Abstract
Gender may be involved in the motivational processing of facial beauty. This study applied a behavioral probe, known to activate brain motivational regions, to healthy heterosexual subjects. Matched samples of men and women were administered two tasks: (a) key pressing to change the viewing time of average or beautiful female or male facial images, and (b) rating the attractiveness of these images. Men expended more effort (via the key-press task) to extend the viewing time of the beautiful female faces. Women displayed similarly increased effort for beautiful male and female images, but the magnitude of this effort was substantially lower than that of men for beautiful females. Heterosexual facial attractiveness ratings were comparable in both groups. These findings demonstrate heterosexual specificity of facial motivational targets for men, but not for women. Moreover, heightened drive for the pursuit of heterosexual beauty in the face of regular valuational assessments, displayed by men, suggests a gender-specific incentive sensitization phenomenon.
Keywords: Reward, Reinforcement, Sex, Motivation, Men, Women, Male, Female, Incentive sensitization, Heterosexual
According to Darwin's sexual selection theory (Darwin, 1871), gender differences in mating strategies and effort reflect adaptive natural selection processes, derived from conflicting motivations for maximizing the number of fertilized women by the abundant sperm vs. entrusting a limited egg supply to the fittest men. Thus, women tend to search for men with social dominance and material goods (Sadalla, Kenrick, & Vershure, 1987), whereas men pursue physiological qualities that indicate fertility, including large breasts, prominent buttocks, and wide pelvises (Buss, 1989; Buss & Schmitt, 1993; Cunningham, 1986; Senior, 2003; Symons, 1979). The motivational appeal of these exaggerated fertility symbols, depicted in the figures of ancient Eastern goddesses of fertility (e.g., Babylonian Ishtar) and still permeating some cultures, has, however, been sublimated over time into more subtle ideals of health and facial beauty (Rhodes, 2006).
Much of the prior research on the motivational role of beauty in mating gender differences is based on self-report measures and ratings of preferences, while fundamental motivational drives to pursue heterosexual beauty have not yet been methodically assessed using rigorous scientific paradigms. For this purpose, it is critical to develop a solid theoretical framework along with accurate measurements of motivated behavior.
Germane here, Berridge and Robinson (2003) advanced a theory that integrates new neurobiological insights into the brain reward system with psychological aspects of motivation. In this model, the authors distinguish between the desirability of reward and emotional experience. The former is defined by the extent to which a particular reward is wanted, whereas the latter refers to the subjective experience of pleasure or pain. Rewards that are “liked” (e.g., evoke pleasure) may not always be desired or “wanted”, as in the case of sexual pursuit of a physically attractive partner in situations where destructive consequences are likely to ensue.
Psychological aspects of “wanting” and “liking” are purportedly mediated via distinct neurobiological pathways, which play different roles within the motivational and reward system. The mesolimbic dopaminergic system may thus be particularly involved in what Berridge and Robinson (2003) term the “incentive salience” of reward, which refers to the “wanting” process by which an organism determines the motivational value of a particular object beyond the emotional experience it evokes. Normally objects with high incentive salience are deemed to capture greater attention resources and lead to expenditure of greater behavioral effort relative to available alternatives of lower motivational value. “Liking” is conveyed to the frontotemporal cortical structures (Berridge, 2003; Kelley, 2004) via distinct opioid neurotransmission within the scattered network of subcortical and brain stem nuclei (Saper, Chou, & Elmquist, 2002).
It has been proposed that some psychopathological conditions (to name a few: substance use disorders, psychosis and Parkinson's disease) with excessive release of- and/or sensitivity to dopamine in the “wanting” system may be associated with aberrant salience attribution, that is to say, incentive sensitization (Berridge, 2006; Elman, Borsook, & Lukas, 2006; Kapur, 2003). For example, in the form of irresistible urges to seek and consume drugs despite their diminished hedonic qualities, incentive sensitization is particularly conspicuous in patients with substance use disorders (Kalivas & Volkow, 2005). However, healthy people may be as well disproportionably sensitized to various stimuli including spiders, snakes, crowds, flights, closed spaces, and many others. In this regard, men could motivationally process heterosexual beauty with heightened incentive salience and, given potentially pathological aspects of this motivational state (Kernberg, 1995), with incentive sensitization.
The purpose of the present study was to determine whether male sex is associated with increased motivational value of heterosexual beauty. We applied a “mating opportunity mindset”-inducing (Wilson & Daly, 2004) facial paradigm that was originally developed by Ariely, Breiter, and Etcoff (Aharon et al., 2001) and was later used by Elman and colleagues (Elman et al., 2005). In this paradigm, participants (1) can either increase or decrease the viewing time of each of the faces by pressing designated keys on the keyboard and (2) rate the attractiveness of human faces that appear on a computer screen. In this way, an objective marker of operant behavior or a motivational value conceptualized as effort expended to pursue beauty (in units of computer key presses) and reflective of internal state can be related to conscious and subjective valuation of beauty's esthetics. Thus, our facial paradigm distinguishes between “wanting” (i.e., number of key presses as a measurement of motivational value) aspects of motivation from “liking” (i.e., ratings of attractiveness indicating subjective valuation of esthetics).
The value of using the key-press and the ratings tasks is a more conclusive interpretation of the findings. Matching increments on both tasks’ measurements with regard to beauty will support the intuitively obvious incentive salience attribution to the objects that are consciously experienced as beautiful (Berridge, 2006). On the other hand, increased key-press numbers without corresponding increases in the attractiveness ratings will suggest incentive sensitization mechanisms (Berridge, 2006; Evans et al., 2006). With these considerations in mind, it was hypothesized that, in comparison to men, women will have similar ratings of heterosexual beauty, but decreased key-press responses to this stimulus. Additionally, given greater bisexual interest among heterosexual women vs. men (Chivers & Bailey, 2005; Diamond, 2003), we expected to find in the former group increased attractiveness ratings and motivation for viewing beautiful female images.
Methods
Participants
For clarity of presentation, study participants are referred to as “men” and “women” and facial images as “male” and “female”. Study subjects comprised healthy individuals, as determined by the Structured Clinical Interview for DSM-IV-TR Axis I Disorders (SCID-I/NP; First, Spitzer, Gibbon, & Williams, 2002). Their self-reported heterosexual status was confirmed with the Klein Sexual Orientation Grid (KSOG; Klein, Sepekoff, & Wolf, 1985) that assessed the sexual self (attraction, fantasy, and behavior), the sexual orientation (emotional preference, social preference, and heterosexual or homosexual lifestyle), and the self-identification using the Likert-type scale raging from 1 (“hetero or other sex only”) to 7 (“same sex or gay only”) as applying to the present, past, or ideal. No subject expressed a greater than “2” rating i.e., “other sex/hetero mostly” on any of the KSOG items.
Men (n = 18) and women (n = 17) groups were matched with respect to age (within 3–4 year range), ethnicity (11 Caucasian, 4 African-American; 2 Hispanic and 1 Asian vs. 11 Caucasian; 3 African-American; 2 Hispanic and 1 Asian) and years of education (within 2–3 year range). According to the SCID-I/NP, no subject met criteria for current or lifetime substance abuse or dependence. Women were studied in the midfollicular phase of their menstrual cycle. All subjects gave written informed consent for participation after the procedures had been fully explained.
Stimuli
Subjects were presented with 80 nonfamous human faces that were selected from print media and classified according to pilot test results in the following four categories of 20 each: average female, beautiful female, average male, and beautiful male. The pictures were digitized at 600 dpi in 8-bit grayscale, spatially downsampled, and cut to fit in an oval “window” sized 310–350 pixels wide by 470 pixels high using Photoshop 4.0 software (Adobe Systems). Examples of categorical facial images used as visual stimuli are presented in Fig. 1.
Fig. 1.
Examples of categorical facial images used as visual stimuli.
Procedures
There were two tasks administered in separate runs: a key-press task, followed by an image attractiveness rating task. For each run, faces were presented in two blocks by gender. The order of the gender blocks was counterbalanced across subjects. Within each gender block, the average and beautiful faces were presented intermingled, in random order.
For the key-press task, subjects were informed that the entire task duration was fixed and independent of their actions but that they could control the viewing time of each individual picture. The default viewing time for an individual facial image was 4 s. However, subjects could either increase or decrease this time by up to 4 s (depending upon the frequency of the key presses) by alternately pressing a keyboard's “n” and “m,” or “z” and “x” keys, respectively. The former key presses were scored as positive and the latter as negative. The average of these values for the 20 pictures in each of the four facial categories yielded a subject's “net” key presses for each category. In addition, each subject's total key presses, i.e., absolute number of key presses, regardless of whether scored positive or negative, during the entire experiment were calculated for use as a covariate.
During the subsequent rating task, the subject rated the attractiveness of the same faces on a visual analog scale ranging from 0 “not attractive at all” to 100 “very attractive.” The averages for the 20 pictures in each of the four facial categories yielded a subject's attractiveness rating for each facial category.
Statistical analyses
Data were analyzed using the statistical package Statistica (StatSoft, Inc., Tulsa, OK). T tests for independent samples were conducted to compare demographic variables. To determine effects of beauty and gender on attractiveness assessments and quantified measures of beauty motivation, i.e., computer key presses, a two-factor analysis of variance (ANOVA), with Gender (men and women) as a between-subjects factor and Face type (average female, beautiful female, average male, and beautiful male) as a within-subjects factor was conducted. When group-by-time interactions were significant, post-hoc Newman–Keuls t tests were performed to determine differences in subjects’ responses to the facial stimuli. Group data were summarized as mean (M) ± standard deviation (SD). All analyses were two-tailed, and a p value < .05 defined statistical significance.
Results
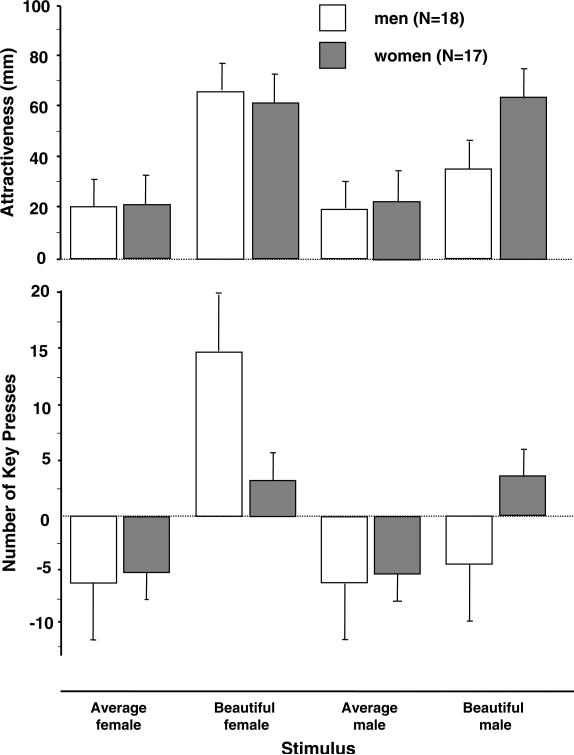
Men and women groups were similar with respect to age [M = 28.7, SD = 8.4 vs. M = 25.0, SD = 3.3; t(33) = 1.60, p = .10] and years of education [M = 15.7, SD = 1.5 vs. 15.8 , SD = 1.5; t(33) = 0.20, p = .84)]. Fig. 2 displays the rating and net key-press data for each category. Overall, the two groups did not differ in the attractiveness ratings [group effect: F(1,33) = 2.92, p = .10], but beautiful faces’ ratings significantly exceeded those of the average faces [Faces Type effect: F(3,99) = 127.90, p < .0001] with men rating significantly lower male beauty [Group by Face Type interaction: F(3,99) = 15.21, p < .0001]. Post-hoc Newman–Keuls t tests revealed accordingly no group differences in the ratings of beautiful females (p = .87) or of heterosexual beauty images (i.e., beautiful females’ ratings by men and beautiful males’ ratings by women; p = .71) and significantly lower ratings of beautiful male images in the men group (p = .005). Men rated beautiful males significantly higher (p < .001) than average males and females, while women gave male and female beauty similar (p = .51) ratings that significantly (p = .0001) exceeded those of the average images.
Fig. 2.
Facial attractiveness ratings by men and women study participants and their performance on the key-press task. Data are presented as mean (SD). The ordinate represents the distance on the visual analog scale of attractiveness, measured in millimeters. Rating data were analyzed with one-way ANOVA, using gender as the grouping factor, and Face Type as the within-subjects factor and post-hoc Newman–Keuls t tests. Key-press data were analyzed with ANCOVA using gender as the grouping factor, Face Type as the within-subjects factor and the absolute total key presses as the covariate; Newman–Keuls t tests were used for post-hoc comparisons.
Significant [t(33) = 2.18, p = .04] men [M = 11.1, SD = 6.2] and women [M = 6.93, SD = 5.1] group differences were detected in the total number of key presses (i.e., absolute number of key presses, regardless of whether scored positive or negative, during the entire experiment). To ascertain that group differences in the net key presses for facial categories did not merely reflect a group difference in general key-press activity, absolute total key presses were used as a covariate in the key-press data analysis. The ANCOVA results yielded a significant group (i.e., Gender) by Face Type interaction [F(3,96) = 7.47, p = .0002], with post-hoc Newman–Keuls t tests showing a higher number of key presses in the male group for beautiful females (p = .002). Men exerted significantly greater (p = .0001) effort (in the unit of computer key-press) to view beautiful female vs. beautiful male faces; this difference was not detected in the women group (p = .90). Men's mean number of key presses for beautiful females was significantly higher than for any other facial category (p = .0001), and no other significant differences in men's key presses were found (p > .90).
Discussion
The results of the current study provide empirical laboratory-based evidence for the motivational value of beauty. Our interpretation that the observed gender differences are related to motivational drives of the viewers (Senior, 2003) rather than to social acceptance or desirability of prolonged viewing behavior towards opposite sexes is supported by recent work demonstrating stronger fMRI activations within motivational/reward regions by female than by male faces for heterosexual men and by male than by female faces for heterosexual women (Ishai, 2007; Kranz & Ishai, 2006; O'Doherty et al., 2003). Moreover, a neuroimaging study employing the same visual stimuli in men only indicated that the key presses, but not the esthetic ratings, were paralleled by activation of distinct brain regions of motivational and reward circuitry (Aharon et al., 2001).
Our data contrast, however, with a prior report on facial beauty processing by healthy subjects (Kranz & Ishai, 2006). In groups of heterosexual men and women (n = 10, each), Kranz and Ishai (2006) observed similar visual processing time and attractiveness ratings of male and female faces. In the present study, men found beautiful male faces less attractive than beautiful female faces, with corresponding increases in the viewing times of the latter. These contrasting data might be accounted for by differences in the study design, such as categorical vs. visual analog types of attractiveness rating scales and passive visual processing vs. operant assessments of motivational effort employed by our study.
The present data render the dissociation between assessments of attractiveness and quantified measures of reward valuation, which the authors, respectively, referred to as “liking” and “wanting” (Aharon et al., 2001), gender specific. Specifically, the results of the rating task qualitatively paralleled those for the key presses (i.e., corresponding increases in both measures) only in the women group. Furthermore, healthy men rated beautiful female faces as highly attractive as healthy women did for beautiful males, but they expended more than quadruple the effort to increase the viewing times of these same faces. This group difference was not explained by the overall level of key-press activity. Our findings are therefore consistent with the conclusions that (1) men assess the attractiveness of heterosexual beautiful faces similarly but derive greater motivational drive (assessed operantly) from viewing them; in other words, in comparison to women, men “wanted” female beautiful faces more then could be explained by their esthetic attributes; and (2) the neural system identified for “wanting” in preclinical studies (Berridge & Robinson, 2003) may be the same system, sensitized in men, for processing female beauty stimuli.
Human sexuality is a broad phenomenon, integrating a complex system of interrelated biological, societal and psychological aspects, each of which exhibits a unique role within the context of sexual behavior. Although it is tempting to suggest that motivational sensitization in men for pursuit of women represents a biology-based phenomenon, similar to exaggerated (relative to hedonic effects) drug “wanting” or craving in addicts (Kalivas & Volkow, 2005), the paradigm employed does not allow us to firmly conclude that. For instance, it remains unclear whether the present findings are a product of innate and/or learned motivation to pursue facial beauty. Moreover, recent work in homosexual individuals has demonstrated that the response to faces is modulated by sexual preference, but not necessarily by gender per se (Ishai, 2007; Kranz & Ishai, 2006). Hence, it would be revealing to perform a follow-up study in homosexuals to examine whether the effect of gender would reverse in this case, or some more complex pattern would emerge.
Another unanswered question raised by this experimental design concerns motivational targets that are more “wanted” by women than by men. Masculine beauty does not seem to be one, as heterosexual women's virtually identical responses to both male and female faces is also seen in other behavioral (e.g., reaction time, valuational assessments and motivational effort) and neuronal (amplitude of the fMRI signal in the orbitofrontal cortex) manifestations (Bray & O'Doherty, 2007; Kranz & Ishai, 2006). An alternative target could be parental investment in care giving and in maximizing offspring survival, particularly in the context of the mother–infant diad (Depue & Morrone-Strupinsky, 2005; Taylor et al., 2000). A prospective survey of 1450 children born with defects revealed the decisive role played by esthetic appearance in the creation and maintenance of mothers’ emotional bonds with biological children (Weiss, 1994). In this study, almost 70% of children abandoned by their parents carried a conspicuous flaw in their appearance that was neither life threatening nor affected intellectual development; only 7% of abandoned children had a serious internal organ (e.g., heart and kidneys) defect. This report underscores the motivational power of beauty and supports the hypothesis that, akin to heterosexual beauty in men, infants’ esthetic appearance may be a potent motivational enhancer for women, expressed as incentive sensitization to this stimulus.
If the hypothesis of normative incentive sensitization is further supported, an important focus for future research will be to determine whether this phenomenon generalizes beyond beauty to other motivational targets (e.g., food and money). Further research is also needed to determine gender differences in the neural substrate underlying incentive sensitization processes and how it may be involved in psychopathologies characterized by gender-specific courses, such as schizophrenia, substance use disorders, and major depression.
An additional issue to consider here, given the predictable preference and pursuit of physically attractive individuals, is that motivational and esthetic aspects of beauty are two closely related concepts. Although disentangling these “original attributes of the sexual object” (Freud, 1946) can be a daunting task (Finlayson, King, & Blundell, 2006), the rationale for the use of our paradigm, juxtaposing subjective esthetic valuations to a physical activity, construed to reflect subliminal internal states, is built upon several lines of neuroimaging and clinical data, including evidence that independently of the tasks’ nature, passive viewing of faces activates both motivational and valuational neural pathways, resulting in differential response patterns to sexually preferred images (Aharon et al., 2001; Kranz & Ishai, 2006), along with differential effects of psychopathology on the performance on these tasks (Elman et al., 2005). Nonetheless, introduction of additional paradigms, targeting other subjective aspects of “liking” responses (Berridge, 2006; Finlayson et al., 2006; Wilson & Daly, 2004; Winkielman, Berridge, & Wilbarger, 2005) than the ones captured by this study may complement our findings.
In conclusion, the data presented here suggest both similarities and differences with regard to processing of beauty by both genders. Healthy men and women displayed similar perception of heterosexual facial attractiveness, with men providing lower ratings for beautiful males. In addition, men expended substantially greater motivational effort for viewing beautiful female images than women for beautiful males. At the same time, women increased the viewing time of both beautiful male and female faces, whereas men concentrated on the beautiful females only. Gender differences in the processing of average faces were not apparent. These data suggest various degrees of facial beauty's motivational value. While, regardless of an image's gender, it appears to be a motivationally salient stimulus for women, men could be incentively sensitized to female beauty. These data call for further research aimed at understanding the distinctive features of motivational systems in men vis-à-vis those of women and their potential role in healthy functioning and in psychopathology.
Acknowledgment
The authors thank Dr. Alumit Ishai for her insightful comments on the manuscript.
Footnotes
This work was supported by Grant D.A. #017959 (I.E.) from the National Institute on Drug Abuse.
References
- Aharon I, Etcoff N, Ariely D, Chabris CF, O'Connor E, Breiter HC. Beautiful faces have variable reward value: fMRI and behavioral evidence. Neuron. 2001;32:537–551. doi: 10.1016/s0896-6273(01)00491-3. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Pleasures of the brain. Brain and Cognition. 2003;52:106–128. doi: 10.1016/s0278-2626(03)00014-9. [DOI] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine's role in reward: The case for incentive salience. Psychopharmacology (Berl) 2006;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. Parsing reward. Trends in Neurosciences. 2003;26:507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Bray S, O'Doherty J. Neural coding of reward-prediction error signals during classical conditioning with attractive faces. Journal of Neurophysiology. 2007;97:3036–3045. doi: 10.1152/jn.01211.2006. [DOI] [PubMed] [Google Scholar]
- Buss DM. Sex differences in human mate preferences: Evolutionary hypotheses tested in 37 cultures. Behavioral and Brain Sciences. 1989;12:1–49. [Google Scholar]
- Buss DM, Schmitt DP. Sexual strategies theory: An evolutionary perspective on human mating. Psychological Review. 1993;100:204–232. doi: 10.1037/0033-295x.100.2.204. [DOI] [PubMed] [Google Scholar]
- Chivers ML, Bailey JM. A sex difference in features that elicit genital response. Biological Psychology. 2005;70:115–120. doi: 10.1016/j.biopsycho.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Cunningham MR. Measuring the physical in physical attractiveness: Quasiexperiments on the sociobiology of female facial beauty. Journal of Personality and Social Psychology. 1986;50:925–935. [Google Scholar]
- Darwin C. The descent of man, and selection in relation to sex. Princeton University Press; Princeton, NJ: 1871. [Google Scholar]
- Depue RA, Morrone-Strupinsky JV. A neurobehavioral model of affiliative bonding: Implications for conceptualizing a human trait of affiliation. Behavioral and Brain Sciences. 2005;28:313–350. doi: 10.1017/S0140525X05000063. [DOI] [PubMed] [Google Scholar]
- Diamond LM. What does sexual orientation orient? A biobehavioral model distinguishing romantic love and sexual desire. Psychological Review. 2003;110:173–192. doi: 10.1037/0033-295x.110.1.173. [DOI] [PubMed] [Google Scholar]
- Elman I, Ariely D, Mazar N, Aharon I, Lasko NB, Macklin ML, Orr SP, Lukas SE, Pitman RK. Probing reward function in post-traumatic stress disorder with beautiful facial images. Psychiatry Research. 2005;135:179–183. doi: 10.1016/j.psychres.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Elman I, Borsook D, Lukas SE. Food intake and reward mechanisms in patients with schizophrenia: Implications for metabolic disturbances and treatment with second-generation antipsychotic agents. Neuropsychopharmacology. 2006;31:2091–2120. doi: 10.1038/sj.npp.1301051. [DOI] [PubMed] [Google Scholar]
- Evans AH, Pavese N, Lawrence AD, Tai YF, Appel S, Doder M, Brooks DJ, Lees AJ, Piccini P. Compulsive drug use linked to sensitized ventral striatal dopamine transmission. Annals of Neurology. 2006;59:852–858. doi: 10.1002/ana.20822. [DOI] [PubMed] [Google Scholar]
- Finlayson G, King N, Blundell JE. Is it possible to dissociate “liking” and “wanting” for foods in humans? A novel experimental procedure. Physiology and Behavior. 2006;90:36–42. doi: 10.1016/j.physbeh.2006.08.020. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. (SCID-I/NP) Biometrics Research, New York State Psychiatric Institute; New York: 2002. Structured clinical interview for DSM-IV-TR axis I disorders, research version, non-patient edition. [Google Scholar]
- Freud S. In: Civilization and its discontents. Joan Riviere, Trans., editor. Hogarth Press; London, UK: 1946. [Google Scholar]
- Ishai A. Sex, beauty and the orbitofrontal cortex. International Journal of Psychophysiology. 2007;63:181–185. doi: 10.1016/j.ijpsycho.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: A pathology of motivation and choice. American Journal of Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kapur S. Psychosis as a state of aberrant salience: A framework linking biology, phenomenology, and pharmacology in schizophrenia. American Journal of Psychiatry. 2003;160:13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Ventral striatal control of appetitive motivation: Role in ingestive behavior and reward-related learning. Neuroscience and Biobehavioral Reviews. 2004;27:765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Kernberg O. Love relations: Normality and pathology. Yale University Press; New Haven, CT: 1995. [Google Scholar]
- Klein F, Sepekoff B, Wolf TJ. Sexual Orientation: A multi-variable dynamic process. Journal of Homosexuality. 1985;11:35–49. doi: 10.1300/J082v11n01_04. [DOI] [PubMed] [Google Scholar]
- Kranz F, Ishai A. Face perception is modulated by sexual preference. Current Biology. 2006;16:63–68. doi: 10.1016/j.cub.2005.10.070. [DOI] [PubMed] [Google Scholar]
- O'Doherty J, Winston J, Critchley H, Perrett D, Burt DM, Dolan RJ. Beauty in a smile: The role of medial orbitofrontal cortex in facial attractiveness. Neuropsychologia. 2003;41:147–155. doi: 10.1016/s0028-3932(02)00145-8. [DOI] [PubMed] [Google Scholar]
- Rhodes G. The evolutionary psychology of facial beauty. Annual Review of Psychology. 2006;57:199–226. doi: 10.1146/annurev.psych.57.102904.190208. [DOI] [PubMed] [Google Scholar]
- Sadalla EK, Kenrick DT, Vershure B. Dominance and heterosexual attraction. Journal of Personality and Social Psychology. 1987;52:730–738. [Google Scholar]
- Senior C. Beauty in the brain of the beholder. Neuron. 2003;38:525–528. doi: 10.1016/s0896-6273(03)00293-9. [DOI] [PubMed] [Google Scholar]
- Saper CB, Chou TC, Elmquist JK. The need to feed: Homeostatic and hedonic control of eating. Neuron. 2002;36:199–211. doi: 10.1016/s0896-6273(02)00969-8. [DOI] [PubMed] [Google Scholar]
- Symons D. The evolution of human sexuality. Oxford University Press; New York, NY: 1979. [Google Scholar]
- Taylor SE, Klein LC, Lewis BP, Gruenewald TL, Gurung RA, Updegraff JA. Biobehavioral responses to stress in females: Tend-and-befriend, not fight-or-flight. Psychological Review. 2000;107:411–429. doi: 10.1037/0033-295x.107.3.411. [DOI] [PubMed] [Google Scholar]
- Weiss M. Conditional love: Parents’ attitudes toward handicapped children. Bergin & Garvey; Westport, CT: 1994. [Google Scholar]
- Wilson M, Daly M. Do pretty women inspire men to discount the future? Proceedings of the Royal Society B: Biological Sciences. 2004;271:S177–S179. doi: 10.1098/rsbl.2003.0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkielman P, Berridge KC, Wilbarger JL. Unconscious affective reactions to masked happy versus angry faces influence consumption behavior and judgments of value. Personality and Social Psychology Bulletin. 2005;31:121–135. doi: 10.1177/0146167204271309. [DOI] [PubMed] [Google Scholar]