Development of synthetic agents that enable specific and sequence-unrestricted targeting of double-stranded DNA (dsDNA) is a long-standing goal of biological chemistry and molecular biology. Efforts are fuelled by the prospect for molecular tools that i) infer and regulate gene function via transcriptional interference, ii) induce genomic repair and recombination, iii) detect target genes, and, iv) treat genetic diseases at the transcriptional level.[1–6] However, unlike RNA-targeting antisense oligonucleotides and siRNA, which are routinely used for transient modulation of gene expression,[7,8] dsDNA-targeting techniques are used much less. This reflects the greater complexity of the target, as well as limitations of classic probe technologies[9–13]: i) triplex-forming oligonucleotides require long homopurine target regions; ii) peptide nucleic acids (PNAs) require non-physiological salinity; and iii) minor-groove binding polyamides are typically only directed against short target regions. These drawbacks have stimulated development of alternative strategies including pseudocomplementary (pc) DNA,[14] pcPNA,[15,16] antigene PNA,[17] antigene locked nucleic acid (LNA),[18] modified γ-PNA,[19,20] zorro LNA,[21] TFOs with engineered nucleobases,[22,23] groove-binding natural products,[24,25] engineered proteins,[26,27] and other oligonucleotide-based approaches.[28,29] Despite these efforts, there remains an urgent and unmet need for probes that enable rapid, specific and efficient mixed-sequence recognition of chromosomal DNA regions (> 15 base pairs) in a wide range of contexts at physiologically relevant conditions, while maintaining desirable “DNA-like” qualities such as aqueous solubility, compatibility with delivery agents, and amenability for large-scale production.
We have explored Invader LNAs as a potential solution toward this end.[30,31] Briefly, Invader LNAs are short DNA duplexes, which are activated for dsDNA-recognition through modification with one or more ‘+1 interstrand zippers’ of 2'-N-(pyren-1-yl)methyl-2'-amino-α-L-LNA X monomers (Figure 1; for a definition of the zipper nomenclature, see the Supporting Information). This monomer arrangement forces pyrene moieties to intercalate into the same region of the probe duplex, resulting in destabilization due to localized duplex unwinding (i.e., formation of ‘energetic hotspots’, Figure 1).[30,31] In contrast, the two strands comprising a probe, display strong affinity toward complementary single-stranded DNA (ssDNA) as intercalation of the pyrene moieties results in formation of stable π-π-stacking interactions with flanking nucleobases upon duplex formation (Figure 1).[30–32] We have previously harnessed the stability difference between Invader LNAs and probe-target duplexes for mixed-sequence recognition of short non-biological dsDNA targets (Figure 1). The process appears to involve partial unwinding of probe and/or target duplexes but does not require full duplex dissociation.[30,31] A related dsDNA-targeting approach, in which DNA duplexes with adjacent incorporations of intercalator-modified non-nucleotide monomers were used to inhibit in vitro transcription in cell-free assays, appeared in the scientific literature[29] after our initial studies[30]; NMR studies have shown that it also relies on intercalator-mediated duplex unwinding for probe destabilization.[33]
Figure 1.
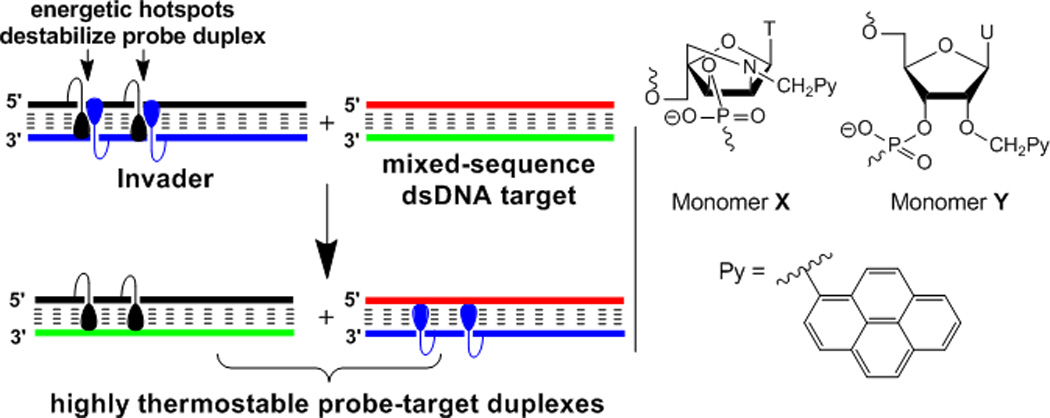
Illustration of the Invader concept for sequence-unrestricted targeting of dsDNA (left, droplets denote intercalating pyrene moieties); structures of first and second generation Invader monomers (right).
Progress with Invader LNAs has been slow due to the limited synthetic availability of the corresponding phosphoramidite of X, which is obtained from diacetone-α-D-glucose in ~3% yield over ~20 steps.[32] More efficient, yet readily accessible, building blocks are needed if the full potential of this approach is to be established. Here, we show that 2'-O-(pyren-1-yl)methyluridine monomer Y (Figure 1) – the corresponding phosphoramidite of which is obtained in only four steps from uridine[34] – is a viable replacement unit, as its pyrene moiety also is predisposed to intercalate into DNA duplexes.[34,35] Moreover, we demonstrate that Invaders comprised of Y monomers enable mixed-sequence recognition of: i) DNA hairpins in cell-free assays, and ii) chromosomal DNA in non-denaturing fluorescence in situ hybridization (nd-FISH) experiments, which establishes proof-of-concept for Invader-mediated mixed-sequence recognition of biological dsDNA.
To establish that Y monomers indeed are Invader LNA mimics, we first studied the thermal denaturation properties of 13-mer DNA duplexes, in which either one or both strands are singly or doubly modified. As expected, Y-modified oligodeoxyribonucleotides (ONs) form very stable duplexes with ssDNA targets compared to reference ONs (ΔTm per modification = 7–11 °C, entries 1–7, first two ΔTm columns, Table 1; ΔΔG293 = −18 to −6 kJ/mol, Table S2). Duplex stabilization is strongly enthalpy-driven, consistent with the formation of energetically favorable stacking interactions between nucleobases and intercalating pyrenes (ΔΔH typically between -33 and -3 kJ/mol, Table S3). In contrast, duplexes with +1 interstrand monomer arrangements (i.e., Invaders) are far less stable (ΔTm/modification −1 to +3 °C, entries 1–5, ‘probe duplex’ column, Table 1; ΔΔG293 = +1 to +12 kJ/mol, Table S2). Invader destabilization is very strongly enthalpy dominated, presumably as hotspot formation perturbs nearby base pairing (ΔΔH = +85 to +129 kJ/mol, Table S3). The special characteristics of Invaders are corroborated by the fact that probe duplexes with other interstrand arrangements of Y monomers are much more stable, as pyrene moieties intercalate into different duplex regions with little influence on each other (ΔTm/mod ~9.5 °C, entries 6–7, ‘probe duplex’ column, Table 1; ΔΔG293 = −13 to −10 kJ/mol, Table S2).
Table 1.
Thermal denaturation temperatures (Tm) and dsDNA-targeting potential () of Y-modified probes.[a]
| Tm [ΔTm] (°C) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | Zipper | ON | Sequence | upper strand vs ssDNA |
lower strand vs ssDNA |
probe duplex |
||
| 1 | +1 | Y1 | 5'-GGYATATATAGGC | 44.5 | 47.5 | 36.5 | −25 | |
| Y4 | 3'-CCAYATATATCCG | [+7.0] | [+10.0] | [−1.0] | ||||
| 2 | +1 | Y2 | 5'-GGTAYATATAGGC | 47.5 | 48.5 | 36.5 | −28 | |
| Y5 | 3'-CCATAYATATCCG | [+10.0] | [+11.0] | [−1.0] | ||||
| 3 | +1 | Y3 | 5'-GGTATATAYAGGC | 47.5 | 46.5 | 35.5 | −28 | |
| Y6 | 3'-CCATATATAYCCG | [+10.0] | [+9.0] | [−2.0] | ||||
| 4 | 2 seq. +1 | Y7 | 5'-GGYAYATATAGGC | 51.5 | 55.5 | 42.0 | −32 | |
| Y9 | 3'-CCAYAYATATCCG | [+14.0] | [+18.0] | [+4.5] | ||||
| 5 | 2 sep. +1 | Y8 | 5'-GGYATATAYAGGC | 52.5 | 55.5 | 49.0 | −33 | |
| Y10 | 3'-CCAYATATAYCCG | [+15.0] | [+18.0] | [+11.5] | ||||
| 6 | +5 | Y2 | 5'-GGTAYATATAGGC | 47.5 | 46.5 | 56.0 | −4 | |
| Y6 | 3'-CCATATATAYCCG | [+10.0] | [+9.0] | [+18.5] | ||||
| 7 | −3 | Y3 | 5'-GGTATATAYAGGC | 47.5 | 48.5 | 57.0 | −8 | |
| Y5 | 3'-CCATAYATATCCG | [+10.0] | [+11.0] | [+19.5] | ||||
ΔTm = change in Tm relative to corresponding reference duplex D1D2 (Tm = 37.5 °C); thermal denaturation curves recorded in medium salt buffer ([Na+] = 110 mM, [Cl−] = 100 mM, pH 7.0 (NaH2PO4/Na2HPO4)), using 1.0 µM of each strand; see main text for definition of ; see Table S2 for ΔG293 values; “seq” and “sep” denotes sequential and separated zippers, respectively.
The thermodynamic dsDNA-targeting potential of Y-modified probes was estimated by calculating the available binding energy for recognition of iso-sequential dsDNA targets (i.e., the process depicted in Figure 1) as . Invaders display far greater dsDNA-targeting potential than probes with other monomer arrangements (compare values, entries 1–5 vs 6–7, Table 1) due to the very large enthalpy differences between probe-target and Invader duplexes (see ΔHrec values, Table S3). Invaders with two hotspots exhibit only slightly greater dsDNA-targeting potential than single hotspot Invaders (compare , entries 4–5 vs 1–3, Table 1) as incorporation of a second hotspot into an Invader probe is mildly stabilizing, especially if hotspots are separated by four base pairs (compare ΔTm and ΔΔG293, entries 1–5, ‘probe duplex’ column, Table 1 and Table S2).
Importantly, Y-modified Invaders display very similar denaturation characteristics as sequence-matched Invader LNAs,[31] demonstrating that the key features of energetic hotspots can be emulated using the more readily accessible 2'-O-(pyren-1-yl)methylribonucleotides.
Next, the dsDNA-targeting characteristics of Y-modified Invaders were studied using an electrophoretic mobility shift assay. A digoxigenin (DIG) labeled DNA hairpin (DH) – comprised of a 13-mer double-stranded stem linked by a T10 loop – was used as a model target (Figure 2a and 2b). The unimolecular nature of the DNA hairpin stabilizes the stem region (compare Tm = 58.0 °C for DH1, Fig. 2b vs Tm = 37.5 °C for D1:D2, Table 1). Room temperature incubation of DH1 with sequence-matched Invaders results in dose-dependent formation of recognition complexes as evidenced by the emergence of bands with lower electrophoretic mobility on non-denaturing PAGE gels (Figure 2c and S2). While all studied Invaders recognize DH1 (15–74% recognition at 200-fold molar excess, Figure 2c and Table S5), Invaders with two sequential hotspots (Y7:Y9) are particularly efficient. We speculate that this motif facilitates probe opening and/or decreases the activation barrier of the recognition process. Subsequent studies have shown that shorter incubation periods or lower probe excess can be used (~50% recognition of DH1 using either 200-fold excess of Y7:Y9 for ~100 min, or ~70-fold excess of Y7:Y9 for ~15h; results not shown).
Figure 2.
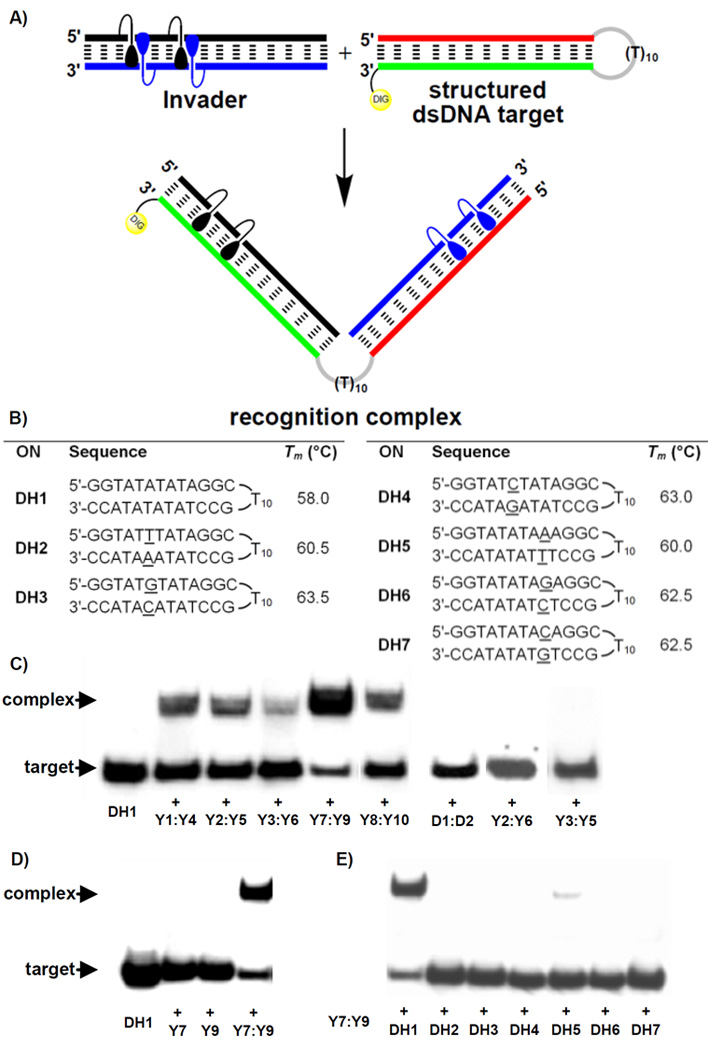
Invader-mediated recognition of DNA hairpins. (a) illustration of recognition process; (b) sequence and Tm's of DNA hairpins with isosequential (DH1) or non-isosequential (DH2–DH7) stems relative to probes (conditions of thermal denaturation experiments, see Table 1); gel electropherograms illustrating: (c) recognition of DH1 using 200-fold molar excess of five different Invaders, (d) incubation of DH1 with 500-fold excess of unmodified D1:D2 or non-Invader probes Y2:Y6 or Y3:Y5, (e) incubation of DH1 with 200-fold molar excess of single-stranded Y7 or Y9 or Invader Y7:Y9; (f) incubation of DH1–DH7 with 200-fold molar excess of Y7:Y9 (<10% recognition observed with DH5). Conditions: separately pre-annealed probes and targets (34.4 nM) were incubated for ~15h at RT in 50 mM HEPES, 100 mM NaCl, 5 mM MgCl2, 10% sucrose, 1.4 mM spermine tetrahydrochloride, pH 7.2; 12% non-denaturing PAGE run at ~4 °C); DIG: digoxigenin.
Importantly, all of the following control experiments failed to produce recognition complexes: i) incubation of DH1 with 500-fold molar excess of unmodified DNA duplex D1:D2 or double-stranded probes with +5 or −3 interstrand monomer arrangements (Y2:Y6 and Y3:Y5, respectively, Figure 2c); ii) incubation of DH1 with 200-fold molar excess of single-stranded Y7 or Y9 (Figure 2d); and iii) incubation of 200-fold molar excess of Invader Y7:Y9 with DNA hairpins DH2–DH7, which harbor fully base-paired but non-isosequential double-stranded stem regions (Figure 2e, one base pair deviation relative to Y7:Y9, see underlined residues in Figure 2b). Moreover, recognition experiments involving DH1 and Y7:Y9, in which either Y7, Y9 or DH1 are DIG-labeled, result in assemblies with identical electrophoretic mobilities (Figure S3), supporting the conclusion that the observed recognition complexes indeed are comprised of both probe strands and the dsDNA target as depicted in Figure 2a.
Thus, the results demonstrate that Invaders, but not their individual strands or probes with other monomer arrangements, display dose-dependent and highly specific recognition of DNA hairpins with mixed-sequence contexts (GC-content ~38%). DNA hairpins play important roles in the regulation of gene expression[36,37] and hairpin-targeting Invaders can therefore be envisioned as molecular tools for the study of these processes.
Encouraged by these results, we set out to study Y-modified Invaders as probes for recognition of chromosomal DNA in non-denaturing FISH experiments. Unlike conventional FISH assays, which require chemical and/or heat-induced denaturation of chromosomal DNA,[38] nd-FISH assays map chromosomal loci at mild conditions using classic dsDNA-targeting agents such as TFOs, PNA or polyamides.[39–44] As discussed earlier, these probes exhibit technical limitations, which renders development of alternative nd-FISH probes desirable.
A unique region within the DYZ-1 satellite (~6×104 repeats) on the bovine (Bos Taurus) Y chromosome was selected as a model target site (NCBI code: M26067; target site: 562–575).[45] We have previously used this site in conjunction with PNA FISH approaches to determine the gender of bovine somatic cells, spermatozoa, and embryos.[46–48] However, a series of LNAs, PNAs and polyamides proved unsuccessful in affording target-specific signals at non-denaturing conditions (results not shown). Three 14-mer Cy3-labeled probes were designed against the DYZ-1 site (Figure 3), i.e., a sequence-matched Invader with three energetic hotspots (Cy3INV) and two controls: an equivalent fully base-paired but triply mismatched Invader (Cy3INVmm) and an unmodified analogue of the sequence-matched Invader (Cy3DNA). The probes exhibit the expected properties, i.e., Cy3INV displays prominent thermodynamic potential for targeting the DYZ-1 site (, Table S7), while Cy3INVmm and Cy3DNA do not (Tables S6 and S7).
Figure 3.
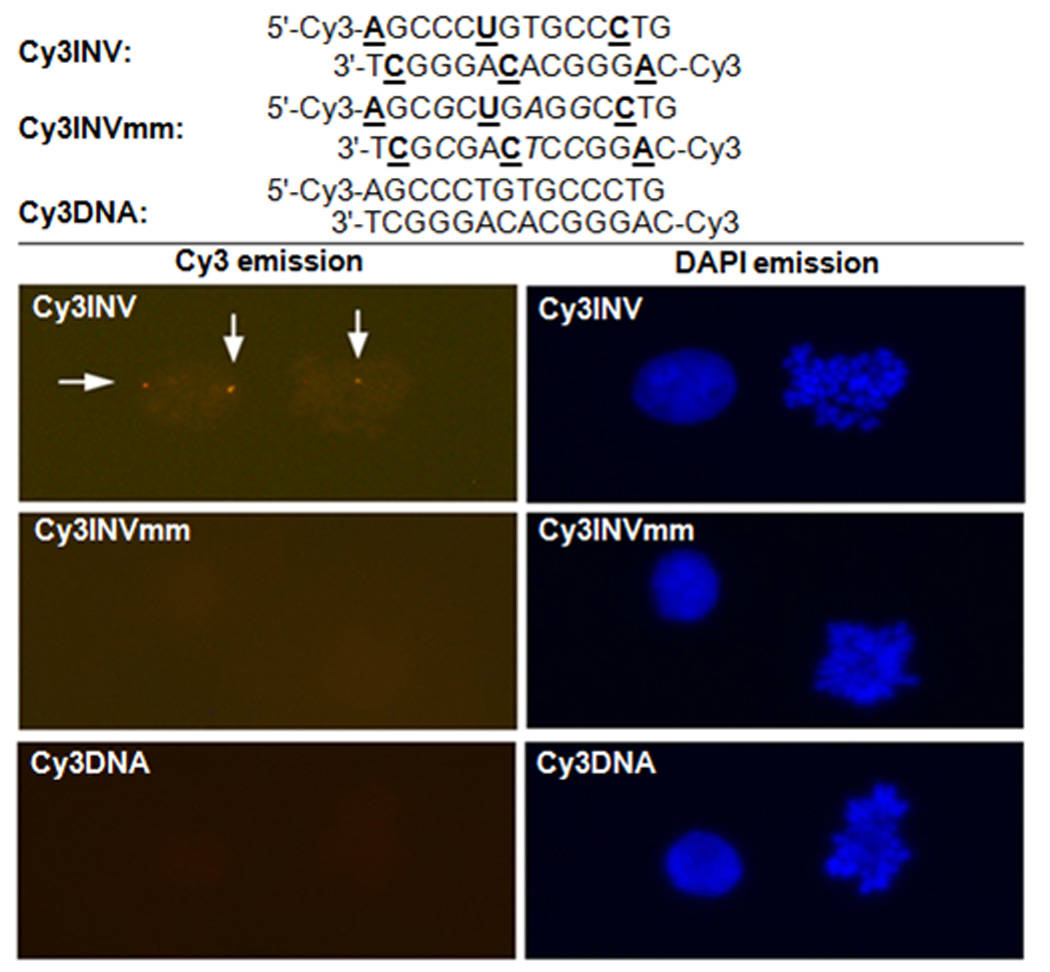
Probe sequences used in - and representative images from - non-denaturing FISH experiments. Cy3INV: sequence-matched Invader, Cy3INVmm: fully base-paired but triply mismatched Invader, and Cy3DNA: unmodified analogue of sequence-matched Invader. Images viewed using Cy3 (left) or DAPI (right) filter settings. A, C and U denote 2'-O-(pyren-1-yl)methyladenosine,[49] 2'-O-(pyren-1-yl)methylcytidine[49] and monomer Y, respectively. Conditions: 38.5 °C, 10 mM Trizma®-HCl, 50mM KCl, pH 8.3, 60 min (Cy3INV) or 180 min (Cy3INVmm/Cy3DNA) incubation. Samples visualized using a fluorescence microscope at 400× magnification.
In line with this, incubation of Cy3INV with fixed interphase nuclei and metaphase spreads from a male bovine kidney cell line (CCL-22) at non-denaturing conditions produces one or two highly localized Cy3-signals per nucleus, consistent with post- and pre-mitotic nuclei (Figure 3). Signal intensity is dose- and time-dependent (Figure S4 and S5). The absence of signals upon incubation with Cy3INVmm or Cy3DNA under identical conditions, demonstrates that Invader-mediated recognition of the DYZ-1 site is highly specific (Figure 3). Control experiments involving nuclei pre-treated with DNase, RNase or proteinase prior to Cy3INV incubation, unequivocally established dsDNA as the molecular target (not shown). The very high labeling efficiency, i.e., the fraction of nuclei displaying localized signals (Figure S4 and S5), is particularly remarkable considering the high GC-content of the target region (~71% GC).
In conclusion, we demonstrate that short DNA duplexes modified with interstrand zippers of 2'-O-(pyren-1-yl)methylribonucleotides, enable efficient and highly specific mixed-sequence recognition of: i) DNA hairpins in cell-free assays, and ii) chromosomal DNA in fixed interphase nuclei and metaphase spreads, which establishes proof-of-concept for Invader-mediated recognition of mixed-sequence target regions in biological dsDNA. Unlike most current DNA-targeting probes, Invaders are devoid of inherent sequence limitations (e.g., polypurine regions), and do not require denaturing incubation conditions (e.g., heat or low ionic strengths). Previously inaccessible DNA target regions may therefore become available for exogenous control, which has exciting implications for karyotyping, in vivo imaging, and gene regulation. Studies aiming at systematically delineating the full potential of the Invader approach and refining it into a general paradigm for mixed-sequence recognition of dsDNA, are ongoing.
Supplementary Material
Footnotes
This study was supported by Award Number R01 GM088697 from the National Institute of General Medical Sciences, National Institutes of Health; Award Number IF13-001 from the Higher Education Research Council, Idaho State Board of Education; NIH Grant Number P20 RR016454 from the INBRE Program of the National Institute of General Medical Sciences and Idaho NSF EPSCoR. We thank Dr. Lee Deobald (EBI Murdock Mass Spectrometry Center, Univ. Idaho) for assistance with mass spectrometric analysis and Dr. Carolyn J. Hovde (Food Science, Univ. Idaho) for access to gel documentation stations.
References
- 1.Rogers FA, Lloyd JA, Glazer PM. Curr. Med. Chem.: Anti-Cancer Agents. 2005;5:319. doi: 10.2174/1568011054222300. [DOI] [PubMed] [Google Scholar]
- 2.Ghosh I, Stains CI, Ooi AT, Segal DJ. Mol. Bio Syst. 2006;2:551. doi: 10.1039/b611169f. [DOI] [PubMed] [Google Scholar]
- 3.Nielsen PE. Chem Bio Chem. 2010;11:2073. doi: 10.1002/cbic.201000346. [DOI] [PubMed] [Google Scholar]
- 4.Mukherjee A, Vasquez KM. Biochimie. 2011;93:1197. doi: 10.1016/j.biochi.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aiba Y, Sumaoka J, Komiyama M. Chem. Soc. Rev. 2011;40:5657. doi: 10.1039/c1cs15039a. [DOI] [PubMed] [Google Scholar]
- 6.Vaijayanthi T, Bando T, Pandian GN, Sugiyama H. Chem Bio Chem. 2012;13:2170. doi: 10.1002/cbic.201200451. [DOI] [PubMed] [Google Scholar]
- 7.Bennett CF, Swayze EE. Annu. Rev. Pharmacol. Toxicol. 2010;50:259. doi: 10.1146/annurev.pharmtox.010909.105654. [DOI] [PubMed] [Google Scholar]
- 8.Watts JK, Corey DR. J. Pathol. 2012;226:365. doi: 10.1002/path.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simon P, Cannata F, Concordet J-P, Giovannangeli C. Biochimie. 2008;90:1109. doi: 10.1016/j.biochi.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Duca M, Vekhoff P, Oussedik K, Halby L, Arimondo PB. Nucleic Acids Res. 2008;36:5123. doi: 10.1093/nar/gkn493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaihatsu K, Janowski BA, Corey DR. Chem. Biol. 2004;11:749. doi: 10.1016/j.chembiol.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 12.Nielsen PE. Chem. Biodiversity. 2010;7:786. doi: 10.1002/cbdv.201000005. [DOI] [PubMed] [Google Scholar]
- 13.Dervan PB, Edelson BS. Curr. Opin. Struct. Biol. 2003;13:284. doi: 10.1016/s0959-440x(03)00081-2. [DOI] [PubMed] [Google Scholar]
- 14.Kutyavin IV, Rhinehart RL, Lukhtanov EA, Gorn VV, Meyer RB, Jr, Gamper HB., Jr Biochemistry. 1996;35:11170. doi: 10.1021/bi960626v. [DOI] [PubMed] [Google Scholar]
- 15.Lohse J, Dahl O, Nielsen PE. Proc. Natl. Acad. Sci. USA. 1999;96:11804. doi: 10.1073/pnas.96.21.11804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishizuka T, Yoshida J, Yamamoto Y, Sumaoka J, Tedeschi T, Corradini R, Sforza S, Komiyama M. Nucleic Acids Res. 2008;36:1464. doi: 10.1093/nar/gkm1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janowski BA, Kaihatsu K, Huffman KE, Schwartz JC, Ram R, Hardy D, Mendelson CR, Corey DR. Nat. Chem. Biol. 2005;1:210. doi: 10.1038/nchembio724. [DOI] [PubMed] [Google Scholar]
- 18.Beane R, Gabillet S, Montaillier C, Arar K, Corey DR. Biochemistry. 2008;47:13147. doi: 10.1021/bi801930p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rapireddy S, Bahal R, Ly DH. Biochemistry. 2011;50:3913. doi: 10.1021/bi2002554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bahal R, Sahu B, Rapireddy S, Lee C-M, Ly DH. Chem Bio Chem. 2012;13:56. doi: 10.1002/cbic.201100646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ge R, Heinonen JE, Svahn MG, Mohamed AJ, Lundin KE, Smith CIE. FASEB J. 2007;21:1902. doi: 10.1096/fj.06-7225com. [DOI] [PubMed] [Google Scholar]
- 22.Rusling DA, Powers VEC, Ranasinghe RT, Wang Y, Osborne SD, Brown T, Fox K. Nucleic Acids Res. 2005;33:3025. doi: 10.1093/nar/gki625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hari Y, Obika S, Imanishi T. Eur. J. Org. Chem. 2012:2875. [Google Scholar]
- 24.Tse WC, Boger DL. Chem. Biol. 2004;11:1607. doi: 10.1016/j.chembiol.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 25.Hamilton PL, Arya DP. Nat. Prod. Rep. 2012;29:134. doi: 10.1039/c1np00054c. [DOI] [PubMed] [Google Scholar]
- 26.Bogdanove AJ, Voytas DF. Science. 2011;333:1843. doi: 10.1126/science.1204094. [DOI] [PubMed] [Google Scholar]
- 27.Gaj T, Gersbach CA, Barbas CF., III Trends. Biotechnol. doi: 10.1016/j.tibtech.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bryld T, Højland TR, Wengel J. Chem. Commun. 2004:1064. doi: 10.1039/b402414a. [DOI] [PubMed] [Google Scholar]
- 29.Filichev VV, Vester B, Hansen LH, Pedersen EB. Nucleic Acids Res. 2005;33:7129. doi: 10.1093/nar/gki1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hrdlicka PJ, Kumar TS, Wengel J. Chem. Commun. 2005:4279. doi: 10.1039/b506986f. [DOI] [PubMed] [Google Scholar]
- 31.Sau SP, Kumar TS, Hrdlicka PJ. Org. Biomol. Chem. 2010;8:2028. doi: 10.1039/b923465a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar TS, Madsen AS, Østergaard ME, Sau SP, Wengel J, Hrdlicka PJ. J. Org. Chem. 2009;74:1070. doi: 10.1021/jo802037v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nielsen CB, Petersen M, Pedersen EB, Hansen PE, Christensen UB. Bioconjugate Chem. 2004;15:260. doi: 10.1021/bc0341932. [DOI] [PubMed] [Google Scholar]
- 34.Karmakar S, Anderson BA, Rathje RL, Andersen S, Jensen T, Nielsen P, Hrdlicka PJ. J. Org. Chem. 2011;76:7119. doi: 10.1021/jo201095p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakamura M, Fukunaga Y, Sasa K, Ohtoshi Y, Kanaori K, Hayashi H, Nakano H, Yamana K. Nucleic Acids Res. 2005;33:5887. doi: 10.1093/nar/gki889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wadkins RM. Curr. Med. Chem. 2000;7:1. doi: 10.2174/0929867003375461. [DOI] [PubMed] [Google Scholar]
- 37.Keene FR, Smith JA, Collins JG. Coord. Chem. Rev. 2009;253:2021. [Google Scholar]
- 38.Levsky JM, Singer RH. J. Cell Sci. 2003;116:2833. doi: 10.1242/jcs.00633. [DOI] [PubMed] [Google Scholar]
- 39.Johnson MD, III, Fresco JR. Chromosoma. 1999;108:181. doi: 10.1007/s004120050367. [DOI] [PubMed] [Google Scholar]
- 40.Schmitt E, Schwarz-Finsterle J, Stein S, Mueller P, Mokhir A, Kraemer R, Cremer C, Hausmann M. Methods Mol. Biol. 2010;659:185. doi: 10.1007/978-1-60761-789-1_13. [DOI] [PubMed] [Google Scholar]
- 41.Molenaar C, Wiesmeijer K, Verwoerd NP, Khazen S, Eils R, Tanke HJ, Dirks RW. EMBO J. 2003;22:6631. doi: 10.1093/emboj/cdg633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Janssen S, Durussel T, Laemmli UK. Mol. Cell. 2000;6:999. doi: 10.1016/s1097-2765(00)00099-x. [DOI] [PubMed] [Google Scholar]
- 43.Gygi MP, Ferguson MD, Mefford HC, Lund KP, O’Day C, Zhou P, Friedman C, van der Engh G, Stolowitz ML, Trask BJ. Nucleic Acids Res. 2002;30:2790. doi: 10.1093/nar/gkf406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silahtaroglua AN, Tommerup N, Vissing H. Mol. Cell. Probes. 2003;17:165. doi: 10.1016/s0890-8508(03)00048-3. [DOI] [PubMed] [Google Scholar]
- 45.Perret J, Shia Y, Fries R, Vassart G, Georges M. Genomics. 1990;6:482. doi: 10.1016/0888-7543(90)90478-d. [DOI] [PubMed] [Google Scholar]
- 46.Bleher R, Erwin W, Paprocki AM, Syverson CM, Koppang R, Didion BA. Reprod. Fertil. Dev. 2008;21:227. [Google Scholar]
- 47.Didion BA, Bleher R. Reprod. Fertil. Dev. 2008;21:229. [Google Scholar]
- 48.Didion B, Erwin W, Bleher R. WO2009/079456A2
- 49.Nakamura M, Shimomura Y, Ohtoshi Y, Sasa K, Hayashi H, Nakano H, Yamana K. Org. Biomol. Chem. 2007;5:1945. doi: 10.1039/b705933g. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


