi: 10.1007/BF00446907. [DOI] [PubMed] [Google Scholar]
- Truitt C. L., Walker J. R. Growth of phages lambda, phiX174, and Ml3 requires the dnaZ (previously dnaH) gene product of Escherichia coli. Biochem Biophys Res Commun. 1974 Dec 11;61(3):1036–1042. doi: 10.1016/0006-291x(74)90259-9. [DOI] [PubMed] [Google Scholar]
- Vapnek D., Lipman M. B., Rupp W. D. Physical properties and mechanism of transfer of R factors in Escherichia coli. J Bacteriol. 1971 Oct;108(1):508–514. doi: 10.1128/jb.108.1.508-514.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P. Y., Iyer V. N. Analogs of the dnaB gene of Escherichia coli K-12 associated with conjugative R plasmids. J Bacteriol. 1978 Jun;134(3):765–770. doi: 10.1128/jb.134.3.765-770.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P. Y., Iyer V. N. Suppression and enhancement of temperature sensitivity of dnaB mutations of Escherichia coli K12 by conjugative plasmids. Plasmid. 1977 Nov;1(1):19–33. doi: 10.1016/0147-619x(77)90005-1. [DOI] [PubMed] [Google Scholar]
- Wechsler J. A., Nüsslein V., Otto B., Klein A., Bonhoeffer F., Herrmann R., Gloger L., Schaller H. Isolation and characterization of thermosensitive Escherichia coli mutants defective in deoxyribonucleic acid replication. J Bacteriol. 1973 Mar;113(3):1381–1388. doi: 10.1128/jb.113.3.1381-1388.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner S. DNA or RNA priming of bacteriophage G4 DNA synthesis by Escherichia coli dnaG protein. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2815–2819. doi: 10.1073/pnas.74.7.2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner S., Hurwitz J. Involvement of escherichia coli dnaZ gene product in DNA elongation in vitro. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1053–1057. doi: 10.1073/pnas.73.4.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins B. M., Hollom S. E. Conjugational synthesis of F lac+ and Col I DNA in the presence of rifampicin and in Escherichia coli K12 mutants defective in DNA synthesis. Mol Gen Genet. 1974;134(2):143–156. doi: 10.1007/BF00268416. [DOI] [PubMed] [Google Scholar]
- Wilkins B. M. Partial suppression of the phenotype of Escherichia coli K-12 dnaG mutants by some I-like conjugative plasmids. J Bacteriol. 1975 Jun;122(3):899–904. doi: 10.1128/jb.122.3.899-904.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright M., Wickner S., Hurwitz J. Studies on in vitro DNA synthesis. Isolation of DNA B gene product from Escherichia coli. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3120–3124. doi: 10.1073/pnas.70.11.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechel K., Bouché J. P., Kornberg A. Replication of phage G4. A novel and simple system for the initiation of deoxyribonucleic acid synthesis. J Biol Chem. 1975 Jun 25;250(12):4684–4689. [PubMed] [Google Scholar]





