Abstract
Obesity is a disorder with complex genetic etiology, and its epidemic is a worldwide problem. Although multiple genetic loci associated with body mass index (BMI), the most common measure of obesity, have been identified in European populations, few studies have focused on Asian populations. Here, we report a genome-wide association study (GWAS) and replication studies with 62,245 East Asian subjects, which identified two novel BMI-associated loci in the CDKAL1 locus at 6p22 (rs2206734, P = 1.4 × 10−11) and the KLF9 locus at 9q21 (rs11142387, P = 1.3 × 10−9), as well as previously reported loci (the SEC16B, BDNF, FTO, MC4R, and GIPR loci; P < 5.0 × 10−8). We subsequently performed gene–gene interaction analysis and identified an interaction (P = 2.0 × 10−8) between SNPs in the KLF9 locus (rs11142387) and the GDF8 locus at 2q32 (rs13034723). These findings should provide useful insights into the etiology of obesity.
Obesity is a major risk factor for a number of chronic diseases, and its recent rise in worldwide prevalence imposes serious medical and economic burdens1. It is well known that obesity is a highly heritable trait and around 40–70% of inter-individual variation is attributable to genetic factors2. Recently, genome-wide association studies (GWASs) have identified dozens of genetic loci associated with body mass index (BMI), the most common measure of obesity3–12. However, most of these studies were conducted in European populations, and few studies have assessed Asian populations5,11, which account for two-thirds of the world’s population. The degree of adiposity and the risks of diseases exacerbated by obesity are greater in Asians than in Europeans when evaluated with the same BMI13. Thus, the study of Asian populations might lead to the identification of novel associated loci and provide novel insight into the genetic architecture of obesity. We report herein a large-scale GWAS and replication studies of BMI examining a total of 62,245 subjects from East Asian populations.
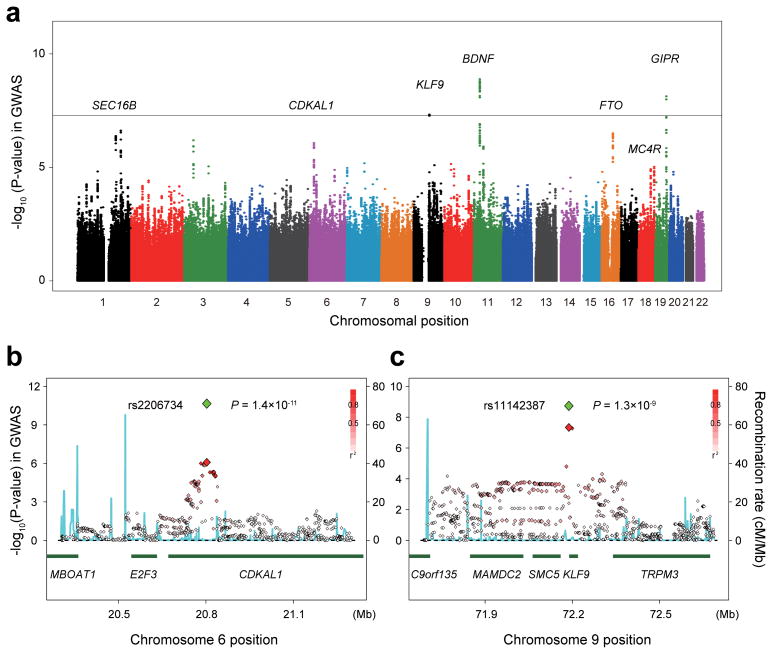
In the GWAS for BMI, we enrolled 26,620 Japanese subjects under the support of the BioBank Japan Project14 (Supplementary Table 1 and Supplementary Figure 1). Stringent quality control criteria, including principal component analysis (PCA) for evaluating potential population stratifications, were applied as previously described15. To extend coverage to the genomic region, whole-genome imputation was performed for SNPs that were not genotyped, and the genotype data of 2,178,018 autosomal SNPs with minor allele frequency (MAF) ≥ 0.01 was obtained. Each SNP was evaluated for association with BMI using a linear regression model, assuming additive effects of allele dosages on the rank-based inverse normal transformed values of BMI. Although no significant population stratification was suggested in our study population (Supplementary Figure 2) or in our previous studies for Japanese15, for robustness we applied genomic control corrections for the results of the GWAS using inflation factor, λGC, of 1.123 (referenced λGC_1000 = 1.005)16. The Quantile-Quantile plot of P-values indicated remarkable discrepancy in its tail from the null hypothesis (Supplementary Figure 3), which suggested the presence of significant associations in this GWAS. We identified significant associations in three chromosomal loci (the KLF9 locus at 9q21, the BDNF locus at 11p14, and the GIPR locus at 19q13) that satisfied the genome-wide significance threshold of P < 5.0 × 10−8 (Table 1 and Figure 1a).
Table 1.
Associations of the GWAS and the replication studies for BMI.
| rsIDa | Chr | Position | Cyto band | Nearest Gene | Class | A1/A2b | East Asian populations
|
European populations (GIANT consortium)g
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GWAS
|
Replication studye
|
Combined
|
Explained variancef | |||||||||||||
| Freq.c | Beta (SE)d | P | Beta (SE)d | P | Beta (SE)d | P | Beta (SE)d | P | ||||||||
| Significantly associated SNPs (P < 5.0 × 10−8) | ||||||||||||||||
| rs12149832 | 16 | 52,400,409 | 16q12 | FTO | intron | A/G | 0.20 | 0.056 (0.011) | 3.2×10−7 | 0.090 (0.011) | 5.1×10−17 | 0.073 (0.008) | 4.8×10−22 | 0.20% | 0.077 (0.005) | 5.6×10−58 |
| rs2030323 | 11 | 27,685,115 | 11p14 | BDNF | intron | C/A | 0.60 | 0.054 (0.008) | 1.3×10−9 | 0.040 (0.008) | 1.8×10−7 | 0.046 (0.006) | 3.8×10−16 | 0.08% | 0.042 (0.006) | 5.7×10−13 |
| rs11671664 | 19 | 50,864,118 | 19q13 | GIPR | intron | G/A | 0.45 | 0.051 (0.008) | 7.4×10−9 | 0.041 (0.009) | 5.6×10−6 | 0.046 (0.006) | 6.8×10−14 | 0.08% | 0.029 (0.009) | 0.0012 |
| rs2206734 | 6 | 20,802,863 | 6p22 | CDKAL1 | intron | C/T | 0.59 | 0.043 (0.008) | 8.3×10−7 | 0.035 (0.008) | 6.2×10−6 | 0.039 (0.006) | 1.4×10−11 | 0.06% | 0.017 (0.006) | 0.0049 |
| rs2331841 | 18 | 55,979,617 | 18q21 | MC4R | intergenic | A/G | 0.25 | 0.045 (0.011) | 1.2×10−5 | 0.047 (0.009) | 1.9×10−7 | 0.046 (0.007) | 1.8×10−11 | 0.08% | 0.035 (0.005) | 1.2×10−13 |
| rs11142387 | 9 | 72,188,152 | 9q21 | KLF9 | intergenic | C/A | 0.46 | 0.048 (0.008) | 4.6×10−8 | 0.028 (0.011) | 0.0084 | 0.040 (0.007) | 1.3×10−9 | 0.04% | 0.003 (0.005) | 0.50 |
| rs516636 | 1 | 176,122,140 | 1q25 | SEC16B | intergenic | A/C | 0.22 | 0.053 (0.011) | 4.2×10−7 | 0.044 (0.014) | 0.0014 | 0.050 (0.008) | 3.4×10−9 | 0.07% | 0.023 (0.027) | 0.40 |
| SNPs with suggestive associations (5.0 × 10−8 ≤ P < 5.0 × 10−5) | ||||||||||||||||
| rs4377469 | 3 | 42,278,078 | 3p22 | CCK | intron | T/G | 0.69 | 0.050 (0.010) | 6.4×10−7 | 0.022 (0.012) | 0.058 | 0.039 (0.007) | 1.6×10−7 | - | 0.018 (0.033) | 0.58 |
| rs10993160 | 9 | 96,108,747 | 9q22 | ZNF169 | intergenic | A/G | 0.83 | 0.061 (0.014) | 7.9×10−6 | 0.041 (0.016) | 0.012 | 0.053 (0.011) | 5.5×10−7 | - | 0.025 (0.012) | 0.035 |
SNPs that satisfied P < 5.0 × 10−5 in the combined study are indicated.
The allele that increased BMI is denoted as allele 1 and is indicated based on forward strand and NCBI Build 36.
Frequency of allele 1.
Effect size of allele 1 on the normalized BMI (mean = 0, standard deviation = 1).
Combined results of three independent replication sets (Supplementary Fig. 1).
Estimated based on the effect sizes in the replication studies and the allele frequencies in HapMap East Asian populations.
Referenced using the results of the genome-wide meta-analysis for BMI in European populations12.
BMI, body mass index; GWAS, Genome-wide association study; SE, standard error.
Figure 1.
Results of the genome-wide association study (GWAS) for BMI. (a) Manhattan plot showing the −log10 (P-values) of the SNPs in the GWAS for BMI in 26,620 Japanese subjects. The genetic loci that satisfied the genome-wide significance threshold of P < 5.0 × 10−8 in the combined study of the GWAS and the replication studies are labeled. The gray horizontal line represents the threshold of P = 5.0 × 10−8. Regional plots of the SNPs (b) in the CDKAL1 locus and (c) in the KLF9 locus. The red diamond-shaped dots represent −log10 (P-values) of the SNPs in the GWAS, and the green dots represent the P-value of the most significantly associated SNP in each of the loci in the combined study. The density of the red color in the small-sized dots represents the r2 value with the most significantly associated SNP of the large-sized red dot. The blue line shows the recombination rates given by the HapMap Phase II East Asian populations (release 22). The lower part indicates the RefSeq genes in the loci.
To further validate the associations identified in the GWAS, we performed replication studies on three independent sets (Supplementary Table 1 and Supplementary Figure 1). The first and second sets consisted of 3,763 and 4,147 Japanese subjects from the BioBank Japan Project14, respectively. The third set consisted of 27,715 subjects enrolled in the concurrently conducted meta-analysis of GWASs for BMI with cohorts of East Asians17. First, 36 SNPs most significantly associated in each of the loci with P < 5.0 × 10−5 in GWAS were evaluated in the first replication set, and then, 11 SNPs with P < 5.0 × 10−5 in the combined study of GWAS and replication set 1 were further evaluated in replication sets 2 and 3. Through the combined results of the GWAS and the replication studies, we identified a total of seven loci that satisfied the genome-wide significance threshold (Table 1), which included the three loci that originally satisfied the threshold in the GWAS. Among the seven identified loci, five were previously identified to be associated with BMI in Europeans (the SEC16B, BDNF, FTO, MC4R, and GIPR loci at 1q25, 11p14, 16q12, 18p21, and 19q13, respectively)3–9,11,12. Associations at the remaining two loci (6p22 and 9q21) have not been previously reported, including the large-scaled study of Europeans12, and were novel findings to our knowledge. The landmark SNP at 6p22 (rs2206734, P = 1.4 × 10−11; Figure 1b) was located in the coding region of CDKAL1, the gene encoding cyclin-dependent kinase 5 (CDK5) regulatory subunit associated protein 1-like 1. The other SNP at 9q21 (rs11142387, P = 1.3 × 10−9; Figure 1c) was located in the promoter region of KLF9, the gene encoding Krüppel-like factor 9 (also known as basic transcription element-binding protein1; BTEB1). The LD block including rs11142387 also covered several genes, such as MAMDC2, SMC5, and TRPM3 (Figure 1c). Although we examined tag CNVs and expression analysis of rs11142387 in the locus using publicly available database, no significant findings were observed (Supplementary Figure 4). Our study demonstrated suggestive associations (5.0 × 10−8 ≤ P < 5.0 × 10−5) in the CCK (cholecystokinin) locus at 3p22 (rs4377469, P = 1.6 × 10−7) and in the ZNF169 (zinc finger protein 169) locus at 9q22 (rs10993160, P = 5.5 × 10−7). Combination of these identified loci (P < 5.0 × 10−8) explained 0.72% of inter-individual variance in BMI, of which the FTO locus explained the largest proportion (0.20%, Table 1).
To evaluate ethnic differences in the genetics of obesity, associations of the loci with confirmed or suggestive associations (P < 5.0 × 10−5) were further evaluated in Europeans by using the results of meta-analysis for 123,865 subjects by the GIANT consortium (Table 1)12. We found the same directional effects of alleles in all the nine evaluated loci. Significant associations were observed in five loci (P < 0.028, false discovery rate (FDR) < 0.05), including the CDKAL1 locus (P = 0.0049), whereas no association could be observed in the KLF9 locus (P = 0.50).
Using our data, we then evaluated the associations of the previously reported BMI-associated loci, most of which had been identified in Europeans (Supplementary Table 2)3–12,18. Our study replicated the associations with the same directional effects of the alleles at 10 loci, including the TMEM18, RBJ-ADCY3-POMC, GNPDA2, FLJ35779-HMGCR, TFAP2B, TRHR, MTCH2, MAP2K5-LBXCOR1, SH2B1-ATP2A1, and BMP2 loci, in addition to the five loci that have been already replicated in our GWAS (P < 0.02, FDR < 0.05). As for the loci reported in Koreans11, we replicated the association in FTO, but not in LOC729076 at 6q24 (Supplementary Table 2).
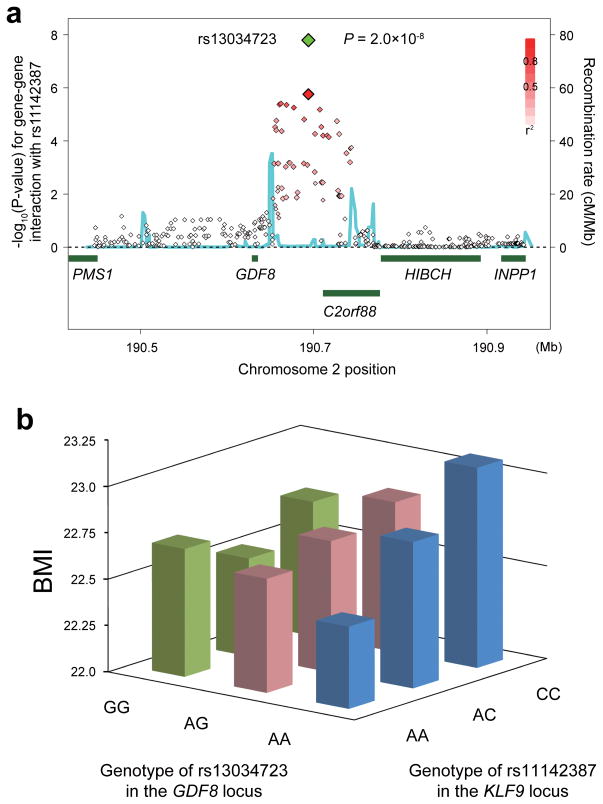
Because obesity is a polygenic trait and epistasis may help dissect its genetic background19, we performed gene–gene interaction analysis of BMI. We evaluated gene–gene interaction assuming additive × additive effects of SNPs20 between each of the seven SNPs confirmed to be associated with BMI and all of the 2,178,018 genome-wide SNPs (Supplementary Figure 5) using the subjects enrolled in the GWAS, and subsequently conducted a replication study. We found an interaction that satisfied the significance threshold (P < 5.0 × 10−8) between rs11142387 at KLF9 and a SNP located in the promoter region of GDF8 (growth and differentiation factor-8, also known as myostatin; MSTN) at 2q32 (rs13034723, P = 2.0 × 10−8; Table 2 and Figure 2a). Interestingly, the association of rs13034723 at the GDF8 locus with BMI was not significant (P = 0.56; Supplementary Table 3). When the association of rs11142387 was stratified by genotypes of rs13034723, a significant association of rs11142387 with BMI was observed in the subjects with AA genotypes at rs13034723 (P = 8.7 × 10−15; Supplementary Table 4 and 5 and Figure 2b), although no significant association was observed for the subjects with AG or GG genotypes (P = 0.0029 and 0.30, respectively).
Table 2.
Gene–gene interaction for BMI between the KLF9 and GDF8 loci
| Independent variables in the regression model
|
Associations with BMI in the regression model
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| rsID | Cytoband | Gene | A1/A2a | GWAS
|
Replication studyc
|
Combined
|
|||
| Beta (SE)b | P | Beta (SE)b | P | Beta (SE)b | P | ||||
| rs11142387d | 9q21 | KLF9 | C/A | 0.088 (0.012) | 8.3×10−14 | 0.117 (0.027) | 1.7×10−5 | 0.093 (0.011) | 1.1×10−17 |
| rs13034723d | 2q32 | GDF8 | A/G | −0.065 (0.015) | 2.8×10−5 | −0.068 (0.029) | 0.018 | −0.065 (0.014) | 1.5×10−6 |
| rs11142387 × rs13034723e | - | - | - | 0.064 (0.013) | 1.7×10−6 | 0.073 (0.025) | 0.0031 | 0.066 (0.012) | 2.0×10−8 |
Based on forward strand and NCBI Build 36.
Effect size of allele 1 on the normalized BMI (mean = 0, standard deviation = 1).
Consisted of replication sets 1 and 2 (Supplementary Fig. 1).
Allele dosage of allele 1 was used as an independent variable.
Product of allele dosages of alleles 1 of rs11142387 and rs13034723 was used as an independent variable.
BMI, body mass index; GWAS, Genome-wide association study; SE, standard error.
Figure 2.
Gene–gene interaction between the KLF9 and GDF8 loci. (a) Regional plots of the SNPs. Diamond-shaped dots represent −log10 (P-values) of the SNPs for gene–gene interaction with the landmark SNP in the KLF9 locus (rs11142387). The green dot indicates the P-value of the most significantly associated SNP in the combined study, and the red dot indicates its P-value in the genome-wide gene–gene interaction analysis. The density of the red color in the small-sized dots represents the r2 value with the most significantly associated SNP of the large-sized red dot. The blue line shows the recombination rates given by the HapMap database. The lower part indicates the RefSeq genes in the locus. (b) Mean BMI values of the subjects stratified with the genotypes of rs13034723 in the GDF8 locus and rs11142387 in the KLF9 locus.
Through the GWAS and the replication studies, we identified two novel loci, CDKAL1 and KLF9, associated with BMI in East Asians. These two loci also demonstrated significant associations with the risk of obesity (BMI ≥ 27.5; P = 1.1 × 10−5 and 4.2 × 10−4; Supplementary Table 6). Compared to the results in Europeans12, the association in the CDKAL1 locus was shared between East Asians and Europeans, but not in the KLF9 locus. Because the study in Europeans12 would have enough power to detect the KLF9 locus (> 99%), under the assumption of the same effect size as East Asians and the allele frequencies in Europeans (= 0.51), ethnic heterogeneity in the effect of the KLF9 locus for BMI would be suggested. We also performed gene–gene interaction analysis and demonstrated an interaction between the KLF9 and GDF8 loci. Although the substantial role of epistasis in polygenic traits has been recognized, approach to elucidate it has been challenging20. Our findings would be one of the initial pieces of evidence for epistatic associations.
Recent studies reported the associations of the CDKAL1 locus with BMI at 8 years of age (rs4712526)21 and birthweight (rs7756992)22, and these SNPs indicated significant associations in our GWAS (P < 5.0 × 10−5). To our knowledge, our study is the first report on the association with adult BMI. The CDKAL1 locus has been reported to be a risk locus of type 2 diabetes (T2D)23,24. We found that the T allele of rs2206734, which decreased BMI, significantly increased T2D risk in our study subjects (P < 1.4 × 10−18; Supplementary Table 6). CDKAL1 risk variants for T2D were associated with decreased insulin secretion23; therefore, the observed effects of the CDKAL1 risk variant on decreasing BMI might be mediated by decreased insulin secretion. Interestingly, a recent study identified the similar patterns of the associations in the GIPR locus25, and we observed that the BMI-decreasing A allele of rs11671664 at GIPR increased T2D risk (P < 1.5 × 10−5; Supplementary Table 6). These findings suggest further studies comprehensively assessing genetic associations with T2D risk, BMI, and insulin secretion should be performed. When the subjects affected with T2D (n = 12,234) were excluded, the association of rs2206734 with BMI was not obvious (effect size = 0.031, P = 1.4 × 10−6). We evaluated the association of the CDKAL1 and GIPR loci with other related traits, including systolic and diastolic blood pressure, and serum lipid levels (total cholesterol; TC, high density lipoprotein cholesterol; HDL-C, low density lipoprotein cholesterol; LDL-C, and triglyceride; TG), although no significant association was observed (α = 0.05; Supplementary Table 6).
KLF9 is a member of zinc-finger transcription factors involved in various physiological processes. A recent study indicated KLF9 as a pro-adipogenic transcription factor acting through transactivation of PPARγ26, a key component of adipocyte differentiation implicated in obesity27. Zobel et al. reported the association of the KLF7 locus with obesity in the Danish population, although we could not test its relevance because the reported SNP, rs7568369, was not polymorphic (Supplementary Table 2)18. It is known that KLF5, a gene belonging to the KLF family and also known as BTEB2, regulates adipocyte differentiation28. Considering these observations, the association of the KLF9 locus with BMI would be plausible from a biological aspect. Contrary to the CDKAL1 locus, no significant associations of KLF9 with T2D risk, or with other related traits, were observed (α = 0.05; Supplementary Table 6).
GDF8 is a member of the transforming growth factor-beta (TGF-β) superfamily that regulates mesenchymal stem cell proliferation29. A loss-of function mutation in GDF8 causes muscle hypertrophy and decreased body fat29,30. In our study, the SNP in the GDF8 locus was not associated with BMI, but its genotypes clearly stratified the association in the KLF9 locus. This would pose the regulatory role of GDF8 on the effect of KLF9 on BMI, and further studies evaluating functional epistasis would be desirable. Notably, Grade et al. identified similar conserved promoter/enhancer architecture in KLF9 and GDF8 through a search of evolutionary conserved regions, which suggests these two genes may form a synexpression group31. Other genes located near GDF8, such as C2orf88, could also be candidates, and the relatively small sample size in the replication studies provided limited evidence.
Wen et al. concurrently reported a genome-wide meta-analysis for BMI using data of eight cohorts of East Asians17. The subjects enrolled in these two studies overlapped due to reciprocal replication approaches, and newly identified loci were shared at CDKAL1, while some loci were specifically identified in each study, such as KLF9. This could be attributed to differences in study designs, effects of different compositions of the populations in discovery phases, and probability of study-specific bias induced by winner’s curse effect.
In summary, our study identified novel associations of the CDKAL1 and KLF9 loci with BMI in East Asians. A gene–gene interaction between the KLF9 and GDF8 loci was also found. Our study should contribute to understanding of the genetic architecture of obesity.
ONLINE METHODS
Subjects
The subjects enrolled in the GWAS (n = 26,620) and the replication sets 1 and 2 (n = 3,763 and n = 4,147, respectively) were obtained from the BioBank Japan Project14 at the Institute of Medical Science, the University of Tokyo, which consisted of patients of 32 diseases (Supplementary Table 1). Subjects with ages < 18 or > 85, with dialysis treatment, or who were determined as being of non-Japanese origin by self-report or by PCA in the GWAS or our previous study15 were not included. Clinical information on the subjects including age (mean ± SD; 61.3 ± 12.9), gender (47.2% for female), and smoking history (49.8% for ever-smoker) were collected by a standard questionnaire. BMI (mean ± SD; 22.7 ± 3.59) were calculated based on self-reported height and weight. BMI based on self-reported data is known to be highly correlated (r > 0.94) with that based on measurements32, and potential bias induced by self-reported data may have little impact on the analysis32,33. All participants provided written informed consent as approved by the ethical committees of the RIKEN Yokohama Institute and the Institute of Medical Science, University of Tokyo. The subjects enrolled in the replication set 3 (n = 27,715) consisted of subjects from the eight cohorts of East Asian populations, and they were enrolled in the discovery stage of the concurrent meta-analysis for BMI17. The subjects in our GWAS were also enrolled in the replication stage of the meta-analysis17.
Genotyping and quality control
We used the data of 32 GWAS performed for the BioBank Japan Project, in which patients of each of the 32 diseases were genotyped (Supplementary Table 1)14. In the GWAS, genotyping was performed using the Illumina HumanHap610-Quad Genotyping BeadChip (Illumina, CA, USA). After excluding the subjects with call rates lower than 0.98, we excluded SNPs with call rates lower than 0.99, SNPs with ambiguous calls, or non-autosomal SNPs. We excluded subjects in close kinships based on estimations using identity-by-state (IBS). We considered the subject pairs with an average proportion of alleles shared by IBS > 1.7 to be in first or second degree of kinship, and excluded the member of the pair with lower call rates. We also excluded subjects whose ancestries were estimated to be distinct from the other subjects using PCA performed by EIGENSTRAT version 2.0. We performed PCA for the genotype data of our study along with the genotype data of unrelated European (CEU), African (YRU), and East Asian (Japanese and Han Chinese; JPT + CHB) individuals obtained from the Phase II HapMap database (release 24)34. Based on the PCA plot, we excluded the outliers in terms of ancestry from JPT + CHB clusters (Supplementary Figure 1). We then excluded the SNPs with MAF < 0.01 or the SNPs with exact P-value of the Hardy-Weinberg equilibrium test < 1.0 × 10−7 and obtained genotype data of 480,103 SNPs for 26,620 subjects.
Genotype imputation was performed using MACH 1.0 in a two step procedure. The JPT and CHB individuals obtained from Phase II HapMap database (release 24)34 were used as references. In the first step, recombination and error rate maps were estimated using 500 subjects randomly selected from the GWAS data. In the second step, genotype imputation of all subjects was conducted using the rate maps estimated in the first step. We excluded the imputed SNPs with MAF < 0.01 or Rsq values < 0.7, and obtained genotype data of 2,178,018 SNPs.
In the replication study sets 1 and 2, we used genotyping data which were performed using the Illumina HumanHap550v3 Genotyping BeadChip and the Illumina HumanOmniExpress Genotyping BeadChip (Illumina, CA, USA), respectively. We applied the same quality control criteria and imputation procedure as GWAS data. Details of the genotyping, quality control, imputation procedure in the replication set 3 are described elsewhere17.
Statistical Analysis
Genome-wide association study and the replication studies of BMI
Rank-based inverse normal transformation was applied to BMI of the subjects. In the GWAS, associations of the SNPs with transformed values of BMI were assessed by linear regression assuming additive effects of allele dosages (bound between 0.0 and 2.0) using mach2qtl software, and genomic control correction was applied35. In the regression model, gender, age, age-squared, smoking history, the affection statuses of the diseases, and the demographic classifications of the medical institutes in Japan where the subjects were enrolled36, were adopted as covariates. For the loci that satisfied P < 5.0 × 10−5 in the GWAS, replication studies were conducted consisting of three replication sets (Supplementary Table 1 and Supplementary Figure 1). In replication sets 1 and 2, the associations of the SNPs were assessed in the same manner as in the GWAS. In replication set 3, we referred the results of the discovery stage of the concurrently conducted genome-wide meta analysis of BMI17. The combined results of the studies were obtained using an inverse-variance method from the summary statistics β and the standard error (SE). Details of examination of tag CNVs and expression analysis in the KLF9 locus using publicly available database for HapMap Phase II East Asian individuals34,37 are described in Supplementary Figure 4. Associations of the SNPs that satisfied P < 5.0 × 10−5 in the combined study of the GWAS and the replication studies were further evaluated using the results of the meta-analysis for BMI in European populations by the GIANT consortium12. For the evaluation of the associations in the previously reported BMI associated loci3–12,18, the loci that exhibited FDR < 0.05 based on the number of loci reported with non-monomorphic SNPs were considered to be significant. The statistical power of the study was estimated using Quanto version 1.2.4.
The inter-individual variance in BMI explained by each of the identified loci (P < 5.0 × 10−8 in the combined study) was estimated using 2f(1−f)β2, where f is the frequency of the variant in HapMap East Asian populations and β is its additive effect size on the BMI obtained from the replication studies. To estimate the variance explained by the combination of the identified loci, we calculated the genetic risk scores for the subjects in the GWAS, by summing the dosages of BMI-increasing alleles carried by the subjects, weighted by the effect sizes of the SNPs obtained from the replication studies. The explained variance was estimated from a linear regression model incorporating the score as the predictor and the covariate-adjusted inverse normal transformed BMI residuals as outcome.
Gene–gene interaction analysis of BMI
Gene–gene interactions of SNPs were evaluated using a multivariate linear regression model assuming additive × additive effects of two SNPs20. Allele dosages of the respective SNPs and the product of the allele dosages were involved in the model in addition to the covariates. The product of the allele dosages was denoted as interaction term. For each of the landmark SNPs in the loci confirmed to be associated with BMI, gene–gene interactions were evaluated with all of the genome-wide SNPs (7 × 2,178,018 SNP pairs; Supplementary Figure 5), and genomic control corrections were applied35. For SNP pairs that demonstrated P < 5.0 × 10−6 for the interaction term, replication studies using replication sets 1 and 2 were performed. The SNP pair that satisfied P < 5.0 × 10−8 in the combined study of GWAS and replication studies was considered to be significant.
Association study of metabolic traits and other related ones
Associations with obesity (BMI ≥ 27.538; 3,058 cases and 31,472 controls), type 2 diabetes (T2D; 6,526 cases and 22,689 controls), systolic and diastolic blood pressure (n = 13,049), total cholesterol (TC; n = 12,565), high density lipoprotein cholesterol (HDL-C; n = 4,924), low density lipoprotein cholesterol (LDL-C; n = 4,219), and triglyceride (TG; n = 9,747) were evaluated using the subjects enrolled in the GWAS and the replication sets 1 and 2 (Supplementary Table 6). In addition to the two novel loci associated with BMI (CDKAL1 and KLF9), we assessed the GIPR locus, where the associations with T2D and its related traits have been reported25. Case-control analysis and analyses of the quantitative traits were performed using logistic and linear regression models including the covariates, respectively. In the association analysis of T2D, subjects not affected with cardiovascular diseases were enrolled as controls, and BMI was additionally incorporated as a covariate.
R statistical software was used for the general analysis. Details of the study design are also indicated in Supplementary Figure 1.
Supplementary Material
Acknowledgments
We thank Kazuyuki Tobe and Minoru Iwata at the First Department of Internal Medicine, Faculty of Medicine, Toyama University, and Hiroshi Hirose at Health Center, Keio University School of Medicine, and all the staff of the Laboratory for Endocrinology and Metabolism, and Statistical Analysis at CGM, RIKEN for their assistance. This study was supported by the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Footnotes
AUTHOR CONTRIBUTIONS
Y.O. and T. Tanaka designed the study and drafted the manuscript. N.H. and M.K. performed the genotyping. Y.O., H.O., A.T., N. Kumasaka, and T. Tsunoda performed the statistical analysis. Y.O. and M.K. managed the clinical information. W.W., R.D., M.J.G., W.Z., N. Kato., J.W., and Q.L. managed the replication study set 3. The GIANT consortium managed the association study in Europeans. S.M., K.Y., Y.N., N. Kamatani, and T. Tanaka supervised the study.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
URLs
The URLs for data presented herein are as follows.
The BioBank Japan Project, http://biobankjp.org
EIGENSTRAT software, http://genepath.med.harvard.edu/~reich/Software.htm
MACH and mach2qtl software, http://www.sph.umich.edu/csg/abecasis/MACH/index.html
International HapMap Project, http://www.hapmap.org
Quanto software, http://hydra.usc.edu/gxe
R statistical software, http://cran.r-project.org
References
- 1.Kopelman PG. Obesity as a medical problem. Nature. 2000;404:635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 2.Maes HH, Neale MC, Eaves LJ. Genetic and environmental factors in relative body weight and human adiposity. Behav Genet. 1997;27:325–351. doi: 10.1023/a:1025635913927. [DOI] [PubMed] [Google Scholar]
- 3.Frayling TM, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu YJ, et al. Genome-wide association scans identified CTNNBL1 as a novel gene for obesity. Hum Mol Genet. 2008;17:1803–1813. doi: 10.1093/hmg/ddn072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chambers JC, et al. Common genetic variation near MC4R is associated with waist circumference and insulin resistance. Nat Genet. 2008;40:716–718. doi: 10.1038/ng.156. [DOI] [PubMed] [Google Scholar]
- 6.Loos RJ, et al. Common variants near MC4R are associated with fat mass, weight and risk of obesity. Nat Genet. 2008;40:768–775. doi: 10.1038/ng.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thorleifsson G, et al. Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nat Genet. 2009;41:18–24. doi: 10.1038/ng.274. [DOI] [PubMed] [Google Scholar]
- 8.Willer CJ, et al. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet. 2009;41:25–34. doi: 10.1038/ng.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyre D, et al. Genome-wide association study for early-onset and morbid adult obesity identifies three new risk loci in European populations. Nat Genet. 2009;41:157–159. doi: 10.1038/ng.301. [DOI] [PubMed] [Google Scholar]
- 10.Liu XG, et al. Genome-wide association and replication studies identified TRHR as an important gene for lean body mass. Am J Hum Genet. 2009;84:418–423. doi: 10.1016/j.ajhg.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho YS, et al. A large-scale genome-wide association study of Asian populations uncovers genetic factors influencing eight quantitative traits. Nat Genet. 2009;41:527–534. doi: 10.1038/ng.357. [DOI] [PubMed] [Google Scholar]
- 12.Speliotes EK, et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet. 2010;42:937–948. doi: 10.1038/ng.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deurenberg P, Deurenberg-Yap M, Guricci S. Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obes Rev. 2002;3:141–146. doi: 10.1046/j.1467-789x.2002.00065.x. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura Y. The BioBank Japan Project. Clin Adv Hematol Oncol. 2007;5:696–697. [PubMed] [Google Scholar]
- 15.Okada Y, et al. Genome-wide association study for C-reactive protein levels identified pleiotropic associations in the IL6 locus. Hum Mol Genet. 2011;20:1224–1231. doi: 10.1093/hmg/ddq551. [DOI] [PubMed] [Google Scholar]
- 16.Freedman ML, et al. Assessing the impact of population stratification on genetic association studies. Nat Genet. 2004;36:388–393. doi: 10.1038/ng1333. [DOI] [PubMed] [Google Scholar]
- 17.Wen W, et al. Meta-analysis identifies common variants associated with body mass index in East Asians. doi: 10.1038/ng.1087. In submission. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zobel DP, et al. Variation in the gene encoding Kruppel-like factor 7 influences body fat: studies of 14 818 Danes. Eur J Endocrinol. 2009;160:603–609. doi: 10.1530/EJE-08-0688. [DOI] [PubMed] [Google Scholar]
- 19.Hinney A, Vogel CI, Hebebrand J. From monogenic to polygenic obesity: recent advances. Eur Child Adolesc Psychiatry. 2010;19:297–310. doi: 10.1007/s00787-010-0096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cordell HJ. Detecting gene-gene interactions that underlie human diseases. Nat Rev Genet. 2009;10:392–404. doi: 10.1038/nrg2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Winkler C, et al. BMI at age 8 years is influenced by the type 2 diabetes susceptibility genes HHEX-IDE and CDKAL1. Diabetes. 2010;59:2063–2067. doi: 10.2337/db10-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andersson EA, et al. Type 2 diabetes risk alleles near ADCY5, CDKAL1 and HHEX-IDE are associated with reduced birthweight. Diabetologia. 2010;53:1908–1916. doi: 10.1007/s00125-010-1790-0. [DOI] [PubMed] [Google Scholar]
- 23.Steinthorsdottir V, et al. A variant in CDKAL1 influences insulin response and risk of type 2 diabetes. Nat Genet. 2007;39:770–775. doi: 10.1038/ng2043. [DOI] [PubMed] [Google Scholar]
- 24.Yamauchi T, et al. A genome-wide association study in the Japanese population identifies susceptibility loci for type 2 diabetes at UBE2E2 and C2CD4A-C2CD4B. Nat Genet. 2010;42:864–868. doi: 10.1038/ng.660. [DOI] [PubMed] [Google Scholar]
- 25.Saxena R, et al. Genetic variation in GIPR influences the glucose and insulin responses to an oral glucose challenge. Nat Genet. 2010;42:142–148. doi: 10.1038/ng.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pei H, Yao Y, Yang Y, Liao K, Wu JR. Kruppel-like factor KLF9 regulates PPARgamma transactivation at the middle stage of adipogenesis. Cell Death Differ. 2011;18:315–327. doi: 10.1038/cdd.2010.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26:439–451. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 28.Oishi Y, et al. Kruppel-like transcription factor KLF5 is a key regulator of adipocyte differentiation. Cell Metab. 2005;1:27–39. doi: 10.1016/j.cmet.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 29.Elkasrawy MN, Hamrick MW. Myostatin (GDF-8) as a key factor linking muscle mass and bone structure. J Musculoskelet Neuronal Interact. 2010;10:56–63. [PMC free article] [PubMed] [Google Scholar]
- 30.Schuelke M, et al. Myostatin mutation associated with gross muscle hypertrophy in a child. N Engl J Med. 2004;350:2682–2688. doi: 10.1056/NEJMoa040933. [DOI] [PubMed] [Google Scholar]
- 31.Grade CV, Salerno MS, Schubert FR, Dietrich S, Alvares LE. An evolutionarily conserved Myostatin proximal promoter/enhancer confers basal levels of transcription and spatial specificity in vivo. Dev Genes Evol. 2009;219:497–508. doi: 10.1007/s00427-009-0312-x. [DOI] [PubMed] [Google Scholar]
- 32.Wada K, et al. Validity of self-reported height and weight in a Japanese workplace population. Int J Obes (Lond) 2005;29:1093–1099. doi: 10.1038/sj.ijo.0803012. [DOI] [PubMed] [Google Scholar]
- 33.Nakamura K, Hoshino Y, Kodama K, Yamamoto M. Reliability of self-reported body height and weight of adult Japanese women. J Biosoc Sci. 1999;31:555–558. doi: 10.1017/s0021932099005556. [DOI] [PubMed] [Google Scholar]
- 34.The International HapMap Consortium. The International HapMap Project. Nature. 2003;426:789–796. doi: 10.1038/nature02168. [DOI] [PubMed] [Google Scholar]
- 35.Devlin B, Roeder K. Genomic control for association studies. Biometrics. 1999;55:997–1004. doi: 10.1111/j.0006-341x.1999.00997.x. [DOI] [PubMed] [Google Scholar]
- 36.Yamaguchi-Kabata Y, et al. Japanese population structure, based on SNP genotypes from 7003 individuals compared to other ethnic groups: effects on population-based association studies. Am J Hum Genet. 2008;83:445–456. doi: 10.1016/j.ajhg.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stranger BE, et al. Population genomics of human gene expression. Nat Genet. 2007;39:1217–1224. doi: 10.1038/ng2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.