Abstract
BACKGROUND
Radiofrequency ablation (RFA) of Barrett’s esophagus (BE) is a common strategy for the prevention of esophageal adenocarcinoma (EAC). After RFA, the ablated esophagus heals on acid suppressive therapy, and is re-populated with a stratified squamous epithelium, referred to as ‘neosquamous epithelium (NSE).’ Because the ability of the NSE to protect the underlying tissue from recurrent insult by reflux is unclear, we assessed the barrier function of NSE by comparing it to that of the native upper squamous epithelium (USE) in subjects having undergone RFA.
METHODS
At varying intervals following RFA, the barrier function of NSE and USE were assessed in endoscopic biopsies by light and electron microscopy, and by measurement of electrical resistance (RT) and fluorescein flux in mini-Ussing chambers. Chamber results were further compared with results from control biopsies (healthy distal esophagus). A claudin expression profile in the tight junctions (TJ) of NSE and USE was determined using qRT-PCR. Differential expression of claudin 4 between NSE and USE was assayed by immunoblots.
RESULTS
USE was histologically normal while NSE showed dilated intercellular spaces and marked eosinophilia. NSE was also more permeable than USE and healthy controls, having lower mean RT and higher fluorescein fluxes. Abnormally low RT values for NSE were unrelated to the time period following RFA (or number of prior RFA sessions), being abnormal even 26 months after RFA. Abnormal permeability in NSE was associated with significantly lower values for claudin-4 and claudin-10 than in USE.
CONCLUSIONS
NSE commonly exhibits defective barrier function. Since this defect will make it vulnerable to injury, inflammation and destruction by acidic and weakly acidic refluxates, it may in part explain incidences of recurrence of BE following ablation.
Introduction
Barrett’s esophagus (BE) is defined as the presence of specialized columnar epithelium lining the distal esophagus(1, 2). It arises in the setting of gastroesophageal reflux disease (GERD) as replacement for the ‘native’ esophageal stratified squamous epithelium. Although structurally and functionally better suited to resist reflux damage than squamous epithelium (3,4), BE is a premalignant lesion that can progress from metaplasia to dysplasia to esophageal adenocarcinoma (EAC) at rates reported to range from ~0.12% to 0.5%/year (1,5). One strategy currently employed to prevent BE progression to EAC is endoscopic ablation using radiofrequency ablation (RFA). This approach has been demonstrated to decrease the incidence of EAC in subjects with BE and high-grade dysplasia (6). Following RFA and while on acid suppressive therapy with proton pump inhibitors (PPIs), ablated segments heal with stratified squamous epithelium, referred to as ‘neosquamous epithelium (NSE)’ to distinguish it from native squamous epithelium (1,6). Although initially limited to eradication of dysplastic BE, there is increasing interest in extending this strategy to those without dysplasia whose risk of EAC is very low (7–9). For RFA to be successful in this latter group will require the long term stability of the replacement NSE, particularly since the NSE is located in an area highly exposed to acidic and weakly acidic gastric contents from ongoing reflux. Of concern, recurrence of BE is common, occurring in approximately 25% of subjects after RFA ablation at 3 years of follow-up (10).
Claudins are a family of proteins resident in the tight junctions of squamous epithelium, which are thought to confer resistance to paracellular transport of ions and other molecules. A total of 24 claudins have been described to date (11). Previous work suggests that the claudin profile, or the composition of the various claudins composing the tight junction, decides the competency of this barrier. For instance, squamous epithelium rich in claudin-4 demonstrates high resistance to paracellular diffusion of ions (12). Therefore, the claudin expression profile might shed light on the quality of the epithelial barrier.
Since to date there is limited information on the integrity of NSE (13) and particularly on its functional integrity as a barrier against noxious luminal contents, e.g. refluxed acid, we assessed the barrier function of NSE by comparing it to that of the ‘native’ upper squamous epithelium (USE) in the same patient. Because of the substantial rate of recurrence of BE after ablation, we hypothesized that the NSE might exhibit impaired epithelial barrier function, and that this impaired function might in part be explained by an altered claudin profile compared to non-ablated squamous tissue.
Materials/Methods
Patients
The integrity of the NSE was prospectively assessed in adult patients who presented to the facilities of the University of North Carolina for endoscopic follow up of ablated dysplastic BE (equally distributed between low and high grade dysplasia) that had been performed using the HALO system (Covidien Medical, Sunnyvale, CA). All patients were maintained on chronic twice-a-day proton pump inhibitors for at least 6 months prior to assays and all had complete intact linings of NSE that arose following radiofrequency ablation for dysplastic BE that was at least 3 cm in length. Inclusion criteria required the absence of any apparent residual Barrett’s epithelium. Paired endoscopic biopsies of healthy-appearing NSE and upper squamous epithelium (USE) were obtained using Maxi Jaw forceps. USE was taken from the proximal esophagus at either 25 cm from the incisors or at least 5 cm above the upper margin of the BE, whichever was more proximal.
Adult controls were patients endoscoped for clinical indications but without signs or symptoms of esophageal disease and an endoscopically normal-appearing esophagus.
Biopsies were placed in formalin fixative for light microscopy of hematoxylin-eosin stained sections and in 2% paraformaldehyde-2% glutaraldehyde for electron microscopy. Other biopsies were placed in cold Ringer solution for immediate transfer to the laboratory. This study was reviewed and approved by the Institutional Review Board at the University of North Carolina at Chapel Hill.
At the laboratory, biopsies were mounted in mini-Ussing transport chambers to determine the total transepithelial electrical resistance (RT) and the mucosal-to-serosal flux of fluorescein as markers of epithelial permeability. RT is a measure of the epithelium’s resistance to flux of ions across it, with higher numbers associated with more resistant epithelium. Fluorescein flux assesses the ease with which fluorescein, a large uncharged molecule, diffuses across the paracellular space, with higher measurements indicating increased diffusion. In some subjects biopsies were fixed in RNA-Later for performance of quantitative recombinant-polymerase chain reaction (qRT-PCR) and, in selected cases, Western blots to evaluate the expression of the claudins as representatives of esophageal epithelial tight junction (TJ) proteins.
Quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR)
Claudin profile by qRT-PCR was performed on endoscopic biopsies. In brief biopsies underwent total RNA isolation using RNeasy kits (Qiagen, Inc., Valencia, CA) per the manufacturer’s protocols. RNA was first treated with TURBO DNase (TURBO DNA-free kit, Ambion, Inc., Austin, TX) and cDNA synthesized from 2.5 μg of treated RNA for each tissue sample using Superscript III reverse transcriptase (Invitrogen Corporation, Carlsbad, CA). Real-time PCR primers were validated primer sets (QuantiTect Primers Assays, Qiagen, Inc., Valencia, CA). Real-time PCR was performed in triplicate with No-RT control for each sample. Reactions consisted of SYBR Green JumpStartTaq ReadyMix for Quantitative PCR (Sigma–Aldrich Co., St. Louis, MO), pre-made primers, and 5 μL of sample (cDNA or control). Amplification was performed in a thermal cycler and following amplification, a melting curve analysis was performed. The cycle at which each sample crossed a fluorescence threshold, Ct, was determined and triplicate values for each cDNA averaged. Eef1a1 served as a control gene for normalization between samples. ZO-1 showed a constant relationship to Eef1a1 in all samples and was also considered a non-changing control. To simplify reporting, gene expression was normalized to ZO-1 expression. Relative expression values were calculated as 2 ΔCt, setting the expression value of ZO-1 to 1.0. If a sample’s signal did not rise above threshold within 37 cycles, it was considered not detectable (ND).
Immunoblots
Biopsies for Western blots were flash frozen in liquid N2 and stored at −80° C. Tissue lysates were prepared by homogenizing tissue with the TissueLyser bead mill (Qiagen, Inc., Valencia, CA) in 20 volumes of a 50 mM Hepes buffer (pH 7.4) with 1% Triton X-100, 0.05% SDS, 0.2% DOC, 150 mM NaCl, 1.5 mM MgCl2, 1 mM EGTA, and Complete EDTA-free Protease Inhibitor Cocktail (Roche Diagnostics Corporation, Indianapolis, IN). Cell debris was removed by a short centrifugation at 5000 rpm. An aliquot of cleared lysate was kept for protein quantitation using the BCA Protein Assay Kit (Pierce Biotechnology, Inc., Rockford IL), and the rest diluted with SDS-Laemmli sample buffer. Methods for electrophoresis and immunoblotting were standard. Antibodies against claudin 4 were purchased from Zymed Laboratories (San Francisco, CA). Secondary antibodies for immunoblots were goat anti-rabbit IRDye 800 (Rockland, Gilbertsville, PA). Signals were detected using an Odyssey Infrared Imaging System (LI-COR, Inc., Lincoln, NE).
Permeability studies in Mini-Ussing chamber
Biopsies were immersed in ice-cold oxygenated Ringer solution and immediately transported to the research laboratory. Specimens were mounted between Lucite rings (aperture diameter, 2 mm, and square area, 0.0314 cm2) in mini-Ussing chambers (Device illustrated in reference #12) and bathed on both sides with 5 ml normal Ringer solution (composition in mmol/L): Na+ 140, Cl− 119.8, K+ 5.2, HCO3− 25, Ca++ 1.2, Mg++ 1.2, HPO42− 2.4, H2PO4− 0.4, 268 mosmol/kg H20, pH 7.4 when gassed with 95%O2/5%CO2 at 37°C. In order to provide informative data for this assay, subjects must have had both proximal and distal biopsies of adequate size to completely seal the aperture. Two sets of electrodes connected the Ringer solutions to voltage clamps (Voltage Current Clamp, MC6; Physiologic Instruments, San Diego, CA) that enabled direct recording of the transmural electrical potential difference (PD) and, by passage of current, the short-circuit current (Isc). The total electrical resistance (RT) is calculated using Ohm’s Law: PD = Isc x RT. All experiments were conducted under open circuit conditions except when transiently switched to the short circuit state for recording of Isc.
After equilibration for 30 min, basal readings of PD, Isc and RT were obtained and the flux of fluorescein, 300 molecular weight, (Sigma, St. Louis, MO) was determined by adding 1 mM fluorescein to the mucosal bath and then sampling the mucosal bath to obtain the initial concentration. The serosal bath was sampled at zero time and at 45 min intervals. Samples were read for the presence of fluorescein using a fluorometer (SLM Amico SPF 500, SLM Instruments, Inc. Urbana IL) and the fluorescence in the serosal samples divided by the fluorescence in the luminal bath x 100 to determine the flux as percent (%) of initial fluorescein concentration. [Note that the values for fluorescence were linear over the range measured in these experiments.]
Data are reported as the mean ± SEM. Statistical significance was accepted at the p<0.05 level and determined using Students t test for continuous data from paired and unpaired samples and Chi square 2×2 table for categorical data.
Results
Patients
The integrity of the NSE was assessed in a total of 37 adult patients (29 Caucasian male and 8 Caucasian female, mean age (65.5 ± 7 years) who agreed to allow esophageal biopsies of both NSE and healthy-appearing upper squamous epithelium (USE) from the esophagus. Endoscopic biopsies of healthy-appearing NSE and USE from the same patients were obtained on average 8.2 ± 2.8 months (range 1–26 months) following their last RFA treatment. No patient had undergone prior endoscopic mucosal resection and the number of RFA sessions prior to participation in the study ranged from 1 to 8 with a mean of 3.7 ± 1.6. Subsets of this population participated in different protocols as described below for the individual analyses. Adult controls, (n=9, 100% Caucasian males, aged 66.7 ± 12 years) for healthy distal esophageal epithelium were patients endoscoped for clinical indications but without signs or symptoms of esophageal disease and an endoscopically normal-appearing esophagus.
Morphology
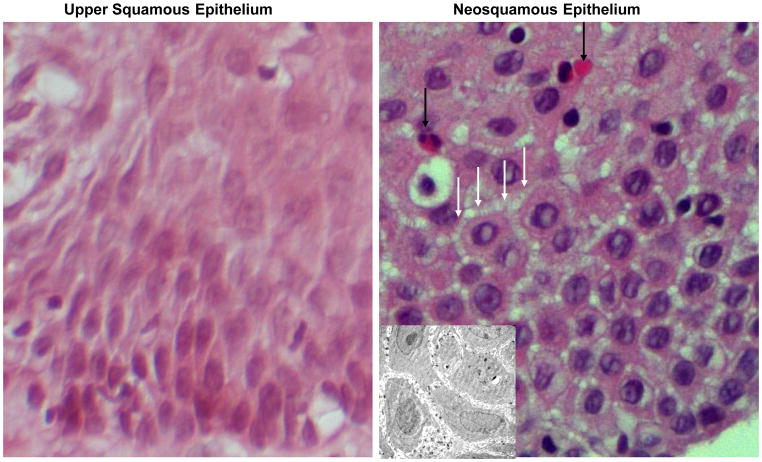
Initially, 13 patients had evaluagted hematoxylin and eosin-stained sections from esophageal biopsies of both NSE and USE from the same patients. Biopsies were reviewed blindly by one of the investigators (RCO) and scored as either being positive or negative for the presence of dilated intercellular spaces. Dilated intercellular spaces were present in 13 of 13 (100%) biopsies of NSE and in 2 of 13 (15%) biopsies of USE, p<0.05 from the same patients (Fig. 1), and this verified in selected cases by performance of transmission electron microscopy (Fig. 1 insert). It was also notable that 7 of 13 (54%) biopsies of NSE had significant eosinophilia compared to 0 of 13 (0%) biopsies of USE, p<0.05.
Fig. 1.
A light photomicrograph of a hematoxylin-eosin stained section of native esophageal upper squamous epithelium (left panel) and neosquamus epithelium from the lower esophagus (right panel). Note that neosquamous epithelium shows dilated intercellular spaces (white arrows) and has a prominent infiltrate of eosinophils (black arrows) when compared with the normal-appearing ‘native’ upper squamous epithelium. Magnification 60X. The INSERT in the right panel is an electron photomicrograph to better illustrate the dilated intercellular spaces in neosquamous epithelium. Magnification 3000X.
Permeability Markers
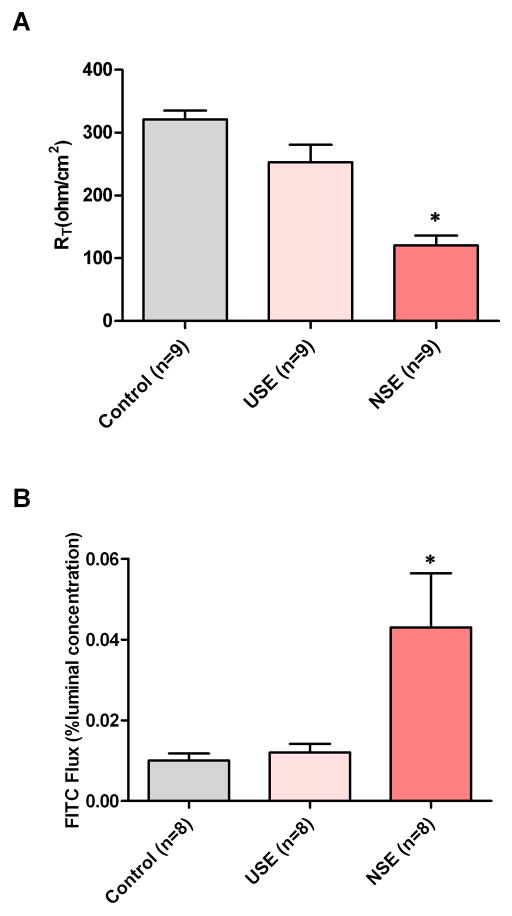
To assess barrier function, paired endoscopic biopsies of NSE and USE were obtained and mounted in mini-Ussing chambers for both measurement of RT and fluorescein flux and these compared with endoscopic biopsies of healthy squamous epithelium from the distal esophagus (controls). Nine subjects had both proximal and distal samples from biopsy of adequate size to be mounted in the chambers. As illustrated in Fig 2A&B, the mean basal value of RT for NSE (120 ± 45.7 ohms.cm2) was significantly lower than RT for USE (252.9 ± 84.2 ohms.cm2; n=9, p<0.05), and for controls (321 ± 42 ohms.cm2; n=9, p<0.05). Similarly, fluorescein flux for NSE (0.043% ± 0.038) was significantly higher than flux for USE (0.010% ± 0.006; n=8, p<0.05), and controls (0.010% ± 0.005; n=8, p<0.05). There where no significant difference between either RT or flux between USE and controls. [Note: although 9 paired biopsies were evaluable for RT of NSE and USE, one biopsy was lost during flux and so only 8 paired biopsies of NSE and USE were evaluable for fluorescein flux.]
Fig. 2.
The transepithelial electrical resistance (RT) (upper panel) and fluorescein flux (lower panel) are illustrated for neosquamous epithelium (NSE), native esophageal upper squamous epithelium (USE) from the same patients, and healthy native distal squamous epithelium from subjects without esophageal disease (controls). Note that NSE has significantly lower RT and higher fluorescein flux values than both USE and healthy controls indicating higher paracellular permeability to ions and uncharged molecules, respectively. * p<0.05 compared to healthy controls.
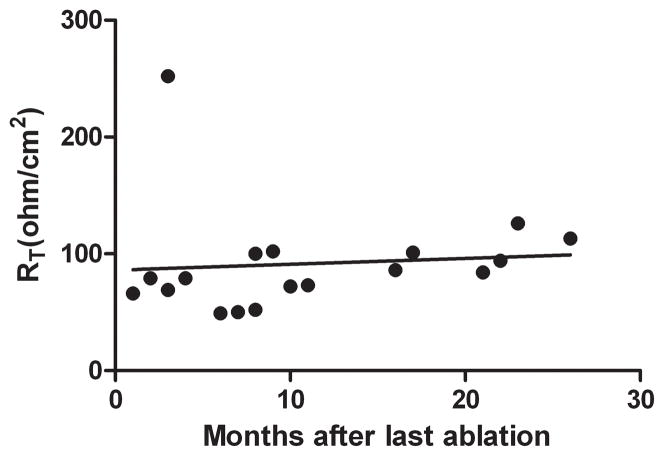
Given that RT was significantly lower for NSE than USE, we questioned whether this was a function of time from RFA, i.e. with the lower values of NSE were simply a reflection of the time needed for complete repair of the epithelium. Therefore, we plotted the RT values for NSE versus time from last RFA treatment for all subjects who had assessment of NSE resistance (n=18; Fig. 3). As shown, RT values for healthy-appearing NSE were obtained from 2–22 months from their last RFA; however there was no correlation between time from RFA and value of RT and specifically, RT did not increase over time from RFA. Moreover, there was no correlation of either RT or fluorescein flux values with number of RFA sessions prior to biopsy of NSE. Specifically, a greater number of sessions did not result in lower values for RT or higher values for fluorescein flux (data not shown).
Fig. 3.
A plot of the transepithelial electrical resistance (RT in ohms. cm2) in NSE versus the time (months) from the last performance of radiofrequency ablation of Barrett’s esophagus. Note that there is no trend for RT to improve with time for NSE (linear regression R2=0. 008; p=0.7).
Claudin Expression
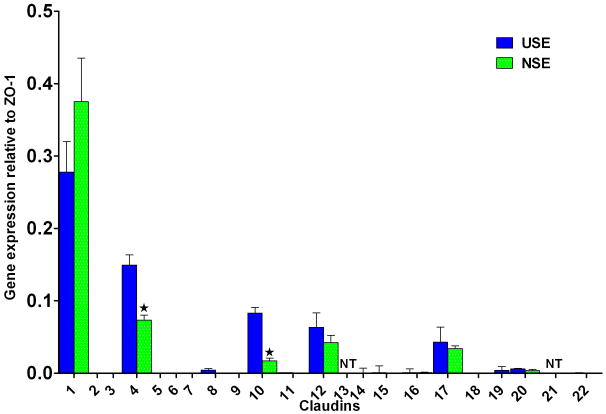
qRT-PCR was performed for 20 claudins on endoscopic biopsies of NSE and USE from the same patients. The claudins tested were: #1 thru #12, #14 thru #20, #22 and #23. Claudin expression is presented as relative the expression of zonula occludens-1 (ZO-1), where ZO-1 is set at 1.0. As shown in Fig. 4, although a big number of claudins were expressed, most were in small amounts with the exception of claudin#1 whose expression levels exceeded 0.25% of ZO-1. Claudin #2 and #6 were undetectable. Notably, NSE exhibited significantly lower levels for claudin #4 and #10 than for USE, and we verified by Western blot (Fig. 5) that the protein expression level of claudin #4 was also lower in NSE than in USE.
Fig. 4.
Claudin gene expression profiles for neosquamous epithelium (NSE) and for native esophageal upper squamous epithelium (USE). Claudin expression levels are referenced to expression levels for ZO-1 which is set at 1.0. Note that expression of claudin-4 and claudin-10 were significantly lower in NSE than in USE while there was no difference in expression of the most prominently expressed claudin, i.e. claudin-1. Error bars = [(2%CV)/100] x [relative expression]; NT = not tested; *p<0.05 compared to USE.
Fig. 5.
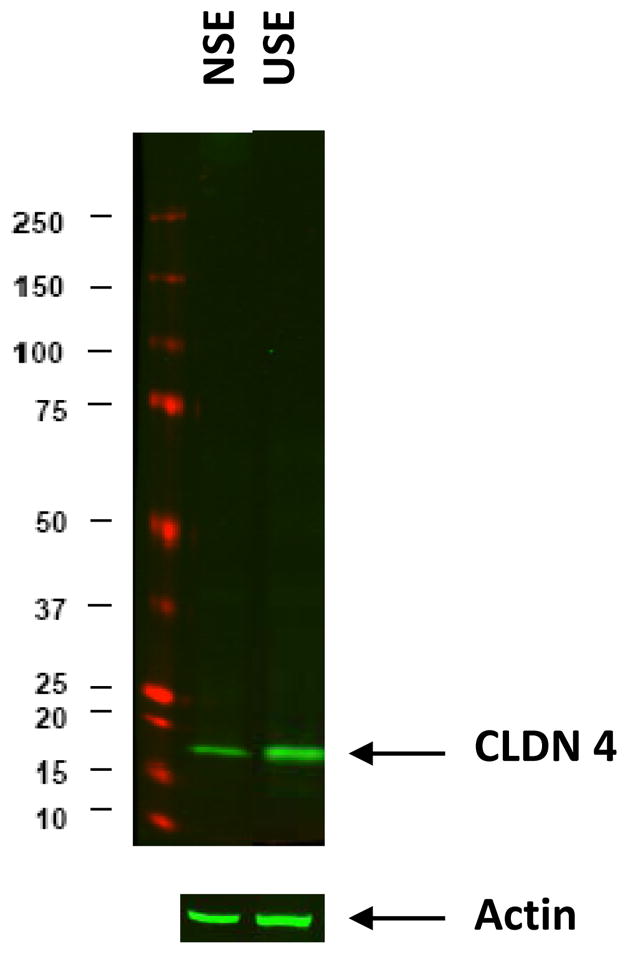
An immunoblot of claudin-4 for neosquamous epithelium (NSE) and for native esophageal upper squamous epithelium (USE). Ten micrograms of protein were loaded in each lane and the actin signal shown to document equal protein loading. Molecular weight standards are displayed on the left in kilodaltons (kDa). Prestained SDS-PAGE standard (wide range; Bio-Rad) was used (red bands). Claudin-4 is shown to be more strongly expressed in USE than NSE.
Discussion
In the present report, we evaluated the barrier function of NSE as a marker of its integrity and compared it to both the ‘native’ USE from the same patient and to native distal esophageal squamous epithelium from controls with healthy esophagus. Native USE was of interest for comparison because it represents the optimum control for the genetic and biologic potential for barrier function of NSE while allowing for differences in environment to be manifest, e.g. exposure to gastric refluxates and previous ablative therapy. Moreover, native USE in the study proved to be an effective surrogate for ‘healthy’ squamous epithelium in that it lacked (in 11 of 13 subjects) dilated intercellular spaces and exhibited mean values for RT and fluorescein flux that were shown to be similar to that of healthy distal esophageal epithelium of controls, and mirrored those we previously published from the distal esophagus of healthy subjects (14). When compared to USE and healthy distal esophageal epithelium of controls, we found that NSE exhibited both morphologic and functional abnormalities indicative of defective barrier function. Specifically, NSE exhibited dilated intercellular spaces (13 of 13 subjects) and compared to both USE and healthy controls had lower RT and higher fluorescein flux values. Taken together, these features support the presence in NSE of a ‘leaky’ paracellular pathway to ions and uncharged molecules. Moreover, based on the persistence of low RT over periods of up to 26 months following RFA, the defective barrier could not be attributed to the time for repair of the ablated segment with NSE – which, as was standard, took place for all patients on twice-a-day PPI therapy.
The reason(s) for the defective barrier function of the NSE observed in this study is unclear. At its simplest, this could represent recurrent insult from GERD. Notably, and despite PPI therapy, the defective barrier in NSE had features similar to the defective barrier function reported in patients with nonerosive reflux disease, i.e. dilated intercellular spaces, low RT and high fluorescein flux (14–16). This raises the possibility that persistence of the defective barrier function in NSE results from ongoing exposure to acidic and/or weakly acidic refluxates (17). While these subjects were maintained on high dose (twice-daily) PPI therapy after ablation, a previous report of 24hr pH monitoring in asymptomatic patients with BE demonstrated pathological esophageal exposure in 62% even while on twice-a-day PPI therapy (18). Because the current study does not feature concurrent pH monitoring, the extent to which abnormal acid exposures account for the observed impaired barrier function cannot be assessed with the present data, and await further study. Of note, eosinophilia was noted in the NSE. Whether this is due to ongoing reflux injury or another process is not clear.
A second possibility is that genetic influences in these patients predispose them to a functionally deficient epithelial barrier. If this were the case, it might not be surprising that the neosquamous epithelium would recapitulate the findings in the native epithelium, which may have predisposed the patients to develop BE in the first place. Finally, it is possible that post-RFA epithelium is qualitatively different, and inferior to native tissue. While the cross-sectional nature of this study makes it impossible to cast light on the pre-ablation tissue, it may be that the epithelium regenerated after injury is different than the native tissue. To what degree one or more of these mechanisms may explain our findings is unclear.
The implication of this work is that despite PPI therapy, the NSE is a “leaky” epithelial barrier, susceptible to recurrent injury. This could presumably be a mechanism for the 20% or greater recurrence rate of BE seen following ablative therapy (6,19). Indeed, in a study by Kahaleh et al the recurrence rate of BE following successful ablation (argon plasma coagulation) has been linked to ongoing acid reflux by showing that BE recurred in 15/18 pts (83%) patients with abnormal (acid) pH monitoring versus only 1/8 patients (12.5%) with normal pH monitoring (20). In addition to recurrence of BE, reflux damage to NSE may have the potential to destabilize any sub-squamous BE beneath it. This might occur either because the loss of barrier function by NSE enables luminal contents greater access to buried BE and/or because the influx of inflammatory cells into the area has byproducts, e.g. oxygen-free radicals, prostaglandins, that expose buried BE to promoters of cell turnover and mutagenesis (21,22). In keeping with this concern is the fact that sub-squamous high grade dysplasia and EAC has been occasionally reported following RFA (23) and that optical coherence tomography studies suggest that sub-squamous BE may be present in as many as 63% of patients (24).
To explore the basis for the defective barrier in NSE, we compared the TJ expression profiles of 20 claudins in NSE to that of USE in the same patients. The results showed that, while claudin 1 levels were not significantly different, NSE had significant reductions in both claudin-4 and claudin-10 compared to USE. The decrease in claudin-4 is especially notable, since previous work by our group demonstrated that claudin-1 and claudin-4 were the two dominant TJ proteins in healthy esophageal squamous epithelium (4), and that reductions in claudin-4 in tissue culture monolayers is accompanied by both lower RT and increased paracellular permeability to cations (25). Moreover, Oshima et al demonstrated in a culture model of stratified squamous epithelium that exposure luminally to high concentrations of acid (pH 2 for 4 hrs) resulted in increased paracellular permeability and this in conjunction with a decline in TJ-associated claudin-4 (26). Given that in the present study, the reduction in claudin-4 expression in NSE was paralleled by a reduction in claudin-4 protein on Western blot in NSE, this may be a mechanism to account for the defective TJ and increased paracellular permeability to ions in NSE seen in our study. Since claudin-4 in squamous epithelium has been shown sensitive to luminal acid (26) and hydrogen ions are the smallest of cations, these data provide a mechanism for the vulnerability of NSE to the destructive effects of luminal acid, since hydrogen ions are able to diffuse more freely into the NSE than into normal squamous tissue.
In summary, this study demonstrates that patients with NSE have defective barrier function and that this defect persists for long periods despite PPI therapy. This defect is manifest by an increase in paracellular permeability to ions, and may be at least partially explained by diminished claudin-4 levels in the tight junctions of the NSE. These defects could result in a vulnerability of NSE to the destructive effects of ongoing exposure to acidic and weakly acidic refluxates. Further, and by extension, the finding of defective barrier function in NSE provides a key element in support of the hypothesis that ongoing exposure to acid reflux may account for the recurrence of BE following RFA and other ablative techniques and potentially to the continued risk of EAC emerging in sub-squamous BE. Whether these defects are reversible by more intensive medical or surgical therapy remains unclear. Furthermore, the extent to which these changes predispose to recurrence of BE following RFA or other ablative techniques, and their implications for risk of neoplasia are not yet determined. Future studies could consider the barrier function of the NSE as a predictor for recurrence of disease following RFA.
Study Highlights.
What is Current Knowledge
Ablation of Barrett’s esophagus (BE) is a commonly-used therapy in patients with BE dysplasia to prevent esophageal adenocarcinoma
Successful therapy is dependent upon healing with stratified (neo)squamous epithelium (NSE)
NSE appears grossly ‘normal’ on endoscopy
What is New Here
NSE exhibits defective barrier function in many subjects and for long periods
This defect is evident by dilated intercellular spaces, low electrical resistance and high fluorescein flux and is associated with abnormal claudin profile
Defective barrier function may render NSE more vulnerable to reflux damage and recurrent BE.
Acknowledgments
Grant Support: This work was funded by a grant from the American Society of Gastrointestinal Endoscopy (NJS); GI Solutions and CSA Medical (NJS)
Disclosures: Roy C. Orlando MD has investigator grant funding from AstraZeneca and Takeda Pharmaceuticals.
Abbreviations
- RFA
Radiofrequency ablation
- BE
Barrett’s esophagus
- NSE
neosquamous epithelium
- USE
upper squamous epithelium
- TJ
tight junction
- EE
esophageal epithelium
- GERD
gastroesophageal reflux disease
- EAC
esophageal adenocarcinoma
- PPI
proton pump inhibitors
- RT
transepithelial resistance
- qRT-PCR
quantitative reverse transcriptase-polymerase chain reaction
Footnotes
Authors Contribution: Biljana Jovov, Roy C. Orlando and Nicholas J. Shaheen were involved in all aspects of this study from experimental design to manuscript writing. Zorka Djukic and Geraldine S. Orlando provided technical expertise and assisted with both data collection and analysis.
References
- 1.Shaheen NJ, Richter JE. Barrett’s oesophagus. Lancet. 2009;373(9666):850–61. doi: 10.1016/S0140-6736(09)60487-6. [DOI] [PubMed] [Google Scholar]
- 2.Spechler SJ. Barrett’s esophagus. In: Orlando RC, editor. Gastroesophageal Reflux Disease. New York: Marcel Dekker, Inc; 2000. pp. 219–258. [Google Scholar]
- 3.Orlando RC. Mucosal Defense in Barrett’s Esophagus. In: Sharma P, Sampliner R, editors. Barrett’s Esophagus and Esophageal Adenocarcinoma. 2. Blackwell Publishing, Ltd; Oxford, UK: 2006. pp. 60–72. [Google Scholar]
- 4.Jovov B, Van Itallie CM, Shaheen NJ, Carson JL, Gambling TM, Anderson JM, Orlando RC. Claudin-18: A Dominant Tight Junction Protein in Barrett’s Esophagus and Likely Contributor to its Acid Resistance. Am J Physiol Gastrointest Liver Physiol. 2007;293(6):G1106–13. doi: 10.1152/ajpgi.00158.2007. [DOI] [PubMed] [Google Scholar]
- 5.Hvid-Jensen F, Pedersen L, Drewes AM, Sørensen HT, Funch-Jensen P. Incidence of adenocarcinoma among patients with Barrett’s esophagus. N Engl J Med. 2011;365(15):1375–83. doi: 10.1056/NEJMoa1103042. [DOI] [PubMed] [Google Scholar]
- 6.Shaheen NJ, Sharma P, Overholt BF, Wolfsen HC, Sampliner RE, Wang KK, et al. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N Engl J Med. 2009;360(22):2277–88. doi: 10.1056/NEJMoa0808145. [DOI] [PubMed] [Google Scholar]
- 7.Roorda AK, Marcus SN, Triadafilopoulos G. Early experience with radiofrequency energy ablation therapy for Barrett’s esophagus with and without dysplasia. Dis Esophagus. 2007;20(6):516–22. doi: 10.1111/j.1442-2050.2007.00728.x. [DOI] [PubMed] [Google Scholar]
- 8.Hernandez JC, Reicher S, Chung D, Pham BV, Tsai F, Disibio G, et al. Pilot series of radiofrequency ablation of Barrett’s esophagus with or without neoplasia. Endoscopy. 2008;40(5):388–92. doi: 10.1055/s-2007-995747. [DOI] [PubMed] [Google Scholar]
- 9.Falk GW. Radiofrequency ablation of Barrett’s esophagus: should everybody get it? Gastroenterology. 2009 doi: 10.1053/j.gastro.2009.04.036. [DOI] [PubMed] [Google Scholar]
- 10.Shaheen NJ, Overholt BF, Sampliner RE, Wolfsen HC, Wang KK, Fleischer DE, Sharma VK, Eisen GM, Fennerty MB, Hunter JG, Bronner MP, Goldblum JR, Bennett AE, Mashimo H, Rothstein RI, Gordon SR, Edmundowicz SA, Madanick RD, Peery AF, Muthusamy VR, Chang KJ, Kimmey MB, Spechler SJ, Siddiqui AA, Souza RF, Infantolino A, Dumot JA, Falk GW, Galanko JA, Jobe BA, Hawes RH, Hoffman BJ, Sharma P, Chak A, Lightdale CJ. Durability of radiofrequency ablation in Barrett’s esophagus with dysplasia. Gastroenterology. 2011 Aug;141(2):460–8. doi: 10.1053/j.gastro.2011.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta IR, Ryan AK. Claudins: unlocking the code to tight junction function during embryogenesis and in disease. Clinical Genetics. 2010;77(4):314–325. doi: 10.1111/j.1399-0004.2010.01397.x. [DOI] [PubMed] [Google Scholar]
- 12.Tobey NA, Argote CM, Vanegas XC, Barlow W, Orlando RC. Electrical Parameters and Ion Species for Active Transport in Human Esophageal Stratified Squamous Epithelium and Barrett’s Specialized Columnar Epithelium. Am J Physiol Gastrointest Liver Physiol. 2007 Jul;293(1):G264–70. doi: 10.1152/ajpgi.00047.2007. [DOI] [PubMed] [Google Scholar]
- 13.Paulson TG, Xu L, Sanchez C, Blount PL, Ayub K, Odze RD, et al. Neosquamous epithelium does not typically arise from Barrett’s epithelium. Clin Cancer Res. 2006;12(6):1701–6. doi: 10.1158/1078-0432.CCR-05-1810. [DOI] [PubMed] [Google Scholar]
- 14.Jovov B, Que J, Tobey NA, Djukic Z, Hogan BLM, Orlando RC. Role of E-cadherin in the Pathogenesis of Gastroesophageal Reflux Disease. Am J Gastroenterology. 2011 Jun;106(6):1039–47. doi: 10.1038/ajg.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tobey NA, Carson JL, Alkiek RA, Orlando RC. Dilated intercellular spaces: a morphological feature of acid reflux-damaged human esophageal epithelium. Gastroenterology. 1996;111:1200–1205. doi: 10.1053/gast.1996.v111.pm8898633. [DOI] [PubMed] [Google Scholar]
- 16.Caviglia R, Ribolsi M, Gentile M, Rabitti C, Emerenziani S, Guarino MP, Petitti T, Cicala M. Dilated intercellular spaces and acid reflux at the distal and proximal oesophagus in patients with non-erosive gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2007;25(5):629–36. doi: 10.1111/j.1365-2036.2006.03237.x. [DOI] [PubMed] [Google Scholar]
- 17.Sifrim D. Acid, weakly acidic and non-acid gastro-oesophageal reflux: differences, prevalence and clinical relevance. Eur J Gastroenterol Hepatol. 2004;16(9):823–30. doi: 10.1097/00042737-200409000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Gerson LB, Shetler K, Triadafilopoulos G. Control of intra-oesophageal and intra-gastric pH with proton pump inhibitors in patients with Barrett’s oesophagus. Dig Liver Dis. 2005;37(9):651–8. doi: 10.1016/j.dld.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 19.Sharma VK, Wang KK, Overholt BF, Lightdale CJ, Fennerty MB, Dean PJ, et al. Balloon-based, circumferential, endoscopic radiofrequency ablation of Barrett’s esophagus: 1-year follow-up of 100 patients. Gastrointest Endosc. 2007;65(2):185–95. doi: 10.1016/j.gie.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 20.Kahaleh M, Van Laethem JL, Nagy N, Cremer M, Deviere J. Long-term follow-up and factors predictive of recurrence in Barrett’s esophagus treated by argon plasma coagulation and acid suppression. Endoscopy. 2002;34(12):950–5. doi: 10.1055/s-2002-35847. [DOI] [PubMed] [Google Scholar]
- 21.Yoshida N. Inflammation and oxidative stress in gastroesophageal reflux disease. J Clin Biochem Nutr. 2007;40(1):13–23. doi: 10.3164/jcbn.40.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eksteen JA, Scott PA, Perry I, Jankowski JA. Inflammation promotes Barrett’s metaplasia and cancer: a unique role for TNFalpha. Eur J Cancer Prev. 2001;10(2):163–6. doi: 10.1097/00008469-200104000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Titi M, Overhiser A, Ulusarac O, Falk GW, Chak A, Wang K, Sharma P. Development of sub-squamous high-grade dysplasia and adenocarcinoma after successful radiofrequency ablation of Barrett’s esophagus. Gastroenterology. 2012 doi: 10.1053/j.gastro.2012.04.051. doi:10,1053/j.gastro.2012.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou C, Tsai TH, Lee HC, Kirtane T, Figueiredo M, Tao YK, Ahsen OO, Adler DC, Schmitt JM, Huang Q, Fujimoto JG, Mashimo H. Characterization of buried glands before and after radiofrequency ablation by using 3-dimensional optical coherence tomography. Gastrointest Endosc. 2012 doi: 10.1016/j.gie.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Itallie C, Rahner C, Anderson JM. Regulated expression of claudin-4 decreases paracellular conductance through a selective decrease in sodium permeability. J Clin Invest. 2001;107(10):1319–27. doi: 10.1172/JCI12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oshima T, Koseki J, Chen X, Miwa H. Acid modulates the squamous epithelial barrier function by modulating the localization of claudins in the superficial layers. Lab Invest. 2012;92:22–31. doi: 10.1038/labinvest.2011.139. [DOI] [PubMed] [Google Scholar]