Abstract
Demand for sustainable materials motivates the development of microorganisms capable of synthesizing products from renewable substrates. A challenge to commercial production of polyhydroxyalkanoates (PHA), microbially derived polyesters, is engineering metabolic pathways to produce a polymer with the desired monomer composition from an unrelated and renewable source. Here, we demonstrate a metabolic pathway for converting glucose into medium-chain-length (mcl)-PHA composed primarily of 3-hydroxydodecanoate monomers. This pathway combines fatty acid biosynthesis, an acyl-ACP thioesterase to generate desired C12 and C14 fatty acids, β-oxidation for conversion of fatty acids to (R)-3-hydroxyacyl-CoAs, and a PHA polymerase. A key finding is that Escherichia coli expresses multiple copies of enzymes involved in β-oxidation under aerobic conditions. To produce polyhydroxydodecanoate, an acyl-ACP thioesterase (BTE), an enoyl-CoA hydratase (phaJ3), and mcl-PHA polymerase (phaC2) were overexpressed in E. coli ΔfadRABIJ. Yields were improved through expression of an acyl-CoA synthetase resulting in production over 15% CDW – the highest reported production of mcl-PHA of a defined composition from an unrelated carbon source.
Keywords: E. coli, Thioesterase, Polyhydroxyalkanoate, Homopolymer, β-oxidation, Dodecanoic acid
1. Introduction
Polyhydroxyalkanoates (PHA) are a class of microbially synthesized polyesters that are produced in large quantities as a form of carbon and energy storage. Natural PHA possesses structural properties that make it attractive as a renewable plastic for select applications. By changing the identity and/or percentage of co-monomers, the structural properties of PHA can be engineered with varying degrees of crystallinity and elasticity (Khanna and Srivastava, 2005). A wide range of hydroxy-acids have been incorporated as monomers into PHA chains when fed to PHA accumulating organisms (Meng et al., 2012; Steinbuchel and Valentin, 1995; Zhou et al., 2011). Unfortunately, this strategy requires a low-cost source of each monomer or monomer precursor (e.g., fatty acids). For this reason, current PHA research is focused on engineering metabolic pathways to produce monomers from unrelated carbon sources such as glucose (Li et al., 2010; Theodorou et al., 2012). Medium-chain-length PHA (mcl-PHA), which consists of fatty acids containing six to fourteen carbons, is an attractive polymer, desired for novel applications in medical devices, cosmetics, and tissue engineering (Chen and Wu, 2005). Bacteria that naturally produce mcl-PHA incorporate monomers derived from either fatty acid biosynthesis or degradation (β-oxidation) pathways. Efforts to enhance the production of mcl-PHA have used metabolic engineering to enhance both pathways.
In fatty acid biosynthesis, an iterative cycle of two carbon elongations in conjunction with a series of reductions yields long-chain fatty acids (≥16 carbons). Intermediates exist as acyl-carrier protein (ACP) bound thioesters and can be substrates for mcl-PHA biosynthesis. It was recently shown that heterologous expression of Pseudomonas putida phaG, phaC and a predicted acyl-CoA synthetase led to accumulation of 400 mg L−1 mcl-PHA in Escherichia coli when grown on glucose as a sole carbon source (Wang et al., 2012). In this instance PhaG functions as an (R)-3-hydroxyacyl-ACP thioesterase to produce free (R)-3-hydroxyalkanoic acids which are ligated to Coenzyme A (CoA) and polymerized by PhaC. Due to the loose substrate specificity of the PhaG thioesterase and the iterative nature of fatty acid metabolism, the resulting mcl-PHA is a hetero-polymer consisting of C6–C14 carbon monomer units.
Alternatively, mcl-PHA can be produced by exogenous feeding of free fatty acids (FFA) or other lipid mixtures (palm oil, soybean oil, etc.). Here, enzymes such as PhaJ act on β-oxidation intermediates to generate a heterogeneous pool of 3-hydroxyacyl-CoA thioesters, substrates for PhaC polymerization, thereby assembling a heterogeneous polymer comprised of a range of chain-lengths equal to or smaller than the fatty acids fed. While mcl-PHA heteropolymers are likely to have utility, a homopolymer of a desired chain length will enable precise control of polymer properties. Recently, both P. putida KT2442 and E. coli were engineered to accumulate mcl-PHA homopolymer when fed specific chain-length fatty acids (Liu et al., 2011; Tappel et al., 2012; Wang et al., 2011). In the case of Pseudomonas, six gene knockouts in β-oxidation (Fig. 1.) and one in PHA biosynthesis were required to produce pure C10 and C14 PHA from C10 and C14 fatty acids respectively and 16% C10-co-84% C12 mcl-PHA from C12 (Liu et al., 2011). Using a similar fatty acid feeding strategy, a second study involving β-oxidation impaired Pseudomonas produced a series of C4-C9 PHA homopolymers including C5 and C7 based polyesters (Wang et al., 2011). In E. coli, deletions in β-oxidation combined with constitutive expression of short-chain fatty acid metabolism and overexpression of mcl-PHA biosynthesis genes allowed for production of homogeneous PHA ranging from 4–14 carbons when cultured on the corresponding fatty acids (Tappel et al., 2012).
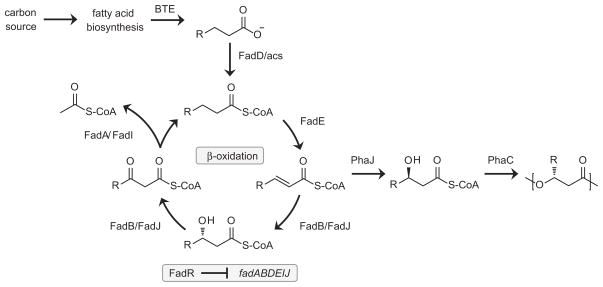
Fig. 1.
Metabolic pathway for mcl-PHA biosynthesis in Escherichia coli. A carbon source (i.e., glucose) is catabolized to acetyl-CoA which enters fatty acid biosynthesis for production of fatty acyl-ACPs. C12 and C14 acyl-ACPs are substrates for the thioesterase, BTE, which catalyzes FFA formation. An acyl-CoA synthetase (e.g., FadD) activates the FFAs for degradation via a partially intact β-oxidation cycle generating enoyl-CoAs which PhaJ hydrates to produce mcl-PHA monomers for polymerization by PhaC. The resulting monomer composition is therefore identical to that of the FFA pool generated by the thioesterase. FadR represses expression of β-oxidation genes in the absence of acyl-CoAs.
A third approach for producing mcl-PHA combines fatty acid biosynthesis and β-oxidation in a hybrid metabolic pathway. Acyl-ACP thioesterases are used to produce FFA of a desired length and β-oxidation converts FFA to PHA monomers. Many thioesterases have been employed for the production of mcl-PHA including TesB (Chung et al., 2009), TesA (Qiu et al., 2005), and the California Bay Laurel (Umbellularia californica) thioesterase (BTE) (Rehm and Steinbuchel, 2001). Expression of BTE in E. coli generates a large pool of C12 and C14 FFA by depleting the pool of long-chain acyl-ACP which regulate the early steps in fatty acid biosynthesis. Co-expression of BTE and a PHA polymerase in E. coli LS1298 (ΔfadB) or RS3097 (ΔfadR) was shown to produce C10 PHA from 3–6% cell dry weight (CDW) when grown on Lysogeny Broth (LB) supplemented with gluconate and acrylic acid (an inhibitor of β-oxidation) (Rehm and Steinbuchel, 2001). However, it remains unclear if other monomer units were present in this study.
In this work, we present a rational approach for producing mcl-PHA homopolymer from an unrelated carbon source (i.e., glucose) in E. coli. We characterized a panel of mutant E. coli strains to determine the impact of β-oxidation enzymes on fatty acid consumption and mcl-PHA synthesis. We characterized two PHA synthases (PhaC) and four enoyl-coA hydratases (PhaJ) for producing mcl-PHA in E. coli, identifying a promising combination for making mcl-PHA. We then examined the impact of different modes of regulating acyl-CoA synthetases on PHA titer. Finally, we engineered a strain of E. coli to produce mcl-PHA with composition matching the product profile of the thioesterase. Our strategy involved constructing a strain of E. coli in which key genes in fatty acid β-oxidation were deleted in conjunction with the overexpression of BTE, phaJ3 and phaC2 from Pseudomonas aeruginosa PAO1 and PP_0763 from P. putida KT2440. The resulting strain produced over 15% CDW mcl-PHA when grown in minimal glucose-based media.
2. Material and methods
2.1. Bacterial strains, reagents, media, and growth conditions
All strains used in this study are listed in Table 1. E. coli DH5α was used to construct and propagate plasmids. E. coli K-12 MG1655 ΔaraBAD was used as the base strain for studying β-oxidation and PHA production. Chemicals and reagents were purchased from Fisher Scientific (Pittsburgh, PA) unless otherwise specified. Enzymes used for cloning were purchased from New England Biolabs (Ipswich, MA). Oligonucleotides were purchased from Integrated DNA Technologies, Inc. (Coralville, IA) and sequences are listed in Table S1. For all growth experiments, single colonies were used to inoculate 5 mL starter cultures that were grown overnight prior to inoculation of experimental cultures. All growth experiments were performed at 37 °C in a rotary shaker (250 rpm). When necessary, cultures were supplemented with 100 μg mL−1 ampicillin and/or 34 μg mL−1 chloramphenicol.
Table 1.
Strains and plasmids used in this study.
| Strain/Plasmid | Relevant genotype/property | Source or Reference |
|---|---|---|
| Strains | ||
| Escherichia. coli K-12 MG1655 | F− λ− ilvG− rfb-50 rph-1 | ECGSC |
| E. coli LS5218 | F+ fadR601 atoC512(Const) | ECGSC |
| E. coli DH10B | F− mcrA Δ(mrr-hsdRMS-mcrBC) Φ80lacZΔM15 ΔlacX74 recA1 endA1 araD139 Δ(ara, leu)7697 galU galK λ− rpsL nupG | Invitrogen |
| E. coli DH5α | F− Φ80lacZΔM15 Δ(lacZYA-argF) U169 recA1 endA1 hsdR17 ( , mk +) phoA supE44 λ− thi−1 gyrA96 relA1 | Invitrogen |
| E. coli DY330 | F− λ− rph-1 INV(rrnD, rrnE) ΔlacU169 gal490 pglΔ8 λcI857 Δ(cro-bioA) | (Yu et al., 2000) |
| Pseudomonas aeruginosa PAO1 | Source for phaC1–2, phaJ1–4 | ATCC BAA-47™ |
| Pseudomonas putida KT2440 | Source for PP_0763 | ATCC 47054™ |
| NRD204 | MG1655 ΔaraBAD::cat | (De Lay and Cronan, 2007) |
| araBAD | MG1655 ΔaraBAD | This work |
| A | MG1655 ΔaraBAD ΔfadA | This work |
| B | MG1655 ΔaraBAD ΔfadB | This work |
| E | MG1655 ΔaraBAD ΔfadE | This work |
| I | MG1655 ΔaraBAD ΔfadI | This work |
| J | MG1655 ΔaraBAD ΔfadJ | This work |
| R | MG1655 ΔaraBAD ΔfadR | This work |
| RA | MG1655 ΔaraBAD ΔfadR ΔfadA | This work |
| RB | MG1655 ΔaraBAD ΔfadR ΔfadB | This work |
| RE | MG1655 ΔaraBAD ΔfadR ΔfadE | This work |
| RI | MG1655 ΔaraBAD ΔfadR ΔfadI | This work |
| RJ | MG1655 ΔaraBAD ΔfadR ΔfadJ | This work |
| AI | MG1655 ΔaraBAD ΔfadA ΔfadI | This work |
| BJ | MG1655 ΔaraBAD ΔfadB ΔfadJ | This work |
| AB | MG1655 ΔaraBAD ΔfadAB | This work |
| IJ | MG1655 ΔaraBAD ΔfadIJ | This work |
| RAI | MG1655 ΔaraBAD ΔfadR ΔfadA ΔfadI | This work |
| RBJ | MG1655 ΔaraBAD ΔfadR ΔfadB ΔfadJ | This work |
| RAB | MG1655 ΔaraBAD ΔfadR ΔfadA ΔfadB | This work |
| RIJ | MG1655 ΔaraBAD ΔfadR ΔfadIJ | This work |
| ABIJ | MG1655 ΔaraBAD ΔfadAB ΔfadIJ | This work |
| RABIJ | MG1655 ΔaraBAD ΔfadR ΔfadAB ΔfadIJ | This work |
| Φ(Ptrc-fadD) | MG1655 ΔaraBAD Φ(Ptrc –fadD) | This work |
| SA01 | MG1655 ΔaraBAD ΔfadR ΔfadIJ fadBA::Φ(Ptrc –BTE) | This work |
| Plasmids | ||
| pCP20 | FLP+, λ cI857+, λ pR Repts, ApR, CmR | (Cherepanov and Wackernagel, 1995) |
| pKD13 | Template plasmid for gene disruption. KanR cassette flanked by FRT sites. AmpR | (Datsenko and Wanner, 2000) |
| pTrc99A | Ptrc promoter, pBR322 origin, AmpR | (Amann et al., 1988) |
| pTrc99A-fadD | fadD cloned as a Kpn I–Xba I fragment into pTrc99a | This work |
| pTrc99A-BTE | pTrc99A carrying BTE under Ptrc control, AmpR | (Hoover et al., 2011) |
| pMSB6 | pTrc99A with altered MCS | This work |
| pMSB6-J1 | pMSB6 containing phaJ1 gene (P. aeruginosa) | This work |
| pMSB6-J2 | pMSB6 containing phaJ2 gene (P. aeruginosa) | This work |
| pMSB6-J3 | pMSB6 containing phaJ3 gene (P. aeruginosa) | This work |
| pMSB6-J4 | pMSB6 containing phaJ4 gene (P. aeruginosa) | This work |
| pBAD33 | PBAD promoter, pACYC origin, CmR | (Guzman et al., 1995) |
| pBAD33-C280* | pBAD33 araE C280* Δ281–292 | (Lee et al., 2007) |
| pBAD33*–C1 | pBAD33-C280* containing phaC1 gene (P. aeruginosa) | This work |
| pBAD33*–C2 | pBAD33-C280* containing phaC2 gene (P. aeruginosa) | This work |
| pDA-JC | pMSB6 containing phaJ3 and phaC2 genes (P. aeruginosa) | This work |
| pDA-JAC | pDA-JC with PP_0763 cloned between phaJ3 and phaC2 | This work |
| pBTE-int | pTrc99A containing BTE with cat-FRT cassette from pKD3 (Datsenko and Wanner, 2000) inserted 5′ of lacIQ | (Youngquist et al., 2012) |
For dodecanoic acid catabolism experiments (Figs. 2a and 3), each strain was cultured in 25 mL of LB to an optical density at 600 nm (OD600) of 1.0. Cultures were centrifuged (1,000 × g for 20 min) and resuspended in 50 mL of M9 minimal media supplemented with 0.25 g L−1 sodium dodecanoate from a 5 g L−1 sodium dodecanoate aqueous stock solution. This amount was chosen because higher levels impaired growth of E. coli MG1655 ΔaraBAD (data not shown). Under these conditions, soluble dodecanoic acid existed in equilibrium with a solid precipitate. After transfer, cultures were incubated at 37 °C with shaking and 2.5 mL culture samples were taken at 24 and 48 h for FAME analysis. In the case of fadD overexpression constructs, 1 mM isopropyl β-D-thiogalactopyranoside (IPTG) was added at an OD600 of 0.02 and again after resuspension in minimal media.
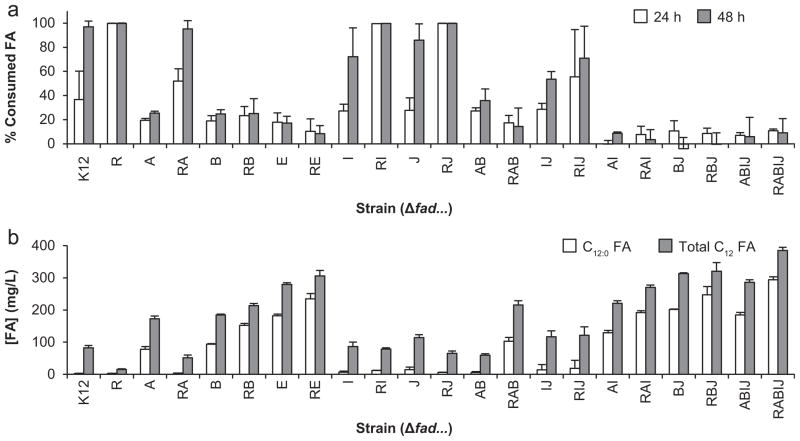
Fig. 2.
Metabolism of dodecanoic acid by a library of E. coli β-oxidation knock-out strains harboring the specific fad deletion(s) indicated on the horizontal axis (e.g., K12=E. coli K-12 MG1655; R=E. coli K-12 MG1655 ΔfadR; etc.). (a) Metabolism of exogenously fed dodecanoic acid after 24 and 48 h of shake flask cultivation as a percent of the initial fatty acid concentration. (b) Metabolism of endogenously synthesized fatty acids in strains with plasmid-based expression of BTE after 48 h of cultivation. Data for both saturated (C12:0) and total C12 (including unsaturated and hydroxy) species are presented.
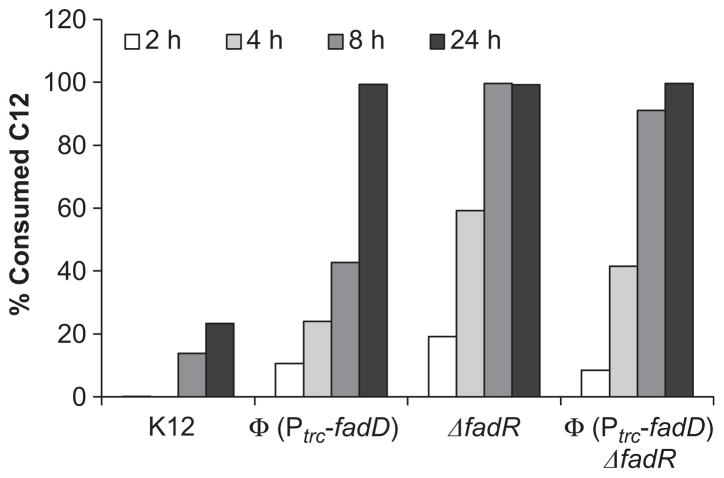
Fig. 3.
Comparison of the effect of a fadR deletion with fadD overexpression via a chromosomal fusion of the trc promoter (Φ(Ptrc-fadD)) on exogenous dodecanoic acid metabolism in E. coli over a 24 h period. Data are presented as a percent of the initial fatty acid concentration.
For dodecanoic acid production experiments (Fig. 2b), each strain was inoculated to OD600 of 0.05 in 5 mL of LB+0.4% (D)-glucose and induced with 1 mM IPTG at an OD600 of 0.2. After induction, cultures were incubated for 48 h at 37 °C with shaking at which point, cultures were harvested for PHA and FAME analysis.
For shake flask experiments summarized by Table 2, 35 mL of LB was inoculated to OD600 0.05 and incubated with shaking until cultures reached OD600 1.0. Cultures were centrifuged (1,000 × g for 20 min) and the cell pellet resuspended in 50 mL M9 minimal media supplemented with 2.5 g L−1 dodecanoic acid and inducer(s) (1 mM IPTG; 0.2% (L)-arabinose). Cultures were harvested at 96 h for PHA and FAME analysis.
Table 2.
GC/MS analysis of the composition of mcl-PHA produced in E. coli LS5218 expressing combinations of two phaC and four phaJ from P. aeruginosa PAO1 after culturing in the presence of exogenous dodecanoic acid.
| Genotype | Cell dry weight (g L−1) | PHA content (wt%) | PHA composition (wt%)
|
|||
|---|---|---|---|---|---|---|
| C6 | C8 | C10 | C12 | |||
| phaC1 phaJ1 | 1.0 | 0.3 | 8.4 | 90.7 | 0.0 | 0.9 |
| phaC1 phaJ2 | 1.2 | 4.4 | 4.8 | 49.6 | 28.9 | 16.8 |
| phaC1 phaJ3 | 1.4 | 10.8 | 3.9 | 43.5 | 33.0 | 19.6 |
| phaC1 phaJ4 | 1.0 | 2.8 | 5.2 | 52.3 | 25.6 | 16.9 |
| phaC1 | 1.1 | 0.6 | 4.7 | 65.1 | 22.0 | 8.3 |
| phaC2 phaJ1 | 1.0 | 2.2 | 34.0 | 54.8 | 6.7 | 4.5 |
| phaC2 phaJ2 | 1.1 | 13.9 | 11.1 | 35.9 | 28.8 | 24.2 |
| phaC2 phaJ3 | 1.1 | 19.1 | 8.2 | 32.3 | 32.2 | 27.3 |
| phaC2 phaJ4 | 0.9 | 9.4 | 9.6 | 35.0 | 29.3 | 26.1 |
| phaC2 | 1.1 | 1.8 | 6.9 | 48.5 | 26.7 | 17.9 |
Note: C6, 3-hydroxyhexanoate; C8, 3-hydroxyoctanoate; C10, 3-hydroxydecanoate; C12, 3-hydroxydodecanoate.
For PHA production experiments detailed in Table 3 and Fig. 4, 50 mL of MOPS+1% (D)-glucose was inoculated to OD600 of 0.05 and induced with 1 mM IPTG at an OD600 of 0.2. After induction, cultures were incubated for 96 h at 37 °C with shaking at which point, cultures were harvested for PHA and FAME analysis. For strains lacking chromosomal expression of BTE, 0.25 g L−1 sodium dodecanoate from a 5 g L−1 sodium dodecanoate aqueous stock solution was added at the time of induction.
Table 3.
GC/MS analysis of the composition of mcl-PHA produced in a series of E. coli β-oxidation deletion strains containing plasmid pDA-JC after culturing in the presence of exogenous dodecanoic acid.
| Relevant genotype | Cell dry weight (g L−1) | PHA content (wt%) | PHA composition (wt%)
|
|||
|---|---|---|---|---|---|---|
| C6 | C8 | C10 | C12 | |||
| ΔfadR | 0.97±09 | 1.71±18 | 4.0 | 30.3 | 34.0 | 31.8 |
| ΔfadRB | 0.96±08 | 0.39±13 | n.d. | 8.3 | 42.4 | 49.3 |
| ΔfadRBJ | 1.10±19 | 0.38±15 | n.d. | n.d. | n.d. | 100.0 |
| ΔfadRABIJ | 0.93±02 | 0.75±03 | n.d. | n.d. | n.d. | 100.0 |
Note: C6, 3-hydroxyhexanoate; C8, 3-hydroxyoctanoate; C10, 3-hydroxydecanoate; C12, 3-hydroxydodecanoate. n.d., not detected.
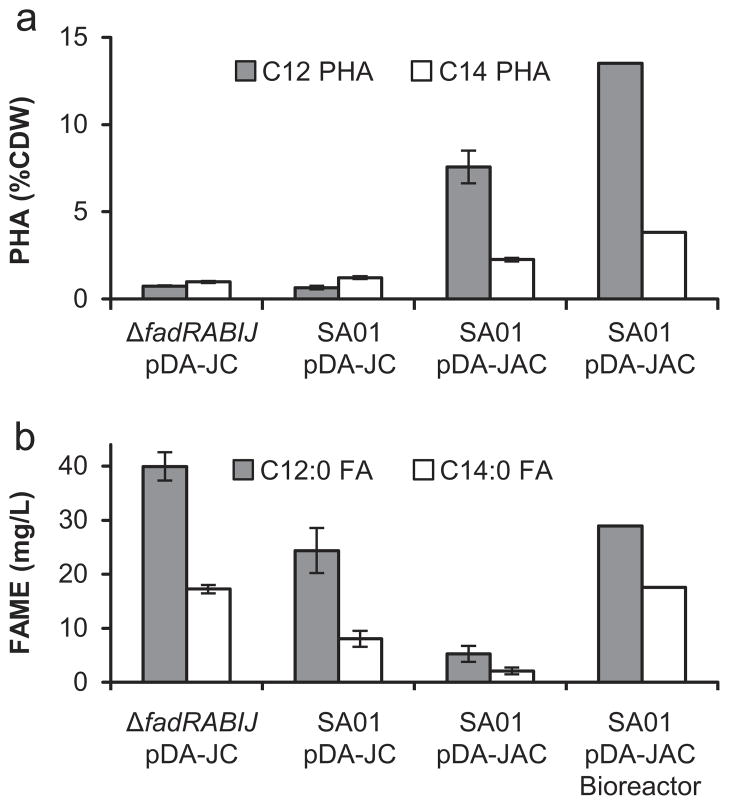
Fig. 4.
Production of mcl-PHA in E. coli in the presence of exogenously fed dodecanoic acid or endogenously produced FFA. (a) Titer of PHA as a percentage of cell dry weight determined by quantifying 3-hydroxy fatty acid methyl esters from a PHA extraction. (b) Titer of fatty acids determined by quantifying fatty acid methyl esters (FAME) from a total lipid extraction. Strain ΔfadRABIJ was cultured in the presence of dodecanoic acid while SA01 (expressing BTE) was capable of endogenous FFA production in glucose minimal media. Please see Table S2 for individual CDW and PHA titer values.
Bioreactor experiments were performed in a 3 L stirred bioreactor (Applikon Biotechnology, Inc., Schiedam, Netherlands) using a 1.0 L working volume. Temperature was maintained at 37 °C using an electric heat blanket and temperature, pH, and dissolved oxygen (DO2) were monitored using specific probes. Vessel pH was maintained at 7.00±0.05 by addition of 1 M NaOH or 1 M HCl solutions. Agitation was provided by a single impeller with the stirrer speed set to 700 rpm. Stirrer speed was occasionally increased to ensure the DO2 content did not decrease below 40% saturation in order to maintain an aerobic environment (Becker et al., 1997; Tseng et al., 1996). Air inflow was maintained at 1.5 L min−1.
Bioreactor experiments were inoculated at an OD600 of 0.05 with a culture of strain SA01 harboring plasmid pDA-JAC grown to an OD600 of ≥2.5 in MOPS minimal media supplemented with 1% glucose. Induction with 1 mM IPTG occurred when the OD600 of the bioreactor reached 0.2. The reactor was operated in batch mode with one addition of 10 g of glucose (50 mL of a 20% (w/v) glucose solution) at 24 h post-induction. The OD600 of the culture was monitored periodically and 15 mL of culture taken every 24 h for FAME and PHA analysis. The contents of the bioreactor were harvested at 96 h post-induction for PHA and FAME analysis.
2.2. Plasmid construction
All plasmids used in this study are listed in Table 1. Plasmid pBAD33-C280* (Lee et al., 2007) was constructed by PCR amplification of plasmid pBAD33 (Guzman et al.,1995) with primers C280*-F/R (Table S1). The PCR product was treated with Dpn I and Xho I digestion and circularized by ligation with T4 DNA ligase. Genomic DNA was isolated from P. putida KT2440 and P. aeruginosa PAO1 with a Wizard® Genomic DNA Purification Kit (Promega). PHA genes phaJ1–4 and phaC1–2 were amplified by PCR from a P. aeruginosa PAO1 genomic DNA template with the respective phaC and phaJ primers (Table S1). PP_0763 was amplified by PCR from a P. putida KT2440 genomic DNA template with primers acs-F/R (Table S1). All constructs were confirmed by DNA sequence analysis. Annotated sequence files for relevant constructs were deposited in GenBank.
2.3. Chromosome engineering
Chromosomal gene deletions were created in E. coli K12 MG1655 ΔaraBAD by P1 transduction (Thomason et al., 2007) using phage lysates generated from members of the KEIO collection (Baba et al., 2006). Deletions of fadBA and fadIJ were generated as described previously using pKD13 as template (Datsenko and Wanner, 2000). Chromosomal integration of a Φ(Ptrc-BTE) expression cassette (a fusion of the IPTG inducible trc promoter with BTE) was constructed as described previously (Youngquist et al., 2012). Briefly, an insertion template was generated by PCR amplification of a fragment comprising lacIQ-Ptrc-BTE-FRT-CmR-FRT from plasmid pBTE-int. Primers contained 40 base pairs of sequence homology to regions of the E. coli chromosome flanking the fadBA locus (Table S1) to guide λ red mediated recombination. To construct the fadD promoter replacement, Φ(Ptrc-fadD), the region consisting of lacIQ-Ptrc-fadD was PCR amplified off of plasmid pTrc-fadD. A region of pKD13 comprising the kanamycin resistance cassette flanked by FRT sites was PCR amplified separately. The two PCR products were stitched together in a third PCR, generating a linear DNA that was integrated onto the chromosome of E. coli DY330 via λ red mediated recombination. For each mutant strain, resistance markers were removed by inducing FLP recombinase encoded on plasmid pCP20 which was subsequently cured by growth at a non-permissive temperature (Datsenko and Wanner, 2000). All chromosomal mutations were verified by colony PCR.
2.4. Fatty acid and PHA extraction and characterization
FAME analysis was performed on 2.5 mL of culture or supernatant as described previously (Lennen et al., 2010). For PHA analysis, cells were harvested by centrifugation (3000 x g for 25 min), washed with 25 mL 1X phosphate buffered saline (PBS), and lyophilized overnight. PHA content was analyzed by GC/MS based on the method of Kato et al. (1996). PHA was converted to the corresponding monomer-esters by combining 2 mL of chloroform and 2 mL of 3% H2SO4 in methanol (v/v) with 10 mg of lyophilized cells in a 10 mL disposable glass centrifuge tube. 50 μL of 10 mg mL−1 pentadecanoic acid in ethanol was added as an internal standard. The mixture was heated at 105 °C in a heat block for 24 h followed by addition of 5 mL of 100 mg mL−1 NaHCO3 in water. The mixture was vortexed and centrifuged (1,000 ×g for 10 min) and the aqueous layer was removed by aspiration. The organic (chloroform) phase (1 μL) was analyzed using a Shimadzu GCMS QP2010S gas chromatograph mass spectrometer equipped with an AOC-20i auto-injector and a Restek Rxi®-5ms column (catalog #13423). The temperature program used was as follows: 60 °C hold for 1 min, ramp from 60 °C to 230 °C at 10 °C per min and a final hold at 230 °C for 10 min. The MS was operated in scanning mode between 35 and 500 m/z.
2.5. PHA purification and nuclear magnetic-resonance spectroscopy
PHA was extracted for analysis by nuclear magnetic-resonance (NMR) as described previously (Jiang et al., 2006) and modified based on communications with Chris Nomura (State University of New York). Briefly, lyophilized cells were washed with methanol to remove fatty acids and other impurities followed by a second lyophilization step. The material was extracted with 120 mL refluxing chloroform in a Soxhlet apparatus followed by evaporation of the chloroform to recover the purified PHA. 10–15 mg of product was dissolved in 1 mL deuterated chloroform and analyzed at room temperature on a Bruker AC-300 spectrometer for 1H NMR and on a Varian Mercury-300 spectrometer for 13C NMR.
3. Results
3.1. Effect of fad deletions on dodecanoic acid catabolism
β-oxidation of fatty acids occurs in three stages. First, FFA are imported across the outer membrane via FadL and activated as CoA thioesters by FadD in the inner membrane. The acyl-CoA thioesters are a key regulatory signal which abrogates the DNA binding ability of FadR. In the absence of acyl-CoAs FadR represses expression of enzymes involved in β-oxidation. Once activated, acyl-CoAs are catabolized to acetyl-CoA via an iterative pathway comprised of four enzymatic reactions (Fig. 1) – acyl-CoA dehydrogenation (FadE), enoyl-CoA hydration (FadB), (3S)-hydroxyacyl-CoA dehydrogenation (FadB), and ketoacyl-CoA thiolation (FadA). Three additional fad genes – fadK, fadI and fadJ have strong sequence homology to fadD, fadA and fadB, respectively and have been shown to be critical for anaerobic beta-oxidation (Campbell et al., 2003). Each cycle ends when FadA (or FadI) cleaves a ketoacyl-CoA to generate an acetyl-CoA and an acyl-CoA reduced in length by two carbons that is the substrate for the next round. Finally, E. coli possesses additional β-oxidation capacity in the ato genes which are responsible for processing short-chain FFAs.
Our metabolic engineering strategy for producing mcl-PHA from endogenously synthesized fatty acids requires the disruption of β-oxidation such that (R)-3-hydroxyacyl-CoA thioesters can be polymerized but not catabolized to acetyl-CoA. We therefore tested the ability of strains harboring various deletions in β-oxidation (fad) genes to catabolize dodecanoic acid after 24 and 48 h of shake flask cultivation (Fig. 2a). The base strain, K12 MG1655 ΔaraBAD, was not observed to completely catabolize all of the dodecanoic acid until 48 h, while a fadR mutant was able to consume all of the dodecanoic acid within 24 h. A fadB deletion, which based on previous reports was expected to greatly impair dodecanoic acid catabolism under aerobic conditions, consumed 20% of the dodecanoic acid. To completely block dodecanoic acid consumption over the course of 48 h, a double knockout, ΔfadB, ΔfadJ strain was required. Similarly, a ΔfadA strain consumed ~20% of the dodecanoic acid while a ΔfadA, ΔfadI double mutant demonstrated negligible dodecanoic acid consumption. The performance of other fad strains and the effect of a fadR deletion combined with these strains, which generally improved the rate of dodecanoic acid metabolism, is shown in Fig. 2a.
To determine if metabolism of exogenously fed dodecanoic acid correlated with metabolism of endogenously produced FFAs, β-oxidation deletion strains were transformed with pTrc99a-BTE and grown for 48 h on LB supplemented with glucose (Fig. 2b). Final fatty acid concentrations and especially saturated dodecanoic acid concentrations correlated with exogenous consumption data (Fig. 2a). Specifically, strains capable of complete consumption of exogenous dodecanoic acid after 48 h accumulated little to no endogenous dodecanoic acid while strains that were the most impaired in exogenous C12 consumption yielded the largest concentrations of endogenous C12 FFA. While FFA uptake has been well studied (DiRusso and Black, 2004), the mechanism of FFA secretion is poorly understood. It should be noted that the data presented in Fig. 2b does not distinguish rates of FFA secretion and reuptake from catabolism of intracellular FFA.
3.2. Effect of fadD regulation on dodecanoic acid catabolism
The proposed mcl-PHA pathway requires the activation of FFA and oxidation by FadE to yield enoyl-CoA thioesters. These genes could be upregulated by increasing the rate of acyl-CoA synthesis (e.g. replacing PfadD with a stronger promoter), removing repression via FadR, or both. Therefore, a fadD overexpression strain was constructed by replacing the native fadD promoter with the strong, IPTG inducible trc promoter (Brosius et al., 1985). Dodecanoic acid consumption in this strain was compared with the base strain, ΔfadR and Φ(Ptrc-fadD) ΔfadR combination strains (Fig. 3). Interestingly, the ΔfadR strain completely consumed the dodecanoic acid after 8 h while complete consumption was not observed for the Φ(Ptrc-fadD) overexpression strain until 24 h. Surprisingly, a Φ(Ptrc-fadD) ΔfadR combination strain consumed dodecanoic acid at a rate in between the Φ(Ptrc-fadD) overexpression and ΔfadR strains. Deletion of fadR may provide the additional benefit of upregulating fadE expression which is required to produce enoyl-CoA thioesters in our mcl-PHA strategy.
3.3. Production of mcl-PHA in fad strains in the presence of exogenous dodecanoic acid
Two PHA biosynthetic enzymes are required to confer E. coli with the ability to synthesize mcl-PHA from enoyl-CoA thioesters, a PHA polymerase (PhaC) and an (R)-specific enoyl-CoA hydratase (PhaJ). P. aeruginosa DSM1707 phaJ1–4 have been previously characterized in E. coli LS5218 (Tsuge et al., 2003). Here, genes from P. aeruginosa PAO1 were selected based on sequence identity with DSM1707 and the ability of this strain to accumulate mcl-PHA. Individual phaJ and phaC clones were co-expressed from plasmids pMSB-6 and pBAD33-C280*, respectively, in LS5218 grown in the presence of exogenous dodecanoic acid as a sole carbon source. All phaJ-phaC combinations yielded mcl-PHA identified as methyl esters of 3-hydroxyacyl-chains after processing (Table 2). The observed acyl-chains ranged in length from C6 to C14 corresponding to mcl-PHA monomers (C6–C12) and components of lipid A (C14). The combination of phaJ3 and phaC2 was selected based on the ability to produce mcl-PHA containing C12 monomer units at yields greater than other combinations tested (Table 2).
Pseudomonas aeruginosa phaC2 was cloned downstream of phaJ3 into pMSB-6 yielding pDA-JC and the plasmid was transformed into a selection of fad deletions strains for mcl-PHA production. Table 3 shows the ability of a ΔfadR, ΔfadRB, ΔfadRBJ and ΔfadRABIJ strains to accumulate mcl-PHA as well as the monomer composition of the resulting polymer. Most notably, ΔfadR and ΔfadRB strains both produced mcl-PHA with a heterogeneous monomer composition, although the fraction of C12 monomers in the ΔfadRB strain was greatly increased over that of the ΔfadR strain. The ΔfadRBJ and ΔfadRABIJ strains were both capable of producing mcl-PHA homopolymer consisting entirely of C12 monomers with the yield of PHA in the ΔfadRABIJ strain slightly improved over that of the ΔfadRBJ strain. This result was consistent with the relative rates of endogenous FFA production (Fig. 2b).
3.4. Accumulation of mcl-PHA in a ΔfadRABIJ strain with endogenous dodecanoic acid production
Expression of the California Bay Laurel (U. californica) thioesterase (BTE) in E. coli results in the accumulation of FFAs composed predominantly (≥80%) of saturated C12 and unsaturated C12:1 species with the remainder comprised mainly of C14 and unsaturated C14:1 FFAs (Voelker and Davies, 1994). A codon optimized version of BTE (Lennen et al., 2010) was integrated into the chromosome of E. coli K-12 MG1655 ΔaraBAD ΔfadR ΔfadIJ into the fadBA locus, resulting in a ΔfadRABIJ strain with one copy of the Φ(Ptrc-BTE) cassette. This strain (SA01) when transformed with pDA-JC and grown in MOPS minimal media supplemented with 1% glucose accumulated mcl-PHA at a % CDW on par with a ΔfadRABIJ strain cultured with exogenous dodecanoic acid (Fig. 4). A significant amount of residual dodecanoic and tetradecanoic acid was also observed indicating that there is room for further pathway optimization.
3.5. Effect of overexpression of PP_0763 on mcl-PHA accumulation in a ΔfadRABIJ strain with endogenous dodecanoic acid production
Given the presence of excess FFA, we hypothesized that the rate of fatty acyl-CoA production was not balanced with FFA synthesis. Therefore, the predicted acyl-CoA synthetase, PP_0763 from P. putida KT2440 was cloned between phaJ3 and phaC2 in pDA-JC resulting in pDA-JAC. Strain SA01 was transformed with pDA-JAC which resulted in the production of 9.8% CDW mcl-PHA, a 5-fold increase compared to the same strain without PP_0763 (Fig. 4, Table S2). When cultured in a 1 L bioreactor, mcl-PHA accumulation increased to 17.3% CDW after 96 h. The identity of the purified product was confirmed to be predominantly polyhydroxydodecanoate by 1H and 13C NMR (Figs. S1, S2).
4. Discussion
4.1. Effect of fad deletions on dodecanoic acid metabolism
Previous work has demonstrated that the ability to use fatty acids ≥C12 as a sole carbon source is lost in the case of deletions in fadB (Dirusso, 1990), however, a fadB(A) phaC+ strain was still capable of aerobic production of mcl-PHA heteropolymer indicating that E. coli can complement fadB activity (Langenbach et al., 1997; Prieto et al., 1999; Qi et al., 1997; Ren et al., 2000; Snell et al., 2002). Furthermore, a fadA insertion mutant was capable of aerobic growth on oleic acid (C18:1) as a sole carbon source after extended incubation (<5 days) on solid media (Campbell et al., 2003), further indicating that additional β-oxidation activity is present. Our data indicate both E. coli ΔfadA and ΔfadB mutants are capable of dodecanoic acid metabolism after 24 h, although with reduced capability compared to WT. Conversely, E. coli ΔfadR ΔfadA catabolized dodecanoic acid more efficiently than WT with nearly complete consumption of the dodecanoic acid after 48 h. As fadR is a negative regulator for fadIJ, it is likely that fadIJ is capable of complementing fadBA and restoring β-oxidation activity to that of WT. However, a ΔfadR ΔfadB strain did not show increased dodecanoic acid catabolism over the 48 h period. Therefore, fadJ may not be able to complement a fadB deletion as effectively as in the case of fadI with fadA.
Deletions of fadI or fadJ had a minor negative effect on dodecanoic acid metabolism compared to WT which is expected if fadBA function as the major contributor to aerobic β-oxidation. Similarly, ΔfadR ΔfadI and ΔfadR ΔfadJ strains were comparable to a ΔfadR strain. An unexpected result was the reduced rate of dodecanoic acid consumption in both a ΔfadBA and ΔfadIJ double knockout compared to WT. These data indicate that functional expression of fadBA is not essential for dodecanoic acid metabolism under the conditions tested. It is important to note that dodecanoic acid metabolism was still active in a ΔfadIJ strain which is in line with previous work that demonstrated both aerobic and anaerobic growth for a ΔfadIJ (yfcYX) strain on oleic acid (Campbell et al., 2003).
Based on the behavior of the aforementioned deletions, it was anticipated that a ΔfadA ΔfadI or ΔfadB ΔfadJ strain would be incapable of C12 metabolism. This result was confirmed for these strains, a ΔfadBA ΔfadIJ strain and for each of the strains when combined with a fadR deletion. These results agree with the observation that an E. coli ΔfadB ΔfadJ strain expressing the phaC polymerase from Pseudomonas oleovorans from a plasmid was incapable of mcl-PHA accumulation (Snell et al., 2002). Recent work in an E. coli LS5218 ΔfadB ΔfadJ strain for PHA homopolymer production on exogenously fed fatty acids corroborates these results as well (Tappel et al., 2012).
4.2. Comparison of fadD overexpression and fadR deletion on dodecanoic acid metabolism
Due to the ability of a fadR deletion to improve the initial rate of C12 metabolism, we hypothesized that overexpression of fadD would result in a similar phenotype. We therefore tested a chromosomal trc promoter fusion with fadD, Φ(Ptrc-fadD), individually and in combination with a ΔfadR strain. Over a 24 h period, we noted that Φ(Ptrc-fadD) was capable of improved C12 consumption compared with WT but was not as efficient as a ΔfadR or Φ(Ptrc-fadD) ΔfadR combination strain. Overexpression of fadD increases the cytoplasmic acyl-CoA pool faster than in WT resulting in faster de-repression of all β-oxidation genes regulated by fadR, while in a ΔfadR strain, there is no repression of β-oxidation genes allowing for faster initial turnover of exogenous fatty acids.
4.3. Effect of soluble vs. membrane associated CoA-synthetases
Although mcl-PHA production in strain SA01 expressing pDA-JC was achieved with a defined composition from a non-fatty acid feedstock, a large amount of endogenously produced FFA remained in the culture broth. Therefore, we hypothesized that the limiting step in PHA biosynthesis was CoA ligation. Or put another way, we hypothesized that intracellular FFAs were leaving the cell at a faster rate than FadD ligation with CoA, the product of which (acyl-CoA) is not exportable. Two models of the CoA synthetase reaction can be envisioned (DiRusso and Black, 2004). First, cytoplasmic FFA, freshly produced by BTE, could be directly bound by a cytosolic FadD and converted to CoA thioesters. Alternatively, cytoplasmic FFA could begin to traverse the inner cell membrane, periplasm, and outer membrane and be re-imported for FadD activation. The import of extracellular fatty acids across the outer membrane is facilitated by FadL. Once across the outer membrane, FFA traverse the periplasm and intercalate into the inner membrane. FFA then bind to the FadD active site and become phosphorylated from an ATP donor. The final CoA ligation, disassociation of FadD from the inner membrane and association of the fatty acyl-CoA with the cytoplasm likely takes place in one concerted event. If the rate of re-import is inferior to continued export (which would be down the concentration gradient), dodecanoic acid could accumulate extracellularly as was observed in our BTE expressing strains. We therefore co-expressed the predicted soluble CoA-synthetase encoded by P. putida gene PP_0763 (acs) which has been shown to be an effective medium-chain-length acyl-CoA synthetase when heterologously expressed in E. coli (Wang et al., 2012). Co-expressing acs with PHA biosynthesis genes in SA01 resulted in a 5-fold increase in mcl-PHA accumulation in shake flasks and a 7.5-fold increase in 3-OH-C12 content. These data support the conclusion that balancing FFA production and CoA activation will be critical to maximizing mcl-PHA yields.
4.4. Bioreactor scale-up of mcl-PHA production from glucose
Our PHA production strategy is the first to produce a defined mcl-PHA from an unrelated carbon source. Our highest mcl-PHA production (17.3% CDW) was achieved by cultivating strain SA01 pDA-JAC in a 1 L bioreactor using a fed-batch strategy. For comparison, prior studies achieved ~6% CDW of an undefined mcl-PHA in E. coli when grown on gluconate (Rehm and Steinbuchel, 2001) and 11.6% CDW of undefined heteropolymer in E. coli grown on glucose (Wang et al., 2012). Finally, recent work in both P. putida and E. coli demonstrated production of mcl-PHA homopolymer in the case of feeding exogenous fatty acids (Liu et al., 2011; Tappel et al., 2012). In putida, 85% C12-co-15% C10 PHA was produced at 9% CDW while a ΔfadR ΔfadB strain of E. coli was capable of making 28.6% CDW C12 homopolymer. Based on the maximum theoretical yield calculations, E. coli is capable of producing 0.32 g (R)-3-hydroxydodecanoic acid per g glucose fed. Thus, further optimization of the described pathway for mcl-PHA biosynthesis should lead to additional improvements in the yield on glucose as a sole carbon source. For example, improvements in PHA biosynthesis could be achieved through expression of alternative polymerases or hydratases with a higher activity for C12 units. Besides fadJ (yfcX), there exist at least five additional genes with homology to fadB on the E. coli chromosome (Park and Lee, 2004). When these genes were overexpressed in E. coli ΔfadB in the presence of a PHA polymerase and LB+0.2% decanoic acid (C10), a 1.3- to 2.0-fold improvement in PHA accumulation (% CDW) was achieved over an empty vector control. Along with fadJ, overexpression of ydbU, paaF and paaG resulted in the greatest improvement. By contrast, no PHA accumulation was detected in E. coli fadB+ under the same conditions. Therefore, these gene products may have a role in both C12 metabolism and PHA biosynthesis in E. coli and overexpression of these genes in addition to or in place of phaJ could improve PHA accumulation.
5. Conclusions
A scheme was presented for the production of mcl-PHA homopolymer from a non-fatty acid related carbon source at up to 17.3% CDW. Examination of a series of β-oxidation deletion strains provided an understanding of knockouts required to completely inhibit iterative degradation of both exogenously fed and endogenously produced fatty acids. Specifically, disruption of both the aerobic and anaerobic pathways (i.e., fadBA or fadIJ) proved essential for the proposed mcl-PHA biosynthesis pathway. Co-expression of phaJ3 and phaC2 from P. aeruginosa PAO1 in E. coli ΔfadRABIJ yielded polyhydroxydodecanoate in the presence of dodecanoic acid feeding. When the plant acyl-ACP thioesterase, BTE, was expressed in this strain, PHA comprised primarily of hydroxydodecanoate monomers was observed. Finally, expression of an additional, soluble CoA-synthetase improved production 5-fold resulting in the highest reported production of mcl-PHA for a scheme involving a thioesterase.
We anticipate that this strategy can be generalized to produce a variety of mcl-PHA homo- and heteropolymers where the resulting monomer composition can be tailored based on the known fatty acid production profile of a particular acyl-ACP thioesterase. If integrated with pathways for converting renewable substrates to acetyl-CoA, processes for synthesizing designer mcl-PHA can be developed. The use of inexpensive feedstocks will ultimately allow renewable, biodegradable PHAs to compete on a cost-basis with analogous, petroleum derived plastics.
Supplementary Material
Acknowledgments
The authors would like to thank Jackie Cooper (University of Wisconsin-Madison) for support with operation and maintenance of the GC/MS equipment, Ryan Tappel and Christopher Nomura (State University of New York) for assistance with a method for PHA purification, Adrián Peña Hueso (Silatronix, Inc.) for facilitating 1H NMR analysis and Michelle Wilson (University of Wisconsin-Madison) for 13C NMR analysis. This work was funded by the DOE Great Lakes Bioenergy Research Center (DOE BER Office of Science DE-FC02–07ER64494), and the Wisconsin Alumni Research Foundation. Daniel E. Agnew is the recipient of a National Institutes of Health Biotechnology Training Program Fellowship (NIH 5 T32 GM08349).
This material is based upon work supported by the National Science Foundation under Grant Nos. NSF CHE-9208463 and NSF CHE-0342998. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Abbreviations
- (mcl)-PHA
(medium-chain-length)-polyhydroxyalkanoate
- LB
Lysogeny Broth
- PCR
polymerase chain reaction
- BTE
California Bay Laurel (Umbellularia californica) Thioesterase
- PBS
phosphate buffered saline
- FAME
fatty acid methyl ester
- GC/MS
gas chromatography mass spectrometry
- ECGSC
Escherichia coli Genetic Stock Center – Yale University
- ACP
acyl-carrier protein
- CoA
coenzyme A
- DO2
dissolved oxygen
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.ymben.2012.08.003.
References
- Amann E, Ochs B, Abel KJ. Tightly regulated tac promoter vectors useful for the expression of unfused and fused proteins in Escherichia coli. Gene. 1988;69:301–315. doi: 10.1016/0378-1119(88)90440-4. [DOI] [PubMed] [Google Scholar]
- Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Sys Biol. 2006;2:11. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker S, Vlad D, Schuster S, Pfeiffer P, Unden G. Regulatory O-2 tensions for the synthesis of fermentation products in Escherichia coli and relation to aerobic respiration. Arch Microbiol. 1997;168:290–296. doi: 10.1007/s002030050501. [DOI] [PubMed] [Google Scholar]
- Brosius J, Erfle M, Storella J. Spacing of the -10 and -35 regions in the tac promoter: effect on its in vivo activity. J Biol Chem. 1985;260:3539–3541. [PubMed] [Google Scholar]
- Campbell JW, Morgan-Kiss RM, Cronan JE. A new Escherichia coli metabolic competency: growth on fatty acids by a novel anaerobic beta-oxidation pathway. Mol Microbiol. 2003;47:793–805. doi: 10.1046/j.1365-2958.2003.03341.x. [DOI] [PubMed] [Google Scholar]
- Chen GQ, Wu Q. The application of polyhydroxyalkanoates as tissue engineering materials. Biomaterials. 2005;26:6565–6578. doi: 10.1016/j.biomaterials.2005.04.036. [DOI] [PubMed] [Google Scholar]
- Cherepanov PP, Wackernagel W. Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene. 1995;158:9–14. doi: 10.1016/0378-1119(95)00193-a. [DOI] [PubMed] [Google Scholar]
- Chung A, Liu Q, Ouyang SP, Wu Q, Chen GQ. Microbial production of 3-hydroxydodecanoic acid by pha operon and fadBA knockout mutant of Pseudomonas putida KT2442 harboring tesB gene. Appl Microbiol Biotechnol. 2009;83:513–519. doi: 10.1007/s00253-009-1919-6. [DOI] [PubMed] [Google Scholar]
- Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lay NR, Cronan JE. In vivo functional analyses of the type II Acyl carrier proteins of fatty acid biosynthesis. J Biol Chem. 2007;282:20319–20328. doi: 10.1074/jbc.M703789200. [DOI] [PubMed] [Google Scholar]
- Dirusso CC. Primary sequence of the Escherichia-coli fadBA operon, encoding the fatty acid-oxidizing multienzyme complex, indicates a high degree of homology to eukaryotic enzymes. J Bacteriol. 1990;172:6459–6468. doi: 10.1128/jb.172.11.6459-6468.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiRusso CC, Black PN. Bacterial long chain fatty acid transport: gateway to a fatty acid-responsive signaling system. J Biol Chem. 2004;279:49563–49566. doi: 10.1074/jbc.R400026200. [DOI] [PubMed] [Google Scholar]
- Guzman LM, Belin D, Carson MJ, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose pbad promoter. J Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover SW, Marner WD, Brownson AK, Lennen RM, Wittkopp TM, Yoshitani J, Zulkifly S, Graham LE, Chaston SD, McMahon KD, Pfleger BF. Bacterial production of free fatty acids from freshwater macroalgal cellulose. Appl Microbiol Biotechnol. 2011;91:435–446. doi: 10.1007/s00253-011-3344-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Ramsay JA, Ramsay BA. Acetone extraction of mcl-PHA from Pseudomonas putida KT2440. J Microbiol Methods. 2006;67:212–219. doi: 10.1016/j.mimet.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Kato M, Bao HJ, Kang CK, Fukui T, Doi Y. Production of a novel copolyester of 3-hydroxybutyric acid and medium chain length 3-hydroxyalkanaic acids by Pseudomonas sp 61-3 from sugars. Appl Microbiol Biotechnol. 1996;45:363–370. [Google Scholar]
- Khanna S, Srivastava AK. Recent advances in microbial polyhydroxyalkanoates. Process Biochem. 2005;40:607–619. [Google Scholar]
- Langenbach S, Rehm BHA, Steinbuchel A. Functional expression of the PHA synthase gene PhaC1 from Pseudomonas aeruginosa in Escherichia coli results in poly(3-hydroxyalkanoate) synthesis. FEMS Microbiol Lett. 1997;150:303–309. doi: 10.1016/s0378-1097(97)00142-0. [DOI] [PubMed] [Google Scholar]
- Lee SK, Chou HH, Pfleger BF, Newman JD, Yoshikuni Y, Keasling JD. Directed evolution of AraC for improved compatibility of arabinose- and lactose-inducible promoters. Appl Environ Microbiol. 2007;73:5711–5715. doi: 10.1128/AEM.00791-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennen RM, Braden DJ, West RM, Dumesic JA, Pfleger BF. A process for microbial hydrocarbon synthesis: overproduction of fatty acids in Escherichia coli and catalytic conversion to alkanes. Biotechnol Bioeng. 2010;106:193–202. doi: 10.1002/bit.22660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZJ, Shi ZY, Jian J, Guo YY, Wu Q, Chen GQ. Production of poly (3-hydroxybutyrate-co-4-hydroxybutyrate) from unrelated carbon sources by metabolically engineered Escherichia coli. Metab Eng. 2010;12:352–359. doi: 10.1016/j.ymben.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Liu Q, Luo G, Zhou XR, Chen GQ. Biosynthesis of poly(3-hydroxydecanoate) and 3-hydroxydodecanoate dominating polyhydroxyalkanoates by beta-oxidation pathway inhibited Pseudomonas putida. Metab Eng. 2011;13:11–17. doi: 10.1016/j.ymben.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Meng DC, Shi ZY, Wu LP, Zhou Q, Wu Q, Chen JC, Chen GQ. Production and characterization of poly(3-hydroxypropionate-co-4-hydroxybutyrate) with fully controllable structures by recombinant Escherichia coli containing an engineered pathway. Metab Eng. 2012;14:317–324. doi: 10.1016/j.ymben.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Park SJ, Lee SY. New fadB homologous enzymes and their use in enhanced biosynthesis of medium-chain-length polyhydroxyalkanoates in fadB mutant Escherichia coli. Biotechnol Bioeng. 2004;86:681–686. doi: 10.1002/bit.20065. [DOI] [PubMed] [Google Scholar]
- Prieto MA, Kellerhals MB, Bozzato GB, Radnovic D, Witholt B, Kessler B. Engineering of stable recombinant bacteria for production of chiral medium-chain-length poly-3-hydroxyalkanoates. Appl Environ Microbiol. 1999;65:3265–3271. doi: 10.1128/aem.65.8.3265-3271.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi QS, Rehm BHA, Steinbuchel A. Synthesis of poly(3-hydroxyalkanoates) in Escherichia coli expressing the PHA synthase gene PhaC2 from Pseudomonas aeruginosa: comparison of PhaC1 and PhaC2. FEMS Microbiol Lett. 1997;157:155–162. doi: 10.1111/j.1574-6968.1997.tb12767.x. [DOI] [PubMed] [Google Scholar]
- Qiu YZ, Han J, Guo JJ, Chen GQ. Production of Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) from gluconate and glucose by recombinant Aeromonas hydrophila and Pseudomonas putida. Biotechnol Lett. 2005;27:1381–1386. doi: 10.1007/s10529-005-3685-6. [DOI] [PubMed] [Google Scholar]
- Rehm BHA, Steinbuchel A. Heterologous expression of the acyl-acyl carrier protein thioesterase gene from the plant Umbellularia californica mediates polyhydroxyalkanoate biosynthesis in recombinant Escherichia coli. Appl Microbiol Biotechnol. 2001;55:205–209. doi: 10.1007/s002530000541. [DOI] [PubMed] [Google Scholar]
- Ren Q, Sierro N, Kellerhals M, Kessler B, Witholt B. Properties of engineered poly-3-hydroxyalkanoates produced in recombinant Escherichia coli strains. Appl Environ Microbiol. 2000;66:1311–1320. doi: 10.1128/aem.66.4.1311-1320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell KD, Feng F, Zhong L, Martin D, Madison LL. YfcX enables medium-chain-length poly(3-hydroxyalkanoate) formation from fatty acids in recombinant Escherichia coli fadB strains. J Bacteriol. 2002;184:5696–5705. doi: 10.1128/JB.184.20.5696-5705.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbuchel A, Valentin HE. Diversity of bacterial polyhydroxyalkanoic acids. FEMS Microbiol Lett. 1995;128:219–228. [Google Scholar]
- Tappel RC, Wang Q, Nomura CT. Precise control of repeating unit composition in biodegradable poly(3-hydroxyalkanoate) polymers synthesized by Escherichia coli. J Biosci Bioeng. 2012;113:480–486. doi: 10.1016/j.jbiosc.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Theodorou EC, Theodorou MC, Kyriakidis DA. Involvement of the AtoSCDAEB regulon in the high molecular weight poly-(R)-3-hydroxybutyrate biosynthesis in phaCAB+ Escherichia coli. Metab Eng. 2012;14:354–365. doi: 10.1016/j.ymben.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Thomason LC, Costantino N, Court DL. E. coli genome manipulation by P1 transduction. In: Ausubel Frederick M, et al., editors. Current Protocols In Molecular Biology. Unit 1.17. Chapter 1. 2007. [DOI] [PubMed] [Google Scholar]
- Tseng CP, Albrecht J, Gunsalus RP. Effect of microaerophilic cell growth conditions on expression of the aerobic (cyoABCDE and cydAB) and anaerobic (narGHJI, frdABCD, and dmsABC) respiratory pathway genes in Escherichia coli. J Bacteriol. 1996;178:1094–1098. doi: 10.1128/jb.178.4.1094-1098.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuge T, Taguchi K, Taguchi S, Doi Y. Molecular characterization and properties of (R)-specific enoyl-CoA hydratases from Pseudomonas aeruginosa: metabolic tools for synthesis of polyhydroxyalkanoates via fatty acid beta-oxidation. Int J Biol Macromol. 2003;31:195–205. doi: 10.1016/s0141-8130(02)00082-x. [DOI] [PubMed] [Google Scholar]
- Voelker TA, Davies HM. Alteration of the specificity and regulation of fatty-acid synthesis of Escherichia coli by expression of a plant medium-chain acyl-acyl carrier protein thioesterase. J Bacteriol. 1994;176:7320–7327. doi: 10.1128/jb.176.23.7320-7327.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HH, Zhou XR, Liu QA, Chen GQ. Biosynthesis of polyhydroxyalkanoate homopolymers by Pseudomonas putida. Appl Microbiol Biotechnol. 2011;89:1497–1507. doi: 10.1007/s00253-010-2964-x. [DOI] [PubMed] [Google Scholar]
- Wang Q, Tappel RC, Zhu CJ, Nomura CT. Development of a new strategy for production of medium-chain-length polyhydroxyalkanoates by recombinant Escherichia coli via inexpensive non-fatty acid feedstocks. Appl Environ Microbiol. 2012;78:519–527. doi: 10.1128/AEM.07020-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngquist JT, Lennen RM, Ranatunga DR, Bothfeld WH, II, WDM, Pfleger BF. Kinetic modeling of free fatty acid production in Escherichia coli based on continuous cultivation of a plasmid free strain. Biotechnol Bioeng. 2012;109:1518–1527. doi: 10.1002/bit.24420. [DOI] [PubMed] [Google Scholar]
- Yu DG, Ellis HM, Lee EC, Jenkins NA, Copeland NG, Court DL. An efficient recombination system for chromosome engineering in Escherichia coli. Proc Natl Acad Sci USA. 2000;97:5978–5983. doi: 10.1073/pnas.100127597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Shi ZY, Meng DC, Wu Q, Chen JC, Chen GQ. Production of 3-hydroxypropionate homopolymer and poly(3-hydroxypropionate-co-4-hydroxybutyrate) copolymer by recombinant Escherichia coli. Metab Eng. 2011;13:777–785. doi: 10.1016/j.ymben.2011.10.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.