Abstract
Progesterone is a key hormone in the regulation of uterine function. In the normal physiological context, progesterone is primarily involved in remodeling of the endometrium and maintaining a quiescent myometrium. When pathologies of the uterus develop, specifically, endometrial cancer and uterine leiomyoma, response to progesterone is usually altered. Progesterone acts through mainly two isoforms of the progesterone receptor (PR), PRA and PRB which have been reported to exhibit different transcriptional activities. Studies examining the expression and function of the PRs in the normal endometrium and myometrium as well as in endometrial cancer and uterine leiomyoma are summarized here. The clinical use of progestins and the transcriptional activity of the PR on genes specific to endometrial cancer and leiomyoma are described. An increased understanding of the differential expression of PRs and response to progesterone in these two diseases is critical in order to develop more efficient and targeted therapies.
I. Introduction
The progesterone receptor (PR) has been the focus of extensive analysis over the past few decades given its significance in reproductive tissues. The uterus is one of the most highly responsive organs to progesterone. Based on PR function, certain modalities of treatment for uterine pathologies have involved synthetic progestins or selective progesterone receptor modulators (SPRM). These compounds have proven to be effective in certain cases of endometrial cancer or uterine leiomyoma. Studies investigating the expression of PRs, and action of progesterone through its receptor in endometrial cancer and leiomyoma are summarized here. A brief description of PR expression and progesterone action in the normal endometrium and myometrium followed by a description of the clinical studies using progestins and SPRMs and the transcriptional activity of the PR on genes specific to endometrial cancer and leiomyoma will be presented.
II. The Uterus
The uterus is the major female reproductive organ where the fetus develops during pregnancy. During development, the uterus develops from the middle to upper portion of the paramesonephric duct, also known as the Mullerian ducts.1 The uterus further organizes into distinct layers: the outer-most layer which consists of smooth muscle is the myometrium and the innermost layer which lines the uterine cavity is the endometrium (Fig. 1A). The endometrium consists of a layer of columnar luminal epithelium supported by cellular stroma containing tubular glands (Fig. 1B). The luminal and glandular cells of the endometrium originate from the paramesonephric duct epithelium while the stroma originates from the mesenchyme surrounding the urogenital ridge. It is also from this mesenchyme that the myometrium forms. The myometrium consists of an organized network of smooth muscle cells with supporting stromal and vascular tissue (Fig. 1B). During pregnancy, the myometrium stretches by expanding the size and number of the smooth muscle cells and contracts in a coordinated fashion during labor. After pregnancy the uterus returns to its nonpregnant size. Both the endometrium and myometrium are highly responsive to the steroid hormones, estrogen and progesterone, and represent one of the most dynamic sites of hormone action during the menstrual cycle and pregnancy.
Fig. 1.
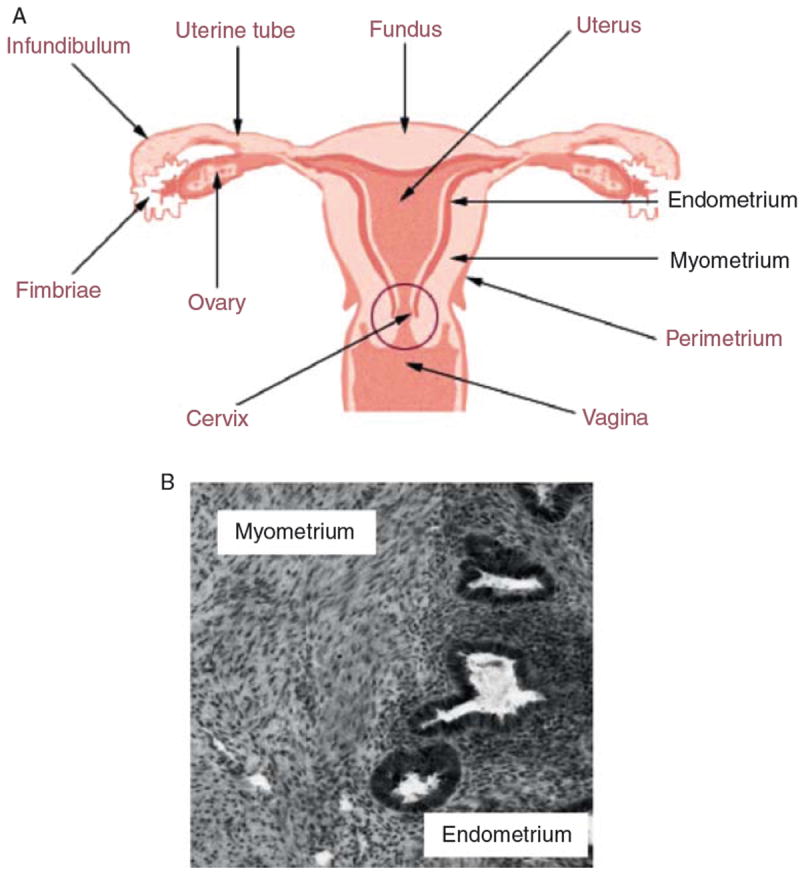
(A) The human uterus is comprised of an outer smooth muscle layer termed the myometrium and the innermost layer which lines the uterine cavity termed the endometrium. (B) Cross section of human uterine tissue shows distinct morphology of the myometrium and endometrium. The myometrium consists of smooth muscle cells with supporting stroma and vasculature. The endometrium consist mainly of epithelial glands and stroma.
III. Progesterone Action on the Endometrium and Myometrium
A. Physiological Response to Progesterone
The ovary is the major source of estrogen and progesterone in the human, synthesizing and secreting these hormones in a cyclical fashion.2 Granulosa cells from developing primary follicles biosynthesize and secrete estrogen and after ovulation these granulosa cells mature to form the corpus luteum which actively secretes progesterone and estrogen during the secretory phase of the menstrual cycle. If there is no pregnancy, the corpus luteum regresses resulting in the decline of estrogen and progesterone levels. If there is a pregnancy, the corpus luteum continues to grow and function for several months, after which time, it will regress as the placenta begins to synthesize estrogen and progesterone.
The endometrium undergoes extensive remodeling in response to ovarian steroid hormones. Estrogen promotes proliferation and growth of the endometrial lining while progesterone antagonizes estrogen driven growth as well as promotes differentiation in preparation of an impending implantation.3 Specifically, when progesterone levels are high during the luteal phase of the menstrual cycle, the glandular epithelium transforms from relatively inactive cells full of free ribosomes to very active polarized cells, containing giant mitochondrial profiles, intracellular deposits of glycogen/glycoprotein-rich material, and a complex intranuclear channel system.4 Morphologically, the glands become tortuous and have large lumens due to increased secretory activity. In parallel, the underlying stroma becomes very edematous as a result of increased capillary permeability and the cells begin to appear large and polyhedral, a transformation process termed decidualization. Decidualization begins in the stroma around the spiral arteries when progesterone levels are high during the midluteal phase, and spreads to the upper two-thirds of the endometrium.5 If embryo implantation occurs, the reaction is intensified and becomes the decidua of pregnancy. The decidualized cell biochemically expresses new proteins and two of the most abundantly secreted proteins are insulin-like growth factor binding protein-1 (IGFBP1) and prolactin (PRL) and as a result are considered markers of decidualization.6 If there is no pregnancy, levels of estrogen and progesterone decline due to the regression of the corpus luteum causing the upper two thirds of the endometrium to be shed.
The overall effect of progesterone on the myometrium is to maintain quiescence in both the pregnant and nonpregnant uterus. The nonpregnant uterus contracts throughout the menstrual cycle.7 During the late follicular phase, a progressive increase in uterine contractility parallels the rise in estradiol levels. After ovulation, during the luteal phase, the uterus undergoes a characteristic period of quiescence, strongly implicating progesterone as a mediator of this quiescence. At menses, after circulating levels of progesterone decrease, contractility increases and participates in the emptying of the uterine contents. In the pregnant uterus, it is well accepted that progesterone keeps the myometrium quiescent in order to promote and sustain pregnancy. Progesterone promotes myometrial relaxation and thought to actively block the transformation of the myometrium to a contractile phenotype. Although circulating levels of progesterone do not decrease before labor onset,8-10 the withdrawal remains a principal mechanism for the control of human parturition. Synthetic progesterone antagonists, such as RU486 initiate myometrial contractions at all stages of pregnancy.11,12
B. Progesterone Receptors
The physiologic actions of progesterone are mediated by interaction with the PR, a member of the nuclear hormone receptor superfamily of ligand-activated transcription factors.13,14 There are two predominant PR isoforms, designated PR-A and PR-B, transcribed from the same gene by two distinct promoters, with the only difference being that human PR-B is larger by an additional 164 amino acids at the amino terminus15-17 (Fig. 2). As a result, PR-A and PR-B have distinct transcriptional activities.18-25 Three activation function (AF) domains have been identified in PR as AF1, AF2, and AF3. In many contexts, PRB functions as an activator of progesterone-responsive genes, while PRA is transcriptionally inactive. In addition, PRA also functions as a strong transdominant repressor of PRB as well as the human estrogen receptor (ER) transcriptional activity.23 The precise mechanism underlying the differential activities of the two PR isoforms is not fully understood. Studies have suggested that PRA and PRB adopt different conformations within the cell which may contribute to its different transactivation functions. Tetel et al.19 demonstrated that the interaction of the amino terminus to the carboxyl terminus in PRB and PRA is different. Giangrande et al.24 demonstrated that PRA is unable to efficiently recruit the coactivators SRC-1 and GRIP1 upon agonist binding despite the fact that both PRA and PRB contain sequences within the LBD that binds coactivators. In addition, PRA interacts efficiently with the corepressor SMRT permitting it to function as a transdominant repressor.24
Fig. 2.
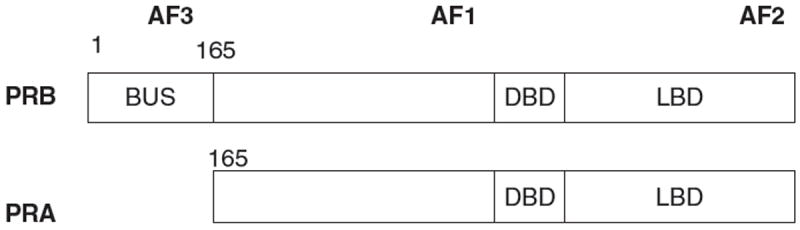
Functional domains of PRB and PRA. PRA lacks the 164 amino acids in the N-terminus and thus the activation function (AF)3 domain. The DNA binding domain (DBD), ligand binding domain (LBD) AF1 and AF2 regions are present in both PRA and PRB.
At the promoter level, PR binds to a palindromic consensus sequence called the progesterone response element (PRE). It is thought that multisite PREs promote a more stable complex.23,26 Surprisingly, a survey of proximal promoter regions of endogenous genes regulated by PR reveal a lack of tandem palindromic PREs and an abundance of PRE half-sites. This suggests that additional mechanisms are in place for PR recruitment to specific sites and activation of genes. Reports have shown that for the glucocorticoid receptor (GR), “composite” response elements which consist of a single hormone response element in tandem with heterologous binding sites leads to synergy between the GR and a variety of factors, including Sp1, NF1, CACCC-box, and AP1.27-29
C. Coregulators of PR
Nuclear receptors recruit the coregulators that perform all of the subsequent reactions needed to induce or repress expression of genes. Coactivators, such as the SRC family (steroid receptor coactivator) and CBP/p300, enhance transcription by liganded receptors while corepressors (such as SMRT and NCOR) repress transcription.30 These coregulators exist and function in large multiprotein complexes which are recruited to the target gene in rapid sequence by nuclear receptors. This complex contains many enzymes that are required for gene expression. Such enzymatic reactions for transcription include chromatin modification and remodeling, initiation of transcription, elongation of RNA chains, RNA splicing, and termination of the transcriptional response. Consequently, it is suggested that genes encoding for coregulators of hormones receptors are the true master genes of eukaryotes.30
It has been shown that upon ligand treatment, PR interacted preferentially with SRC-1, which recruited CBP and significantly enhanced acetylation at K5 of histone H4.31 In contrast, activated GR preferentially associated with SRC-2 (TIF-2/GRIP-1), which subsequently recruited pCAF and led to specific modification of histone H3, suggesting that specific coactivators are differentially recruited by different steroid hormone receptors which then recruit distinct histone acetyltransferases to modulate the transcription of steroid-responsive genes.
Investigation of the SRC family of coactivators, which consist of SRC-1, SRC-2, and SRC-3 in mice has demonstrated its relevance in steroid responsive tissues. SRC-1 knockout mice, although viable exhibited decreased responses to steroid hormone treatment.32 Ablation of SRC-2 resulted in a partial lethal phenotype due to death in the first month of life.33 Those that survived to adulthood showed slowed growth and hypofertility due to placental defects. Female SRC-3 null mice have reduced fertility. However, SRC-3 is not expressed in the mouse endometrium and the uteri of SRC-3−/− mice are able to undergo the artificially induced decidual reaction.34 Thus, it was concluded that SRC1 and SRC2 are the critical members of the p160 coactivator family for the regulation of uterine function.34 Recent studies have demonstrated the role of SRC-2 in the adult uterus using a floxed allele of SRC-2 crossed to the PR-Cre mouse which abrogated SRC-2 function only in cell lineages that express the PR.35 Absence of SRC-2 in PR-positive uterine cells was shown to result in infertility due to an early block in embryo implantation. The uterus of these mice was unable to undergo the necessary cellular and molecular changes that precede complete progesterone-induced decidual progression. The expression of a number of decidualization markers, Bmp2, Cox2, and follistatin was significantly reduced in the partially decidualized PR(Cre/+) SRC-2(flox/flox) mice. While Bmp2 induction was negligible, Cox2 and follistatin were partially induced, which was suggested to be due to SRC-2 being essential for the induction of pathways that lead to Bmp2 expression but that additional coregulators may be required for elaborating the Cox2 and follistatin expression pathways. The incomplete decidual response shown in both the PRCre/+SRC-2flox/flox mouse and the SRC-1KO32 suggests that both SRC coregulators may be required together in PR-mediated signaling cascades that result in a fully decidualized uterus. To support this hypothesis, removal of SRC-1 in these PR(Cre/+) SRC-2(flox/flox) mouse uterus resulted in the complete absence of a decidual response, confirming that both uterine SRC-2 and -1 cooperate in progesterone-initiated transcriptional programs.
It is also widely observed that other transcription factors are able to interact with and modulate function of nuclear receptors. Reports have shown that for GR, PR, and ER-alpha, other transcription factors, including members of Sp, NF, CACCC-box, and AP1 families can bind to response elements that occur in tandem to the nuclear receptor response element allowing synergy/antagonism between the steroid receptors and the other transcription factors.27-29 In the absence of canonical PRE sequences, PR can tether to some transcription factors such as Sp1, Ap-1, Stat5, p65 subunit of NF-κB,36-38 and FOXO1.39,40 Through mouse models, it has been demonstrated that FKBPs, which are immunophilins interact with PR and influence its localization.41 Specifically, FKBP4 and FKBP5 interact with PR and are expressed in the uterine stroma during implantation. Furthermore, the FKBP4 knockout mice are infertile due to the inability to support implantation or undergo adequate decidualization.
In one pioneering study, a genome-wide scan of chromosomes 21 and 22 was performed in order to identify ER binding regions.42 In doing so, the authors discovered that FOXA1 was necessary for mediating the estrogen response in breast cancer cells. Furthermore, the FOXA1 binding site was the most conserved motif proximal to the regions that had an ER element. Another forkhead protein, from a different subfamily, FOXO1 can interact with steroid hormone receptors such as ER-alpha, retinoic acid receptor, thyroid hormone receptor, and PR and elicits either repressive or activating effects on nuclear receptor mediated gene expression.39,43,44 In endometrial fibroblasts, FOXO1 and PR interacted with each other and bound to tandem DNA sequences in the IGFBP1 promoter.39,40 In addition, FOXO1 modulated PR transactivation of a PRE responsive reporter.39 Gene array studies revealed that many of the genes significantly regulated by FOXO1 during decidualization of endometrial stromal cells are also dependent on PR.40 Given that both transcription factors are involved in important cellular processes in the endometrium associated with growth inhibition and differentiation, the cross talk between these two molecules may be an important mode of endometrial remodeling.
D. Progesterone Receptors in the Endometrium and Myometrium
Progesterone is central to the remodeling that occurs in the endometrium for uterine receptivity and acts on both the epithelial and stromal compartments. Studies in mice with selective ablation of PR isoforms revealed that PR-A is necessary for ovulation and modulates the antiproliferative effects of progesterone in the uterus while PR-B is required for normal mammary gland development and function.45,46 Recent evidence has confirmed the existence of a functional third isoform, designated PR-C, which appears to play a critical role in the onset of parturition.47 The presence of multiple PR isoforms potentially increases the specificity and versatility of hormone action in a target tissue. PRs A and B are expressed in cells of the endometrium and its expression is dependent on the hormonal status and cell type. In the glandular epithelium, PR expression is stimulated by estrogen during the proliferative phase but is downregulated by its own ligand in the secretory phase. Prior to ovulation, PR-A and PR-B levels are approximately equivalent in glandular epithelium but only PR-B persists in these cells in the mid-secretory phase, suggesting that PR-B is most important for the progesterone driven phenotypic changes in the glands at this time. In the stroma, PR-A is the dominant isoform throughout the cycle.48-50 Conditions associated with endometrial pathology such as endometrial cancer is due to inadequate progesterone response as subsequently described.
Myometrial expression of PR is less dynamically regulated than the endometrium during the menstrual cycle. Studies have demonstrated that both PRA and PRB are expressed and levels are consistent throughout the menstrual cycle.51,52
IV. Endometrial Cancer
Endometrial cancer is the most common cancer of the female reproductive tract, with estimated 39,080 new cases diagnosed in 2007. Despite the frequent detection of early-stage cancers and the evolving use of adjuvant chemotherapy for advanced disease, the death rate from this malignancy has increased and currently claims 7400 lives among US women per year.53 The incidence of endometrial cancer is rising as life expectancy increases and as key risk factors, including obesity, become more prevalent. A better understanding of the pathophysiology underlying endometrial cancer is the first step to identifying key biomarkers that can improve diagnostic efforts and prevent development of this disease.
Endometrial cancer is diagnosed by pathological examination. Approximately 80% of all endometrial carcinomas are endometrioid type, which arise from endometrial glands (Fig. 3A). The malignant phenotype and the varying degrees of differentiation are easily recognizable by microscopy.54 Endometrioid carcinoma is graded histologically from grade 1 to grade 3 depending on the percentage of solid nonsquamous areas and cytological atypia.55 Most endometrioid carcinomas are well to moderately differentiated and present alongside hyperplastic endometrium. These tumors, also known as type 1 endometrial carcinomas, are associated with chronic exposure to estrogen and a lack of opposing progesterone. One source of estrogen is fat tissue, in which peripheral androgens act as aromatase substrates to produce estrone. Estrone is then converted to estradiol by 17-hydroxysteroid dehydrogenase.56 Through the androgen-to-estrone pathway, postmenopausal women produce approximately 100 mg of estrone per day, or more if they are obese.57 Chronic estrogen exposure is also exacerbated by comorbid conditions in obese women, namely hypertension and diabetes. Polycystic ovarian syndrome (PCOS) is also associated with higher levels of estrogen and androgen, as well as low levels of opposing progesterone, leading to a higher risk of endometrial cancer in these patients. Finally, the long-term use of tamoxifen, with its paradoxical estrogen agonist effect in the uterus, can also lead to type I endometrial cancer.58
Fig. 3.
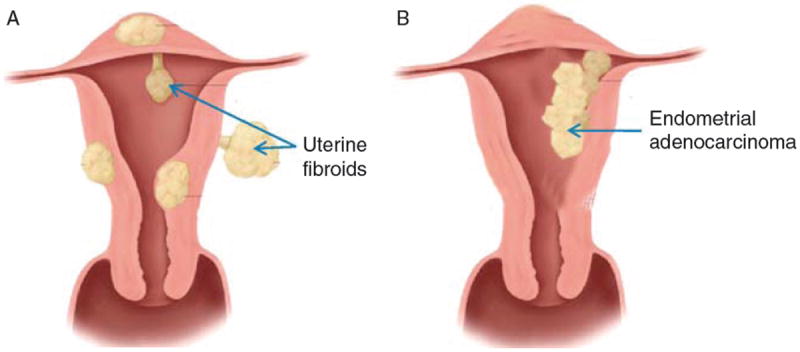
Endometrial cancer and uterine leiomyoma. (A) Endometrial adenocarcinoma arises from the endometrial glands exhibiting malignant behavior. (B) Uterine leiomyomas arise from benign overgrowth of smooth muscle cells and can range dramatically in size.
Type II endometrial cancer occurs primarily in elderly postmenopausal women, and is neither related to estrogen nor preceded by endometrial hyperplasia. Type II tumors are high grade tumors with serous or clear-cell morphology and carry a poor prognosis. Other morphological variants of endometrial cancer are placed into this category but occur at much lower rates.58,59 At the molecular level, type 1 tumors are commonly associated with abnormalities of DNA-mismatch repair genes, including k-ras, PTEN, and beta-catenin. Type 2 tumors are associated with abnormalities of p53 and HER2/neu, although they are not present in all cases.58
V. Progesterone Receptor Action in Endometrial Cancer
A. Progestin Therapy in Women
Studies have proven the efficacy of progesterone in protecting the endometrium against the hyperplastic effects of estradiol by inducing glandular and stromal differentiation.60 Accordingly, progestins play an essential and effective role in the management of endometrial hyperplasia.61,62 In a study of 52 postmenopausal women diagnosed with atypical hyperplasia or hyperplasia without atypia, 90% of patients had complete remission after treatment with 40 mg megestrol acetate per day for 42 months.63 However, close follow-up is usually recommended in women who are treated with progestins especially for atypical hyperplasia since there is a significant increased risk of progression to carcinoma.64 Progestins have been used as adjuvant for endometrial cancer in hopes to prevent recurrence. However, several studies have shown that progestin treatment is not beneficial to the overall survival of women postsurgery.65,66 Progestins are also used as primary therapy, especially for premenopausal women as a fertility-sparing treatment. Approximately 25% of endometrial cancer cases affect premenopausal women, particularly in the setting of obesity, chronic anovulation, and polycystic ovary syndrome.62,67-70 Progestin therapy would only be given when the tumor is well differentiated with positive receptors. There are few studies looking at the efficacy of progestin treatment in these women with the majority of published studies being case reports. In a review of articles published between Jan 1966 and Jan 2007 describing patients with endometrial cancer treated with hormonal therapy, 133 patients were identified, who were treated for an average duration of 6 months, and who demonstrated an average response time of 12 weeks.71 Of these 133 patients, 51% demonstrated a lasting complete response, 25% showed a temporary response, and 24% never responded to treatment. It is evident that a larger study is required to demonstrate the true benefit of progestin therapy in endometrial cancer. In regards to sparing fertility, there is no doubt that progestin therapy can be used;72 however, close follow-up is required because of the substantial rate of recurrence.
B. Progesterone Receptors in Endometrial Cancer
Morphological and biochemical evaluations demonstrated that in endometrial cancer, PRA is localized to the nucleus, even in the absence of progesterone.73 In contrast, a large proportion of PRB is cytoplasmic in the absence of ligand, but is rapidly translocated to the nucleus in the presence of progesterone. All endometrial cancer specimens demonstrated cytoplasmic PRB in 50% or more of the cells, and five of the seven tumors that were moderately to poorly differentiated demonstrated no PRB staining in the nuclei. Nuclear PRB was thus significantly associated with increasing tumor differentiation. PRA and PRB exhibit different activating properties and mediate the transcription of different sets of genes in endometrial cancer cells. Smid-Koppman et al.74 demonstrated that in the presence of progesterone, PRB expressing Ishikawa cells displayed almost complete inhibition of cell growth, while PRA expressing Ishikawa cells only displayed 50% inhibition of cell growth. In an additional study by Hanekamp et al.,75 it was demonstrated that while PRB expressing Ishikawa cells caused more tumor growth in vivo than PRA expressing Ishikawa cells, tumor growth was inhibited after administration of MPA only in the tumors expressing PRB. There is an ongoing debate as to the PR status in endometrial tumors with one study suggesting that PRB is predominant in advanced endometrial tumors,76 another study pointing to the loss of both isoforms in advanced endometrial cancer,77 and a third study that indicates only PRA is expressed in poorly differentiated endometrial carcinoma cell lines.78
C. Genes Regulated by Progestins in Endometrial Cancer
Nevertheless, in vitro studies have clearly demonstrated the efficacy of progestins to influence endometrial cancer cell behavior. When endometrial cancer cells are transfected with specifically PR-A or PR-B, progesterone can promote cell cycle inhibition, endometrial cancer cell invasion, differentiation to a secretory phenotype, induction of replicative senescence, and can down-regulate the expression of cellular adhesion molecules.79,80 Regulation of genes such as cyclin D1, matrix metalloproteinase-1 (MMP-1), -2, -7, and -9, and Ets-1 in response to progestins have been implicated to mediate the inhibition of cell growth and invasiveness.81 Primary cells from endometrial tumors also respond to progestins by significantly reducing proMMP-9, proMMP-2, and MMP-2 release.82 Progestins have been shown to induce glycodelin expression in Ishikawa cells83-85 which causes inhibition of G1/S progression and upregulation of CDKIs thereby reducing cell proliferation.86 Progestins can increase FOXO1 protein levels in Ishikawa cells, specifically through PRB87 and promote cell cycle arrest and apoptosis in these cells. Interestingly, levels of FOXO1 protein are dramatically lower in 77%88 or 95.9%87 of endometrial tumor tissues studied compared to normal tissues from cycling endometrium. Shiozawa et al.89 reported that p27 expression in hyperplastic epithelia was negligible before MPA treatment, whereas it was greatly increased after treatment. Watanabe et al. demonstrated that it is through PRB that p21 and p27 expression increases.90 Microarray studies revealed that short term (4 h) and high dose (30 μg/ml)) exposure of Ishikawa cells to progesterone result in 247 differentially expressed genes of which 126 were upregulated and 121 were downregulated. Of these, 135 genes were involved in biological processes like cell cycle, cell proliferation and differentiation, developmental processes, immune responses, intracellular protein traffic, and transport.91 Hanekamp et al.92 reported that MPA inhibits expression of several metastasis-related genes in a set of endometrial cancer cell lines. Treatment of Hec50co cells transfected with PR with progesterone for 12 h significantly regulated genes associated with cell signaling, DNA remodeling, apoptosis, tumor-suppressor, and transcription factors. Interestingly, there was a consistent modulation of cytokines consistent to an antiinflammatory environment. Specifically, proinflammatory genes such as TNFalpha, IL-1beta, and MCP-1/MCAF-1 were downregulated and antiinflammatory genes such as TRAP1 and SMAD4 were induced93. Progestins have been shown to modulate proteins in the apoptotic cascade in human endometrial precancers. Women with hyperplasia treated with either system MPA or a levonorgestrel intrauterine device exhibited increased apoptosis in the glandular cells with decreased expression of the antiapoptotic genes, Bcl-2 and BAX.94 Overexpression of PRA and PRB in endometrial cancer cells resulted in a significant progesterone-dependent inhibition of expression of a cadre of cellular adhesion molecules, including fibronectin, integrin alpha3, integrin beta1, integrin beta3, and cadherin 6.79,95 Thus, it is apparent that progesterone, through PRA and PRB modulate genes that are involved in processes associated with cell cycle, apoptosis, cell adhesion, differentiation, and inflammation in order to regulate endometrial cancer cell behavior.
D. Transcriptional Activity of Progesterone Receptors in Endometrial Cancer
It has been demonstrated that transcriptional activation by progesterone can involve other transcription factors. For example, liganded PR decreases the transcriptional activity of the activating protein-1 (AP-1) transcription factor family, and in particular, c-Jun. In addition, progesterone strongly inhibited total AP-1 as well as c-Jun recruitment to the cyclin D1 promoter, whereas it enhanced AP-1 occupancy on the p53 and p21 promoters, as shown by chromatin immunoprecipitation assays. This study concluded that in endometrial cancer cells, modulation of AP-1 activity is a potential pathway of progesterone-induced growth inhibition in endometrial cancer cells.96
Another mechanism of progesterone action has been proposed to involve inhibition of NFkappaB transcriptional activity. Specifically, expression of A20 and ABIN-2 were induced through PRB and these factors bind in a complex and inhibit NFkappaB transcriptional activity.97 EMSAs revealed the complete inhibition of NFkappaB dimer binding to DNA by both PRA and PRB. The inhibition of NFkappaB and its tumorigenic inflammatory and antiapoptotic effects by PR may be one pathway by which progesterone treatment is effective against endometrial hyperplasia and cancer.
Glycodelin (GdA) is a progesterone induced gene in normal endometrial epithelial cells and endometrial cancer cells. Studies have shown that ligand-activated PR stimulates GdA promoter activity through functional Sp1 sites.98 As on numerous other genes, PR can tether to Sp1 to regulate promoters that do not have PRE sequences.
In another study, it was shown that progesterone upregulated COMT protein expression in Ishikawa cells primarily through PRA. COMT converts genotoxic catecholestrogens to anticarcinogenic methoxyestrogens (2-ME2) in the endometrium. COMT promoter activity was differentially regulated by the three half-site PREs. Accordingly, high doses of 2-ME2 inhibited Ishikawa cell proliferation.99
A novel mechanism for PR-A and PR-B mediated gene transcription in the uterus has been proposed to involve selected KLF members. Specifically, Kruppel-like factor 9 (also known as BTEB1) interacts with ligand-activated PRB to increase PRB transactivity. This facilitates the recruitment of the transcriptional integrator CREB-binding protein within the PR dimer, and is dependent on the structure of the ligand bound by PRB. By contrast, BTEB1 does not influence agonist bound PRA transactivity, but augments PRA inhibition of PRB-mediated transactivation. Also, BTEB1 potentiates ligand-independent PRA transcriptional activity in the presence of CREB-binding protein. Similar observations were made with the BTEB1-related family members Krüppel-like family (KLF) 13/FKLF2/BTEB3 and Sp1 on PRB transactivity.100
VI. Conclusions and Perspectives of Progesterone Action in Endometrial Cancer
Endometrial adenocarcinoma is highly associated with unopposed estrogen action. The significance of progesterone in preventing estrogen-driven proliferation is underlined by its efficacy in eradicating endometrial hyperplasia and some endometrial cancers. While its role in preventing endometrial cancer may involve independent mechanisms to those that promote tumor cell death and regression, it is obvious that progesterone action is complex and involves numerous pathways and players. Despite the limitations of in vitro systems, which utilize endometrial cancer cell lines that have been propagated over many years, endometrial cancer cell behavior in response to progestins and the specific genes that are regulated have proven to be remarkably similar in these cell lines as those grown in xenograft models as well as in the tumor behavior from women. Thus far, in vivo and in vitro studies have shown that progesterone, through its receptor can regulate genes associated with cell cycle, apoptosis, cell adhesion, differentiation, and inflammation. Progesterone binds to either receptor A or B, which can then bind to specific sequences on promoters, in the presence of numerous other coregulators. Depending on the PR isoform and the predominant coregulators it associates with, progesterone can enhance or repress transcription of genes. The complexity of PR action is demonstrated by its differential action depending on the promoter region, length of progesterone exposure, and cell type. Further investigation is required to elucidate mechanisms of PR action in endometrial cancer.
It is also noteworthy that progesterone is key in differentiating the endometrial stroma. While many studies have focused on progesterone action in the stroma in the normal cycling uterus as it pertains to pregnancy and fertility, very little is known on its role in the stroma as it pertains to endometrial cancer. Evidence is strong that progestins are effective in treating endometrial hyperplasia and some endometrial cancers and studies have demonstrated that progestins can regulate endometrial cancer cell behavior. Given the significant role that progesterone plays in the stroma, it would be worth investigating how progesterone acts through the stroma to influence endometrial cancer cells. Thus, progesterone responsiveness may be dictated not only by the hyperplastic or malignant epithelium but also by the stroma.
VII. Uterine Leiomyoma
Uterine fibroids, also known as leiomyomas, are benign tumors originating from the myometrium. They are composed of smooth muscle cells and large amounts of extracellular matrix (ECM) (Fig. 3B). These tumors can range from a few millimeters to over 20 cm in size. Leiomyomas are common and can occur in up to 77% of women.101 The incidence in African-American women is 60% at age 35 and over 80% by age 50 whereas Caucasian women have an incidence of 40% by age 35 and almost 70% by age 50.102 Although the tumors are considered benign, they cause significant morbidity, pain and discomfort, and excessive menstrual bleeding. Risk factors for leiomyomas include early menarche, family history, ethnicity, increased body mass index, and tissue injury. Leiomyomas are the primary indication for over 200,000 hysterectomies in the USA.103 Studies have identified possible factors responsible for the development of leiomyomas, including chromosome rearrangements, congenitally elevated ERs, hormonal changes, and injury.104 Once the disease has set in, hormones and growth factors play a prominent role in the growth and expansion of leiomyomas.
To date, medical treatments for leiomyomas are limited and this is due to the fact that the mechanisms regulating the development and growth of these tumors remain unclear. There exists only one FDA approved drug for the treatment of uterine leiomyoma and thus there is a desperate need for new treatments for one of the most prevalent chronic public health problems in US women. It is hoped that a better understanding of leiomyomas at the molecular level would lead to a more effective treatment of this disease.
VIII. Progesterone Receptor Action in Leiomyoma
A. Relevance of Progesterone in Uterine Leiomyomas
Although the initial steps in the pathogenesis of uterine fibroids are most likely due to chromosomal aberrations and/or the effects of specific genes,105 their development is highly dependent on ovarian steroid hormones. Traditionally, estrogen has been considered the major mitogenic factor in the uterus. However, a growing body of evidence from biochemical, histological, clinical, and pharmacological studies indicates that progesterone and PR play a key role in uterine fibroid growth and development.106 Several investigators have shown an increased concentration of both PR-A and PR-B in leiomyoma tissue compared with adjacent myometrium.51,107,108 Furthermore, there was an increase in mitotic activity in fibroid tissue relative to the adjacent myometrial tissue during the luteal phase109 and after treatment with medroxyprogesterone acetate.110 Increased expression of the proliferation marker Ki67 in leiomyoma compared with the normal myometrium has also been described, and its upregulation was linked to progesterone.107 Epidermal growth factor (EGF) mRNA was increased in leiomyomata only during the secretory phase of the cycle, suggesting that progesterone, not estrogen, controls the expression of this important growth factor.111 In addition, in vitro studies showed that progesterone suppresses apoptosis and stimulates proliferation of leiomyoma cells.112-116 Progesterone markedly increased BCL2 protein expression in primary leiomyoma cell cultures.112-116
Clinical studies with both progestins and RU486 indicate that progesterone may be at least as important as estrogen for regulating fibroid growth. When used as add-back therapy in combination with GnRH agonists, the synthetic progestins medroxyprogesterone acetate and norethindrone attenuate or reverse the inhibitory effects of GnRH agonists on leiomyoma size.117,118 The effects of pregnancy on leiomyoma size have been studied as a possible model for in vivo exposure to high levels of progesterone.119-122 The greatest increase in volume of uterine leiomyomata occurred before the 10th week of gestation.119 Those investigators who followed the leiomyoma size longitudinally after the first trimester, however, did not observe a further difference between the second and third trimester.120-122
The strongest current evidence for possible in vivo mitogenic effect of progesterone on leiomyoma growth comes from clinical trials indicating that four different antiprogestins, RU486, asoprisnil, proellex, and CDB2914 consistently reduced tumor size.123-131 The original studies of Murphy and coworkers in the 1990s suggested that RU486 might be used in the medical management of uterine leiomyomata. Pilot studies indicated that the size of leiomyomata decreased significantly after treatment with RU486.123-125 Early studies indicated that different doses of RU486 decreased leiomyoma size as well as associated excessive uterine bleeding.126 A similar endometrial histology, characterized by hyperplastic glands and stroma, was observed in patients treated with the antiprogestins RU486 and asoprisnil.127 It was subsequently shown that asoprisnil also acts primarily as a progesterone antagonist in the endometrium.132 A number of investigators have attempted to avoid the side effect of endometrial hyperplasia by decreasing the dose of RU486 to 5 mg/day; this dose has been shown to successfully decrease leiomyoma size and uterine bleeding associated with these tumors.125,130,131 Importantly, treatment with RU486 given at a dose of 5 mg/day did not cause endometrial hyperplasia.131 Despite the number of mechanisms proposed for these effects,133-143 a full understanding of the pathophysiology responsible for progesterone-dependent growth and the mechanisms underlying the observed therapeutic effects of antiprogestins remain unclear.
B. Progesterone Action on Genes Associated with Proliferation, Apoptosis, and ECM Deposition
Although data focusing on the genes regulated in leiomyoma by progesterone are limited, studies investigating differential gene expression in leiomyomas during the menstrual cycle have provided groundwork for identifying those that are influenced by steroid hormones. In one study, the temporal and spatial expression of proliferative and proapoptotic molecules that could participate in leiomyoma pathogenesis was determined.144 For example, levels of Fas ligand (FasL) protein, which is associated with apoptosis, was higher during the secretory phase compared with the proliferative phase in the leiomyoma. Furthermore, higher expression of FasL was found in the leiomyoma compared to myometrium. Levels of proliferating cell nuclear antigen (PCNA), which is associated with cell proliferation, was higher during the proliferative phase in leiomyoma and levels were higher than that of paired myometrium. Lower PTEN expression, which is the phosphatase that is associated with the PI3K/AKT pathway, was detected in the leiomyoma compared to the myometrium. In this study, it was speculated that the higher FasL level in the leiomyoma may correspond to suppression of local immunity by inducing apoptosis of immune cells, while a higher level of PCNA and a lower level of PTEN may be related to increased mitogenesis and decreased apoptosis in leiomyoma. Other studies have demonstrated that leiomyoma tissues have higher PCNA levels than myometrium throughout the menstrual cycle.145 Furthermore, treatment with estradiol or progesterone increases PCNA expression in leiomyoma cells compared to controls.112 Asoprisnil, a SPRM decreased the PCNA positive rate in cultured leiomyoma cells with no difference in myometrial cells.146
The antiapoptotic bcl-2 gene in leiomyoma has been investigated by several groups. It has been demonstrated that bcl-2 is more highly expressed in leiomyoma than myometrium.115,147,148 Progesterone and estrogen regulate bcl-2 expression differently. Progesterone upregulates bcl-2 mRNA while estrogen downregulates bcl-2 protein.115 Furthermore, Yin et al.149 found that liganded PR binds to the bcl-2 promoter and enhances bcl-2 transcription in primary cultured leiomyoma cells. Overexpression of the dominant negative ER in cultured leiomyoma cells decreased bcl-2.150 It has been speculated that this reduction in ER activity results in decreased PR expression and hence a decrease in bcl-2 expression. Asoprisnil decreased antiapoptotic bcl-2 with a corresponding increase in TUNEL staining, cleaved caspase 3, and cleaved PARP supporting a role for PR in preventing apoptosis in these cells.133,146
ECM components are of high interest in leiomyoma pathology due to large quantities of matrix proteins found in leiomyoma. It has been demonstrated that certain ECM components and proteins are regulated by steroid hormones. Collagen type I and III mRNAs were upregulated in leiomyoma compared to myometrium during the proliferative phase of the menstrual cycle.151 In concert with this is the higher expression of MMPs in leiomyoma compared to myometrium during the secretory phase, while tissue inhibitors of MMPs (TIMPs) are more highly expressed in leiomyoma during the proliferative phase.152 Collagen binding protein fibrododulin (FMOD), which is involved in collagen fibril network formation, is more highly expressed in leiomyoma and myometrium during the proliferative phase of the menstrual cycle.153 Levens et al.153 also demonstrated that GnRHa treatments decreased FMOD. Asoprisnil treatment of primary leiomyoma cultures decreased TIMP1, TIMP2, collagen I, and collagen III while increasing MMP-1, MT1-MMP, EMMPRIN supporting that progesterone may be in involved in ECM deposition and turnover.154
Recently, investigators have uncovered that miRNA’s may play a role in leiomyoma pathogenesis. MicroRNAs are small noncoding RNA’s that inhibit translation mostly through binding to target mRNA 3′ UTR. Marsh et al.155 and Wang et al.156 suggested that certain miRNA’s are differentially expressed in leiomyoma compared to myometrium. Wang et al.156 focused on the let-7 family of miRNA’s and found that certain let-7 family miRNA’s may be correlated with tumor size. Pan et al.157 also found that miRNA’s are differentially expressed in leiomyoma compared to myometrium and are regulated by sex steroids. More research is needed to address target genes of differentially regulated miRNA’s.
C. Growth Factor Regulation in Leiomyoma by Progesterone
Given the growth properties of leiomyomas, the expression and regulation of growth factors have been studied. Here, the regulation of these growth factors by both progesterone and estrogen is highlighted. Steroid hormones can regulate EGF and its receptor (EGFR) which have been implicated in leiomyoma growth. Both the local growth factor and its receptor are expressed in leiomyoma and myometrial tissues.158 During the secretory phase of the menstrual cycle, EGF mRNA is higher in leiomyoma than myometrium.111 Interestingly, progesterone does not increase EGF-R while increasing EGF and estrogen increases EGFR but does not increase EGF.145 This supports previous results159 that progesterone treatment but not estradiol increased EGF mRNA. In addition, asoprisnil decreased EGF mRNA.134,146 A dominant negative ER decreased immunoreactive EGF in cultured primary and immortalized leiomyoma cells.150 These data suggest that in the case of EGF and EGFR, estrogen and progesterone alter the response to the same growth factor pathway in different ways.
Insulin like growth factors IGF-I, IGF-II, and IGF-II receptor but not receptor type I have been detected in leiomyoma tissues at levels higher than the myometrium.160,161 Studies show that IGF-I treatment can increase leiomyoma proliferation.162-164 IGF-I gene expression was most abundant in leiomyomata obtained during the late proliferative phase of the cycle and was undetectable in leiomyomata from hypoestrogenic patients. SPRM, asoprisnil, decreased IGF-I mRNA in leiomyoma while having no effect in myometrial cells.134,146 IGF-II mRNA expression did not vary with phase of the menstrual cycle.165 Expression levels of IGF-II receptor were not altered with progesterone and estrogen treatments in cultured leiomyoma cells.166
Both platelet derived growth factor (PDGF) and receptor are expressed in leiomyoma and myometrium and have been implicated in leiomyoma growth.167 PDGF is a potent mitogen for smooth muscle cells and leiomyoma cells.168-170 While it has been shown that leiomyoma tissues express higher levels of PDGF-A and B chain mRNA levels compared to matched myometrial tissue,161,171 other studies show conflicting observations.144,172-174 Women treated with GnRHa exhibited a reduction in uterine volume which was statistically related to the decrease in PDGF expression.165 While estrogen can upregulate PDGF in cultured leiomyoma cells,164 progesterone has also been implicated in regulating PDGF expression as shown by the increased expression of PDGF-BB expression during the secretory phase compared to the proliferative phase of the menstrual cycle in leiomyoma tissue.171
The transforming growth factor beta (TGF-beta) family increases the expression of ECM components and are involved in reproductive tissue development and growth.175,176 Since fibroid tumors are composed mostly of ECM, examining connections between leiomyoma growth and TGF-beta cytokines and receptors have been of interest. Consistent expression of TGF-beta receptors type I–II and TGF-beta 1, 2, 3 have been found in myometrium177,178 although expression in leiomyoma remains discrepant. For example, two studies showed increased expression of TGF-beta 1 mRNA in leiomyoma compared to myometrium, while another group showed the contrary.168,177,179 In addition, TGF-beta 2, 3, and their receptors have been found to be more highly expressed in leiomyoma than in myometrium.177 The expression of TGF-beta 3 has been more consistent demonstrating higher expression in leiomyoma compared to myometrium.180 Furthermore, highest levels of TGF-beta 3 were found during the secretory phase of the menstrual cycle suggesting progesterone involvement.168,181 Accordingly, the SPRM, asoprisnil, decreased TGF-beta 3 mRNA in leiomyoma cells.134,146 Treatment with estrogen and progesterone differentially altered TGF-beta levels in myometrium and leiomyoma.139,180
D. Activation of Signaling Pathways in Leiomyoma by Progesterone and Estrogen
The role of nuclear hormone receptors in activating signaling pathways as a mechanism for leiomyoma tumor growth have been proposed in recent years. In concert with the hormonally regulated growth factors described in the above sections EGF, FGF, IGF-I, HGF, and PDGF receptors were found to be highly expressed in leiomyoma tissues compared to myometrium in a receptor tyrosine kinase array.161 Specifically, expression of IGF-IR beta was more abundant in leiomyoma as well as phosphorylated IGF-IR and downstream effectors were more highly activated in leiomyoma. The mitogen activated protein kinase (MAPK) pathway regulates a variety of proteins involved in apoptosis and cell growth.182 Estradiol has been shown to rapidly activate the MAPK pathway in primary leiomyoma cells, including the rapid protein tyrosine phosphorylation of a subset of intracellular proteins, such as GAP, PI3K, and PLCgamma.183 Interestingly, activation of this pathway was related to E2-induced PDGF secretion. In this study, it was proposed that PDGF, alone or in association with other growth factors, is the main growth factor involved in the proliferation response of leiomyoma cells to E2 stimulation. In accordance to this, ER-alpha phosphorylation was higher in leiomyoma tissues derived from patients in the proliferative phase of the menstrual cycle and this correlated with an increased phosphorylation of p44/p42 MAPK proteins in leiomyoma.184 In addition, phosphorylated p44/42 colocalized with ER-alpha phosphorylated on serine 118, suggesting that MAPK can phosphorylate ER-alpha in leiomyoma. ER-alpha can also bind to the p85-alpha regulatory subunit of PI3K, allowing for PI3K activation in MCF-7 cells.185
There is increasing evidence that progesterone also has rapid, membrane initiated effects independent of gene transcription to alter production of second messenger and cell signal transduction pathway. Some of these rapid nongenomic effects of progesterone have been shown to be mediated through the same nuclear PR that regulates gene transcription.186,187 Pioneering work by Edwards’ group187 demonstrated that PRB can directly bind to the SH3 domain of Src kinase and thereby activate the kinase. Similarly, progesterone-mediated regulation of the PI3K/AKT pathway has been demonstrated in breast cancer cells as well as in rat endometrial stromal cells.186-190 Boonyaratanakornkit et al.187 showed that p85 can interact with PR in a GST-pull-down system. Recently, it was shown that progesterone can rapidly phosphorylate AKT in leiomyoma cells.191 AKT phosphorylation was abrogated by PR antagonist RU 486 and PI3K inhibitor LY290004 in primary leiomyoma cells. Furthermore, the downstream targets of AKT, FOXO1, and GSK3 beta were phosphorylated upon progestin treatment indicating activation of down-stream signaling components. In leiomyoma, protein levels of AKT as well as the phosphorylated form were higher than myometrium and phosphor-AKT levels dropped in leiomyoma samples taken from menopausal women.147 In concert with this is deactivated PTEN, a negative regulator of PI3K which was also increased in leiomyoma compared to myometrium, although these differences were less dramatic in menopause.192 GnRHa therapy decreased PI3K activity and AKT phosphorylation supporting that AKT activation is hormone dependent.193 Given the involvement of the AKT pathway in cell proliferation and survival, its activation by progesterone may be another mechanism by which this hormone promotes leiomyoma growth.
Although estrogen and progesterone have distinct functions, the two hormones and their receptors interact with one another. Hodges et al. found that R5020 and MPA inhibited estradiol induced proliferation of ELT3 cells and inhibited ER activated gene transcription. These results suggest that liganded PR transdominantly suppresses ER signaling in leiomyoma. Of note is that estradiol increased expression of both PR isoforms.194 Accordingly, overexpression of dominant-negative ER decreased PR expression in human leiomyoma cells.150
IX. Conclusions and Perspectives on Progesterone Action in Uterine Leiomyoma
The importance of progesterone in promoting leiomyoma growth was initially substantiated by clinical studies as described above. The effectiveness of antiprogestins and SPRMs in reducing leiomyoma size provides strong support of progesterone being mitogenic in leiomyoma. Since the field of progesterone action in leiomyoma is one that has been understudied, it remains unclear as to how progesterone promotes growth of these tumors. Thus far, many studies have demonstrated that expression of genes and proteins are similar in leiomyoma compared to matched myometrium, however, it is the levels of expression and regulation during the menstrual cycle that differ. Whether it is demonstrated by the differential expression of genes during the menstrual phase or in response to progestin or antiprogestin treatment in vivo and in vitro, it is apparent that progesterone promotes expression of genes associated with growth and survival of leiomyoma. Regulation of growth factors and their receptors by progesterone have been proposed as another mode of progesterone action. At the transcriptional level, there are very few studies investigating the role of PR on promoters of genes in leiomyoma. Studying the recruitment of coregulators to various PR binding regions has given insight to how PR functions at the transcriptional level in leiomyomas. Finally, the rapid effects of progesterone on signaling molecules in leiomyoma provide yet another mode of progesterone action on leiomyoma growth. The involvement of classical PR in mediating these rapid effects are physiologically significant and whether progesterone membrane receptors are involved in progesterone mediated activation of kinases are unknown. Given the high incidence of leiomyoma in women, the morbidity that is associated with this disease and the financial burden of over 200,000 hysterectomies per year in the US alone, it is imperative that alternate therapies are developed. This can only be done with a better understanding of the molecular mechanisms associated with this disease.
X. Future Directions
The significance of progesterone in the uterus is indisputable. While its action remains complex and context-specific, it is crucial to decipher how PR works in uterine pathologies in order to be able to treat these diseases effectively. The stark contrast in the physiological response to progesterone in endometrial cancer compared to leiomyoma has important clinical implications when using progestins or antiprogestins as a mode of therapy. A clear example is the use of RU486 for decreasing leiomyoma size, which although effective, can promote endometrial hyperplasia. More information is needed on the differential action of PR in endometrial cancer cells and leiomyoma cells. Comparative studies on the mechanisms of action of PR in epithelial cells and the mesenchymal-derived fibroblasts would provide further insight to the differences in PR action in these two diseases. Alternatively, given the differing response to progesterone in the breast and the endometrium, it would be worthwhile to conduct studies comparing PR action in breast and endometrial cancers. At the molecular level, since PR action is dictated by coregulators, an extensive analysis of proteins that complex with PR on different gene promoters after specific times of progesterone treatment and then combined with gene expression studies would be informational. Global analysis of PR binding regions using chromatin immunoprecipitation techniques, identification of coregulators using mass spectrometry, and analysis of gene expression using microarray combined together with bioinformatics analysis would be one effective approach to decipher differential PR action in uterine cells. Although unraveling the complexity of PR may seem daunting and insurmountable, the information gathered thus far provides solid groundwork to tackle this challenge. Use of innovative and state-of-the-art technology will be key in moving this field forward.
References
- 1.Kurita T, Nakamura H. Embryology and anatomy of the uterus. In: Aplin J, Fazleabas S, Glasser S, Giudice L, editors. The Endometrium, Molecular, Cellular, and Clinical Perspectives. 2. Harwood Academic Publishers; 2007. [Google Scholar]
- 2.Norman AW, Litwack G. Hormones. Orlando, FL: Academic Press; 1987. [Google Scholar]
- 3.Clarke CL, Sutherland RL. Progestin regulation of cellular proliferation. Endocr Rev. 1990;11:266–301. doi: 10.1210/edrv-11-2-266. [DOI] [PubMed] [Google Scholar]
- 4.Dockery P, Li TC, Rogers AW, Cooke ID, Lenton EA. The ultrastructure of the glandular epithelium in the timed endometrial biopsy. Hum Reprod. 1988;3:826–34. doi: 10.1093/oxfordjournals.humrep.a136793. [DOI] [PubMed] [Google Scholar]
- 5.Wynn RM. Ultrastructural development of the human decidua. Am J Obstet Gynecol. 1974;118:652–70. doi: 10.1016/s0002-9378(16)33740-1. [DOI] [PubMed] [Google Scholar]
- 6.Gellersen B, Brosens J. Cyclic AMP and progesterone receptor cross-talk in human endometrium: a decidualizing affair. J Endocrinol. 2003;178:357–72. doi: 10.1677/joe.0.1780357. [DOI] [PubMed] [Google Scholar]
- 7.de Ziegler D, Bulletti C, Fanchin R, Epiney M, Brioschi PA. Contractility of the nonpregnant uterus: the follicular phase. Ann N Y Acad Sci. 2001;943:172–84. doi: 10.1111/j.1749-6632.2001.tb03801.x. [DOI] [PubMed] [Google Scholar]
- 8.Boroditsky RS, Reyes FI, Winter JS, Faiman C. Maternal serum estrogen and progesterone concentrations preceding normal labor. Obstet Gynecol. 1978;51:686–91. [PubMed] [Google Scholar]
- 9.Tulchinsky D, Hobel CJ, Yeager E, Marshall JR. Plama estradiol, estriol, and progesterone in human pregnancy. II. Clinical applications in Rh-isoimmunization disease. Am J Obstet Gynecol. 1972;113:766–70. doi: 10.1016/0002-9378(72)90556-x. [DOI] [PubMed] [Google Scholar]
- 10.Walsh SW, Stanczyk FZ, Novy MJ. Daily hormonal changes in the maternal, fetal, and amniotic fluid compartments before parturition in a primate species. J Clin Endocrinol Metab. 1984;58:629–39. doi: 10.1210/jcem-58-4-629. [DOI] [PubMed] [Google Scholar]
- 11.Haluska GJ, Cook MJ, Novy MJ. Inhibition and augmentation of progesterone production during pregnancy: effects on parturition in rhesus monkeys. Am J Obstet Gynecol. 1997;176:682–91. doi: 10.1016/s0002-9378(97)70570-2. [DOI] [PubMed] [Google Scholar]
- 12.Selinger M, Mackenzie IZ, Gillmer MD, Phipps SL, Ferguson J. Progesterone inhibition in mid-trimester termination of pregnancy: physiological and clinical effects. Br J Obstet Gynaecol. 1987;94:1218–22. doi: 10.1111/j.1471-0528.1987.tb02325.x. [DOI] [PubMed] [Google Scholar]
- 13.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–9. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robinson-Rechavi M, Escriva Garcia H, Laudet V. The nuclear receptor superfamily. J Cell Sci. 2003;116:585–6. doi: 10.1242/jcs.00247. [DOI] [PubMed] [Google Scholar]
- 15.Gronemeyer H, Meyer ME, Bocquel MT, Kastner P, Turcotte B, Chambon P. Progestin receptors: isoforms and antihormone action. J Steroid Biochem Mol Biol. 1991;40:271–8. doi: 10.1016/0960-0760(91)90192-8. [DOI] [PubMed] [Google Scholar]
- 16.Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H, et al. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J. 1990;9:1603–14. doi: 10.1002/j.1460-2075.1990.tb08280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lessey BA, Alexander PS, Horwitz KB. The subunit structure of human breast cancer progesterone receptors: characterization by chromatography and photoaffinity labeling. Endocrinology. 1983;112:1267–74. doi: 10.1210/endo-112-4-1267. [DOI] [PubMed] [Google Scholar]
- 18.McDonnell DP, Shahbaz MM, Vegeto E, Goldman ME. The human progesterone receptor A-form functions as a transcriptional modulator of mineralocorticoid receptor transcriptional activity. J Steroid Biochem Mol Biol. 1994;48:425–32. doi: 10.1016/0960-0760(94)90190-2. [DOI] [PubMed] [Google Scholar]
- 19.Tetel MJ, Giangrande PH, Leonhardt SA, McDonnell DP, Edwards DP. Hormone-dependent interaction between the amino- and carboxyl-terminal domains of progesterone receptor in vitro and in vivo. Mol Endocrinol. 1999;13:910–24. doi: 10.1210/mend.13.6.0300. [DOI] [PubMed] [Google Scholar]
- 20.Tung L, Abdel-Hafiz H, Shen T, Harvell DM, Nitao LK, Richer JK, et al. Progesterone receptors (PR)-B and -A regulate transcription by different mechanisms: AF-3 exerts regulatory control over coactivator binding to PR-B. Mol Endocrinol. 2006;20:2656–70. doi: 10.1210/me.2006-0105. [DOI] [PubMed] [Google Scholar]
- 21.Tung L, Mohamed MK, Hoeffler JP, Takimoto GS, Horwitz KB. Antagonist-occupied human progesterone B-receptors activate transcription without binding to progesterone response elements and are dominantly inhibited by A-receptors. Mol Endocrinol. 1993;7:1256–65. doi: 10.1210/mend.7.10.8123133. [DOI] [PubMed] [Google Scholar]
- 22.Vegeto E, Shahbaz MM, Wen DX, Goldman ME, O’Malley BW, McDonnell DP. Human progesterone receptor A form is a cell- and promoter-specific repressor of human progesterone receptor B function. Mol Endocrinol. 1993;7:1244–55. doi: 10.1210/mend.7.10.8264658. [DOI] [PubMed] [Google Scholar]
- 23.Chi T, Lieberman P, Ellwood K, Carey M. A general mechanism for transcriptional synergy by eukaryotic activators. Nature. 1995;377:254–7. doi: 10.1038/377254a0. [DOI] [PubMed] [Google Scholar]
- 24.Giangrande PH, Kimbrel EA, Edwards DP, McDonnell DP. The opposing transcriptional activities of the two isoforms of the human progesterone receptor are due to differential cofactor binding. Mol Cell Biol. 2000;20:3102–15. doi: 10.1128/mcb.20.9.3102-3115.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wagner BL, Norris JD, Knotts TA, Weigel NL, McDonnell DP. The nuclear corepressors NCoR and SMRT are key regulators of both ligand- and 8-bromo-cyclic AMP-dependent transcriptional activity of the human progesterone receptor. Mol Cell Biol. 1998;18:1369–78. doi: 10.1128/mcb.18.3.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ellwood K, Huang W, Johnson R, Carey M. Multiple layers of cooperativity regulate enhanceosome-responsive RNA polymerase II transcription complex assembly. Mol Cell Biol. 1999;19:2613–23. doi: 10.1128/mcb.19.4.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miner JN, Yamamoto KR. The basic region of AP-1 specifies glucocorticoid receptor activity at a composite response element. Genes Dev. 1992;6:2491–501. doi: 10.1101/gad.6.12b.2491. [DOI] [PubMed] [Google Scholar]
- 28.Prefontaine GG, Lemieux ME, Giffin W, Schild-Poulter C, Pope L, LaCasse E, et al. Recruitment of octamer transcription factors to DNA by glucocorticoid receptor. Mol Cell Biol. 1998;18:3416–30. doi: 10.1128/mcb.18.6.3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schule R, Muller M, Kaltschmidt C, Renkawitz R. Many transcription factors interact synergistically with steroid receptors. Science. 1988;242:1418–20. doi: 10.1126/science.3201230. [DOI] [PubMed] [Google Scholar]
- 30.O’Malley BW. Coregulators: from whence came these “master genes”. Mol Endocrinol. 2007;21:1009–13. doi: 10.1210/me.2007-0012. [DOI] [PubMed] [Google Scholar]
- 31.Li X, Wong J, Tsai SY, Tsai MJ, O’Malley BW. Progesterone and glucocorticoid receptors recruit distinct coactivator complexes and promote distinct patterns of local chromatin modification. Mol Cell Biol. 2003;23:3763–73. doi: 10.1128/MCB.23.11.3763-3773.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu J, Qiu Y, DeMayo FJ, Tsai SY, Tsai MJ, O’Malley BW. Partial hormone resistance in mice with disruption of the steroid receptor coactivator-1 (SRC-1) gene. Science. 1998;279:1922–5. doi: 10.1126/science.279.5358.1922. [DOI] [PubMed] [Google Scholar]
- 33.Gehin M, Mark M, Dennefeld C, Dierich A, Gronemeyer H, Chambon P. The function of TIF2/GRIP1 in mouse reproduction is distinct from those of SRC-1 and p/CIP. Mol Cell Biol. 2002;22:5923–37. doi: 10.1128/MCB.22.16.5923-5937.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeong JW, Lee KY, Han SJ, Aronow BJ, Lydon JP, O’Malley BW, et al. The p160 steroid receptor coactivator 2, SRC-2, regulates murine endometrial function and regulates progesterone-independent and -dependent gene expression. Endocrinology. 2007;148:4238–50. doi: 10.1210/en.2007-0122. [DOI] [PubMed] [Google Scholar]
- 35.Mukherjee A, Soyal SM, Fernandez-Valdivia R, Gehin M, Chambon P, Demayo FJ, et al. Steroid receptor coactivator 2 is critical for progesterone-dependent uterine function and mammary morphogenesis in the mouse. Mol Cell Biol. 2006;26:6571–83. doi: 10.1128/MCB.00654-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bamberger AM, Bamberger CM, Gellersen B, Schulte HM. Modulation of AP-1 activity by the human progesterone receptor in endometrial adenocarcinoma cells. Proc Natl Acad Sci USA. 1996;93:6169–74. doi: 10.1073/pnas.93.12.6169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gronemeyer H. Transcription activation by estrogen and progesterone receptors. Annu Rev Genet. 1991;25:89–123. doi: 10.1146/annurev.ge.25.120191.000513. [DOI] [PubMed] [Google Scholar]
- 38.Kalkhoven E, Wissink S, van der Saag PT, van der Burg B. Negative interaction between the RelA(p65) subunit of NF-kappaB and the progesterone receptor. J Biol Chem. 1996;271:6217–24. doi: 10.1074/jbc.271.11.6217. [DOI] [PubMed] [Google Scholar]
- 39.Kim JJ, Buzzio OL, Li S, Lu Z. Role of FOXO1A in the regulation of insulin-like growth factor-binding protein-1 in human endometrial cells: interaction with progesterone receptor. Biol Reprod. 2005;73:833–9. doi: 10.1095/biolreprod.105.043182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takano M, Lu Z, Goto T, Fusi L, Higham J, Francis J, et al. Transcriptional cross talk between the forkhead transcription factor forkhead box O1A and the progesterone receptor coordinates cell cycle regulation and differentiation in human endometrial stromal cells. Mol Endocrinol. 2007;21:2334–49. doi: 10.1210/me.2007-0058. [DOI] [PubMed] [Google Scholar]
- 41.Li X, O’Malley BW. Unfolding the action of progesterone receptors. J Biol Chem. 2003;278:39261–4. doi: 10.1074/jbc.R300024200. [DOI] [PubMed] [Google Scholar]
- 42.Carroll JS, Liu XS, Brodsky AS, Li W, Meyer CA, Szary AJ, et al. Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell. 2005;122:33–43. doi: 10.1016/j.cell.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 43.Schuur ER, Loktev AV, Sharma M, Sun Z, Roth RA, Weigel RJ. Ligand-dependent interaction of estrogen receptor-alpha with members of the forkhead transcription factor family. J Biol Chem. 2001;276:33554–60. doi: 10.1074/jbc.M105555200. [DOI] [PubMed] [Google Scholar]
- 44.Zhao HH, Herrera RE, Coronado-Heinsohn E, Yang MC, Ludes-Meyers JH, Seybold-Tilson KJ, et al. Forkhead homologue in rhabdomyosarcoma functions as a bifunctional nuclear receptor-interacting protein with both coactivator and corepressor functions. J Biol Chem. 2001;276:27907–12. doi: 10.1074/jbc.M104278200. [DOI] [PubMed] [Google Scholar]
- 45.Mulac-Jericevic B, Lydon JP, DeMayo FJ, Conneely OM. Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc Natl Acad Sci USA. 2003;100:9744–9. doi: 10.1073/pnas.1732707100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mulac-Jericevic B, Mullinax RA, DeMayo FJ, Lydon JP, Conneely OM. Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science. 2000;289:1751–4. doi: 10.1126/science.289.5485.1751. [DOI] [PubMed] [Google Scholar]
- 47.Condon JC, Hardy DB, Kovaric K, Mendelson CR. Up-regulation of the progesterone receptor (PR)-C isoform in laboring myometrium by activation of nuclear factor-kappaB may contribute to the onset of labor through inhibition of PR function. Mol Endocrinol. 2006;20:764–75. doi: 10.1210/me.2005-0242. [DOI] [PubMed] [Google Scholar]
- 48.Garcia E, Bouchard P, De Brux J, Berdah J, Frydman R, Schaison G, et al. Use of immunocytochemistry of progesterone and estrogen receptors for endometrial dating. J Clin Endocrinol Metab. 1988;67:80–7. doi: 10.1210/jcem-67-1-80. [DOI] [PubMed] [Google Scholar]
- 49.Mylonas I, Jeschke U, Shabani N, Kuhn C, Kunze S, Dian D, et al. Steroid receptors ERalpha, ERbeta, PR-A and PR-B are differentially expressed in normal and atrophic human endometrium. Histol Histopathol. 2007;22:169–76. doi: 10.14670/HH-22.169. [DOI] [PubMed] [Google Scholar]
- 50.Snijders MP, de Goeij AF, Debets-TeBaerts MJ, Rousch MJ, Koudstaal J, Bosman FT. Immunocytochemical analysis of oestrogen receptors and progesterone receptors in the human uterus throughout the menstrual cycle and after the menopause. J Reprod Fertil. 1992;94:363–71. doi: 10.1530/jrf.0.0940363. [DOI] [PubMed] [Google Scholar]
- 51.Nisolle M, Gillerot S, Casanas-Roux F, Squifflet J, Berliere M, Donnez J. Immunohistochemical study of the proliferation index, oestrogen receptors and progesterone receptors A and B in leiomyomata and normal myometrium during the menstrual cycle and under gonadotrophin-releasing hormone agonist therapy. Hum Reprod. 1999;14:2844–50. doi: 10.1093/humrep/14.11.2844. [DOI] [PubMed] [Google Scholar]
- 52.Vienonen A, Miettinen S, Blauer M, Martikainen PM, Tomas E, Heinonen PK, et al. Expression of nuclear receptors and cofactors in human endometrium and myometrium. J Soc Gynecol Investig. 2004;11:104–12. doi: 10.1016/j.jsgi.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 53.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 54.Clement PB, Young RH. Endometrioid carcinoma of the uterine corpus: a review of its pathology with emphasis on recent advances and problematic aspects. Adv Anat Pathol. 2002;9:145–84. doi: 10.1097/00125480-200205000-00001. [DOI] [PubMed] [Google Scholar]
- 55.Creasman WT, Eddy GL. Recent advances in endometrial cancer. Semin Surg Oncol. 1990;6:339–42. doi: 10.1002/ssu.2980060608. [DOI] [PubMed] [Google Scholar]
- 56.Judd HL, Judd GE, Lucas WE, Yen SS. Endocrine function of the postmenopausal ovary: concentration of androgens and estrogens in ovarian and peripheral vein blood. J Clin Endocrinol Metab. 1974;39:1020–4. doi: 10.1210/jcem-39-6-1020. [DOI] [PubMed] [Google Scholar]
- 57.Kirschner MA, Schneider G, Ertel NH, Worton E. Obesity, androgens, estrogens, and cancer risk. Cancer Res. 1982;42:3281s–5s. [PubMed] [Google Scholar]
- 58.Munstedt K, Grant P, Woenckhaus J, Roth G, Tinneberg HR. Cancer of the endometrium: current aspects of diagnostics and treatment. World J Surg Oncol. 2004;2:24. doi: 10.1186/1477-7819-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ryan AJ, Susil B, Jobling TW, Oehler MK. Endometrial cancer. Cell Tissue Res. 2005;322:53–61. doi: 10.1007/s00441-005-1109-5. [DOI] [PubMed] [Google Scholar]
- 60.Cohen CJ, Rahaman J. Endometrial cancer. Management of high risk and recurrence including the tamoxifen controversy. Cancer. 1995;76:2044–52. doi: 10.1002/1097-0142(19951115)76:10+<2044::aid-cncr2820761323>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 61.Jadoul P, Donnez J. Conservative treatment may be beneficial for young women with atypical endometrial hyperplasia or endometrial adenocarcinoma. Fertil Steril. 2003;80:1315–24. doi: 10.1016/s0015-0282(03)01183-x. [DOI] [PubMed] [Google Scholar]
- 62.Randall TC, Kurman RJ. Progestin treatment of atypical hyperplasia and well-differentiated carcinoma of the endometrium in women under age 40. Obstet Gynecol. 1997;90:434–40. doi: 10.1016/s0029-7844(97)00297-4. [DOI] [PubMed] [Google Scholar]
- 63.Gal D, Edman CD, Vellios F, Forney JP. Long-term effect of megestrol acetate in the treatment of endometrial hyperplasia. Am J Obstet Gynecol. 1983;146:316–22. doi: 10.1016/0002-9378(83)90754-8. [DOI] [PubMed] [Google Scholar]
- 64.Ferenczy A, Gelfand M. The biologic significance of cytologic atypia in progestogen-treated endometrial hyperplasia. Am J Obstet Gynecol. 1989;160:126–31. doi: 10.1016/0002-9378(89)90103-8. [DOI] [PubMed] [Google Scholar]
- 65.Lai CH, Huang HJ. The role of hormones for the treatment of endometrial hyperplasia and endometrial cancer. Curr Opin Obstet Gynecol. 2006;18:29–34. doi: 10.1097/01.gco.0000192994.37965.c6. [DOI] [PubMed] [Google Scholar]
- 66.Martin-Hirsch PL, Lilford RJ, Jarvis GJ. Adjuvant progestagen therapy for the treatment of endometrial cancer: review and meta-analyses of published randomised controlled trials. Eur J Obstet Gynecol Reprod Biol. 1996;65:201–7. doi: 10.1016/0301-2115(95)02359-3. [DOI] [PubMed] [Google Scholar]
- 67.Benshushan A. Endometrial adenocarcinoma in young patients: evaluation and fertility-preserving treatment. Eur J Obstet Gynecol Reprod Biol. 2004;117:132–7. doi: 10.1016/j.ejogrb.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 68.Crissman JD, Azoury RS, Barnes AE, Schellhas HF. Endometrial carcinoma in women 40 years of age or younger. Obstet Gynecol. 1981;57:699–704. [PubMed] [Google Scholar]
- 69.Gallup DG, Stock RJ. Adenocarcinoma of the endometrium in women 40 years of age or younger. Obstet Gynecol. 1984;64:417–20. [PubMed] [Google Scholar]
- 70.Lowe MP, Bender D, Sood AK, Davis W, Syrop CH, Sorosky JI. Two successful pregnancies after conservative treatment of endometrial cancer and assisted reproduction. Fertil Steril. 2002;77:188–9. doi: 10.1016/s0015-0282(01)02937-5. [DOI] [PubMed] [Google Scholar]
- 71.Chiva L, Lapuente F, Gonzalez-Cortijo L, Carballo N, Garcia JF, Rojo A, et al. Sparing fertility in young patients with endometrial cancer. Gynecol Oncol. 2008;111:S101–4. doi: 10.1016/j.ygyno.2008.07.056. [DOI] [PubMed] [Google Scholar]
- 72.Ushijima K, Yahata H, Yoshikawa H, Konishi I, Yasugi T, Saito T, et al. Multicenter phase II study of fertility-sparing treatment with medroxyprogesterone acetate for endometrial carcinoma and atypical hyperplasia in young women. J Clin Oncol. 2007;25:2798–803. doi: 10.1200/JCO.2006.08.8344. [DOI] [PubMed] [Google Scholar]
- 73.Leslie KK, Stein MP, Kumar NS, Dai D, Stephens J, Wandinger-Ness A, et al. Progesterone receptor isoform identification and subcellular localization in endometrial cancer. Gynecol Oncol. 2005;96:32–41. doi: 10.1016/j.ygyno.2004.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Smid-Koopman E, Blok LJ, Kuhne LC, Burger CW, Helmerhorst TJ, Brinkmann AO, et al. Distinct functional differences of human progesterone receptors A and B on gene expression and growth regulation in two endometrial carcinoma cell lines. J Soc Gynecol Investig. 2003;10:49–57. [PubMed] [Google Scholar]
- 75.Hanekamp EE, Kuhne LM, Grootegoed JA, Burger CW, Blok LJ. Progesterone receptor A and B expression and progestagen treatment in growth and spread of endometrial cancer cells in nude mice. Endocr Relat Cancer. 2004;11:831–41. doi: 10.1677/erc.1.00844. [DOI] [PubMed] [Google Scholar]
- 76.Fujimoto J, Ichigo S, Hori M, Nishigaki M, Tamaya T. Expression of progesterone receptor form A and B mRNAs in gynecologic malignant tumors. Tumour Biol. 1995;16:254–60. doi: 10.1159/000217942. [DOI] [PubMed] [Google Scholar]
- 77.Arnett-Mansfield RL, deFazio A, Wain GV, Jaworski RC, Byth K, Mote PA, et al. Relative expression of progesterone receptors A and B in endometrioid cancers of the endometrium. Cancer Res. 2001;61:4576–82. [PubMed] [Google Scholar]
- 78.Kumar NS, Richer J, Owen G, Litman E, Horwitz KB, Leslie KK. Selective down-regulation of progesterone receptor isoform B in poorly differentiated human endometrial cancer cells: implications for unopposed estrogen action. Cancer Res. 1998;58:1860–5. [PubMed] [Google Scholar]
- 79.Dai D, Wolf DM, Litman ES, White MJ, Leslie KK. Progesterone inhibits human endometrial cancer cell growth and invasiveness: down-regulation of cellular adhesion molecules through progesterone B receptors. Cancer Res. 2002;62:881–6. [PubMed] [Google Scholar]
- 80.Ueda M, Fujii H, Yoshizawa K, Abe F, Ueki M. Effects of sex steroids and growth factors on migration and invasion of endometrial adenocarcinoma SNG-M cells in vitro. Jpn J Cancer Res. 1996;87:524–33. doi: 10.1111/j.1349-7006.1996.tb00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Saito T, Mizumoto H, Tanaka R, Satohisa S, Adachi K, Horie M, et al. Overexpressed progesterone receptor form B inhibit invasive activity suppressing matrix metalloproteinases in endometrial carcinoma cells. Cancer Lett. 2004;209:237–43. doi: 10.1016/j.canlet.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 82.Di Nezza LA, Jobling T, Salamonsen LA. Progestin suppresses matrix metalloproteinase production in endometrial cancer. Gynecol Oncol. 2003;89:325–33. doi: 10.1016/s0090-8258(03)00089-1. [DOI] [PubMed] [Google Scholar]
- 83.Jaffe RC, Ferguson-Gottschall SD, Gao W, Beam C, Fazleabas AT. Histone deacetylase inhibition and progesterone act synergistically to stimulate baboon glycodelin gene expression. J Mol Endocrinol. 2007;38:401–7. doi: 10.1677/JME-06-0030. [DOI] [PubMed] [Google Scholar]
- 84.Uchida H, Maruyama T, Nagashima T, Asada H, Yoshimura Y. Histone deacetylase inhibitors induce differentiation of human endometrial adenocarcinoma cells through up-regulation of glycodelin. Endocrinology. 2005;146:5365–73. doi: 10.1210/en.2005-0359. [DOI] [PubMed] [Google Scholar]
- 85.Uchida H, Maruyama T, Ono M, Ohta K, Kajitani T, Masuda H, et al. Histone deacetylase inhibitors stimulate cell migration in human endometrial adenocarcinoma cells through up-regulation of glycodelin. Endocrinology. 2007;148:896–902. doi: 10.1210/en.2006-0896. [DOI] [PubMed] [Google Scholar]
- 86.Ohta K, Maruyama T, Uchida H, Ono M, Nagashima T, Arase T, et al. Glycodelin blocks progression to S phase and inhibits cell growth: a possible progesterone-induced regulator for endometrial epithelial cell growth. Mol Hum Reprod. 2008;14:17–22. doi: 10.1093/molehr/gam081. [DOI] [PubMed] [Google Scholar]
- 87.Ward EC, Hoekstra AV, Blok LJ, Hanifi-Moghaddam P, Lurain JR, Singh DK, et al. The regulation and function of the forkhead transcription factor, Forkhead box O1, is dependent on the progesterone receptor in endometrial carcinoma. Endocrinology. 2008;149:1942–50. doi: 10.1210/en.2007-0756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Goto T, Takano M, Albergaria A, Briese J, Pomeranz KM, Cloke B, et al. Mechanism and functional consequences of loss of FOXO1 expression in endometrioid endometrial cancer cells. Oncogene. 2008;27:9–19. doi: 10.1038/sj.onc.1210626. [DOI] [PubMed] [Google Scholar]
- 89.Shiozawa T, Nikaido T, Nakayama K, Lu X, Fujii S. Involvement of cyclin-dependent kinase inhibitor p27Kip1 in growth inhibition of endometrium in the secretory phase and of hyperplastic endometrium treated with progesterone. Mol Hum Reprod. 1998;4:899–905. doi: 10.1093/molehr/4.9.899. [DOI] [PubMed] [Google Scholar]
- 90.Watanabe J, Watanabe K, Jobo T, Kamata Y, Kawaguchi M, Imai M, et al. Significance of p27 as a predicting marker for medroxyprogesterone acetate therapy against endometrial endometrioid adenocarcinoma. Int J Gynecol Cancer. 2006;16(Suppl 1):452–7. doi: 10.1111/j.1525-1438.2006.00418.x. [DOI] [PubMed] [Google Scholar]
- 91.Paulssen RH, Moe B, Gronaas H, Orbo A. Gene expression in endometrial cancer cells (Ishikawa) after short time high dose exposure to progesterone. Steroids. 2008;73:116–28. doi: 10.1016/j.steroids.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 92.Hanekamp EE, Kuhne EC, Smid-Koopman E, de Ruiter PE, Chadha-Ajwani S, Brinkmann AO, et al. Loss of progesterone receptor may lead to an invasive phenotype in human endometrial cancer. Eur J Cancer. 2002;38(Suppl 6):S71–2. doi: 10.1016/s0959-8049(02)00294-0. [DOI] [PubMed] [Google Scholar]
- 93.Davies S, Dai D, Wolf DM, Leslie KK. Immunomodulatory and transcriptional effects of progesterone through progesterone A and B receptors in Hec50co poorly differentiated endometrial cancer cells. J Soc Gynecol Investig. 2004;11:494–9. doi: 10.1016/j.jsgi.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 94.Vereide AB, Kaino T, Sager G, Orbo A. Bcl-2, BAX, and apoptosis in endometrial hyperplasia after high dose gestagen therapy: a comparison of responses in patients treated with intrauterine levonorgestrel and systemic medroxyprogesterone. Gynecol Oncol. 2005;97:740–50. doi: 10.1016/j.ygyno.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 95.Dai D, Kumar NS, Wolf DM, Leslie KK. Molecular tools to reestablish progestin control of endometrial cancer cell proliferation. Am J Obstet Gynecol. 2001;184:790–7. doi: 10.1067/mob.2001.113844. [DOI] [PubMed] [Google Scholar]
- 96.Dai D, Litman ES, Schonteich E, Leslie KK. Progesterone regulation of activating protein-1 transcriptional activity: a possible mechanism of progesterone inhibition of endometrial cancer cell growth. J Steroid Biochem Mol Biol. 2003;87:123–31. doi: 10.1016/j.jsbmb.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 97.Davies S, Dai D, Feldman I, Pickett G, Leslie KK. Identification of a novel mechanism of NF-kappaB inactivation by progesterone through progesterone receptors in Hec50co poorly differentiated endometrial cancer cells: induction of A20 and ABIN-2. Gynecol Oncol. 2004;94:463–70. doi: 10.1016/j.ygyno.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 98.Gao J, Mazella J, Seppala M, Tseng L. Ligand activated hPR modulates the glycodelin promoter activity through the Sp1 sites in human endometrial adenocarcinoma cells. Mol Cell Endocrinol. 2001;176:97–102. doi: 10.1016/s0303-7207(01)00450-6. [DOI] [PubMed] [Google Scholar]
- 99.Salih SM, Salama SA, Jamaluddin M, Fadl AA, Blok LJ, Burger CW, et al. Progesterone-mediated regulation of catechol-O-methyl transferase expression in endometrial cancer cells. Reprod Sci. 2008;15:210–20. doi: 10.1177/1933719107310398. [DOI] [PubMed] [Google Scholar]
- 100.Zhang XL, Zhang D, Michel FJ, Blum JL, Simmen FA, Simmen RC. Selective interactions of Kruppel-like factor 9/basic transcription element-binding protein with progesterone receptor isoforms A and B determine transcriptional activity of progesterone-responsive genes in endometrial epithelial cells. J Biol Chem. 2003;278:21474–82. doi: 10.1074/jbc.M212098200. [DOI] [PubMed] [Google Scholar]
- 101.Cramer SF, Patel A. The frequency of uterine leiomyomas. Am J Clin Pathol. 1990;94:435–8. doi: 10.1093/ajcp/94.4.435. [DOI] [PubMed] [Google Scholar]
- 102.Day Baird D, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100–7. doi: 10.1067/mob.2003.99. [DOI] [PubMed] [Google Scholar]
- 103.Farquhar CM, Steiner CA. Hysterectomy rates in the United States 1990–1997. Obstet Gynecol. 2002;99:229–34. doi: 10.1016/s0029-7844(01)01723-9. [DOI] [PubMed] [Google Scholar]
- 104.Parker WH. Etiology, symptomatology, and diagnosis of uterine myomas. Fertil Steril. 2007;87:725–36. doi: 10.1016/j.fertnstert.2007.01.093. [DOI] [PubMed] [Google Scholar]
- 105.Williams AJ, Powell WL, Collins T, Morton CC. HMGI(Y) expression in human uterine leiomyomata. Involvement of another high-mobility group architectural factor in a benign neoplasm. Am J Pathol. 1997;150:911–8. [PMC free article] [PubMed] [Google Scholar]
- 106.Cermik D, Arici A, Taylor HS. Coordinated regulation of HOX gene expression in myometrium and uterine leiomyoma. Fertil Steril. 2002;78:979–84. doi: 10.1016/s0015-0282(02)03366-6. [DOI] [PubMed] [Google Scholar]
- 107.Brandon DD, Bethea CL, Strawn EY, Novy MJ, Burry KA, Harrington MS, et al. Progester-one receptor messenger ribonucleic acid and protein are overexpressed in human uterine leiomyomas. Am J Obstet Gynecol. 1993;169:78–85. doi: 10.1016/0002-9378(93)90135-6. [DOI] [PubMed] [Google Scholar]
- 108.Englund K, Blanck A, Gustavsson I, Lundkvist U, Sjoblom P, Norgren A, et al. Sex steroid receptors in human myometrium and fibroids: changes during the menstrual cycle and gonadotropin-releasing hormone treatment. J Clin Endocrinol Metab. 1998;83:4092–6. doi: 10.1210/jcem.83.11.5287. [DOI] [PubMed] [Google Scholar]
- 109.Kawaguchi K, Fujii S, Konishi I, Nanbu Y, Nonogaki H, Mori T. Mitotic activity in uterine leiomyomas during the menstrual cycle. Am J Obstet Gynecol. 1989;160:637–41. doi: 10.1016/s0002-9378(89)80046-8. [DOI] [PubMed] [Google Scholar]
- 110.Tiltman AJ. The effect of progestins on the mitotic activity of uterine fibromyomas. Int J Gynecol Pathol. 1985;4:89–96. doi: 10.1097/00004347-198506000-00001. [DOI] [PubMed] [Google Scholar]
- 111.Harrison-Woolrych ML, Charnock-Jones DS, Smith SK. Quantification of messenger ribonucleic acid for epidermal growth factor in human myometrium and leiomyomata using reverse transcriptase polymerase chain reaction. J Clin Endocrinol Metab. 1994;78:1179–84. doi: 10.1210/jcem.78.5.8175976. [DOI] [PubMed] [Google Scholar]
- 112.Maruo T, Matsuo H, Samoto T, Shimomura Y, Kurachi O, Gao Z, et al. Effects of progesterone on uterine leiomyoma growth and apoptosis. Steroids. 2000;65:585–92. doi: 10.1016/s0039-128x(00)00171-9. [DOI] [PubMed] [Google Scholar]
- 113.Kurachi O, Matsuo H, Samoto T, Maruo T. Tumor necrosis factor-alpha expression in human uterine leiomyoma and its down-regulation by progesterone. J Clin Endocrinol Metab. 2001;86:2275–80. doi: 10.1210/jcem.86.5.7469. [DOI] [PubMed] [Google Scholar]
- 114.Matsuo H, Kurachi O, Shimomura Y, Samoto T, Maruo T. Molecular bases for the actions of ovarian sex steroids in the regulation of proliferation and apoptosis of human uterine leiomyoma. Oncology. 1999;57(Suppl 2):49–58. doi: 10.1159/000055275. [DOI] [PubMed] [Google Scholar]
- 115.Matsuo H, Maruo T, Samoto T. Increased expression of Bcl-2 protein in human uterine leiomyoma and its up-regulation by progesterone. J Clin Endocrinol Metab. 1997;82:293–9. doi: 10.1210/jcem.82.1.3650. [DOI] [PubMed] [Google Scholar]
- 116.Maruo T, Matsuo H, Shimomura Y, Kurachi O, Gao Z, Nakago S, et al. Effects of progesterone on growth factor expression in human uterine leiomyoma. Steroids. 2003;68:817–24. doi: 10.1016/j.steroids.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 117.Carr BR, Marshburn PB, Weatherall PT, Bradshaw KD, Breslau NA, Byrd W, et al. An evaluation of the effect of gonadotropin-releasing hormone analogs and medroxyprogesterone acetate on uterine leiomyomata volume by magnetic resonance imaging: a prospective, randomized, double blind, placebo-controlled, crossover trial. J Clin Endocrinol Metab. 1993;76:1217–23. doi: 10.1210/jcem.76.5.8496313. [DOI] [PubMed] [Google Scholar]
- 118.Friedman AJ, Daly M, Juneau-Norcross M, Rein MS, Fine C, Gleason R, et al. A prospective, randomized trial of gonadotropin-releasing hormone agonist plus estrogen-progestin or progestin “add-back” regimens for women with leiomyomata uteri. J Clin Endocrinol Metab. 1993;76:1439–45. doi: 10.1210/jcem.76.6.8501148. [DOI] [PubMed] [Google Scholar]
- 119.Rosati P, Exacoustos C, Mancuso S. Longitudinal evaluation of uterine myoma growth during pregnancy. A sonographic study. J Ultrasound Med. 1992;11:511–5. doi: 10.7863/jum.1992.11.10.511. [DOI] [PubMed] [Google Scholar]
- 120.Aharoni A, Reiter A, Golan D, Paltiely Y, Sharf M. Patterns of growth of uterine leiomyomas during pregnancy. A prospective longitudinal study. Br J Obstet Gynaecol. 1988;95:510–3. doi: 10.1111/j.1471-0528.1988.tb12807.x. [DOI] [PubMed] [Google Scholar]
- 121.Neiger R, Sonek JD, Croom CS, Ventolini G. Pregnancy-related changes in the size of uterine leiomyomas. J Reprod Med. 2006;51:671–4. [PubMed] [Google Scholar]
- 122.Hammoud AO, Asaad R, Berman J, Treadwell MC, Blackwell S, Diamond MP. Volume change of uterine myomas during pregnancy: do myomas really grow? J Minim Invasive Gynecol. 2006;13:386–90. doi: 10.1016/j.jmig.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 123.Murphy AA, Kettel LM, Morales AJ, Roberts VJ, Yen SS. Regression of uterine leiomyomata in response to the antiprogesterone RU 486. J Clin Endocrinol Metab. 1993;76:513–7. doi: 10.1210/jcem.76.2.8432797. [DOI] [PubMed] [Google Scholar]
- 124.Murphy AA, Morales AJ, Kettel LM, Yen SS. Regression of uterine leiomyomata to the antiprogesterone RU486: dose-response effect. Fertil Steril. 1995;64:187–90. [PubMed] [Google Scholar]
- 125.Eisinger SH, Meldrum S, Fiscella K, le Roux HD, Guzick DS. Low-dose mifepristone for uterine leiomyomata. Obstet Gynecol. 2003;101:243–50. doi: 10.1016/s0029-7844(02)02511-5. [DOI] [PubMed] [Google Scholar]
- 126.Murphy LL, Mahesh VB. Selective release of follicle-stimulating hormone and luteinizing hormone by 5à-dihydroprogesterone and 3à,5à- tetrahydroprogesterone in pregnant mare’s serum gonadotropin- primed immature rats exposed to constant light. Biol Reprod. 1985;32:795–803. doi: 10.1095/biolreprod32.4.795. [DOI] [PubMed] [Google Scholar]
- 127.Williams AR, Critchley HO, Osei J, Ingamells S, Cameron IT, Han C, et al. The effects of the selective progesterone receptor modulator asoprisnil on the morphology of uterine tissues after 3 months treatment in patients with symptomatic uterine leiomyomata. Hum Reprod. 2007 doi: 10.1093/humrep/dem026. [DOI] [PubMed] [Google Scholar]
- 128.Steinauer J, Pritts EA, Jackson R, Jacoby AF. Systematic review of mifepristone for the treatment of uterine leiomyomata. Obstet Gynecol. 2004;103:1331–6. doi: 10.1097/01.AOG.0000127622.63269.8b. [DOI] [PubMed] [Google Scholar]
- 129.Chwalisz K, Larsen L, Mattia-Goldberg C, Edmonds A, Elger W, Winkel CA. A randomized, controlled trial of asoprisnil, a novel selective progesterone receptor modulator, in women with uterine leiomyomata. Fertil Steril. 2007;87:1399–412. doi: 10.1016/j.fertnstert.2006.11.094. [DOI] [PubMed] [Google Scholar]
- 130.Eisinger SH, Bonfiglio T, Fiscella K, Meldrum S, Guzick DS. Twelve-month safety and efficacy of low-dose mifepristone for uterine myomas. J Minim Invasive Gynecol. 2005;12:227–33. doi: 10.1016/j.jmig.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 131.Fiscella K, Eisinger SH, Meldrum S, Feng C, Fisher SG, Guzick DS. Effect of mifepristone for symptomatic leiomyomata on quality of life and uterine size: a randomized controlled trial. Obstet Gynecol. 2006;108:1381–7. doi: 10.1097/01.AOG.0000243776.23391.7b. [DOI] [PubMed] [Google Scholar]
- 132.Madauss KP, Grygielko ET, Deng SJ, Sulpizio AC, Stanley TB, Wu C, et al. A structural and in vitro characterization of asoprisnil: a selective progesterone receptor modulator. Mol Endocrinol. 2007;21:1066–81. doi: 10.1210/me.2006-0524. [DOI] [PubMed] [Google Scholar]
- 133.Chen W, Ohara N, Wang J, Xu Q, Liu J, Morikawa A, et al. A novel selective progesterone receptor modulator asoprisnil (J867) inhibits proliferation and induces apoptosis in cultured human uterine leiomyoma cells in the absence of comparable effects on myometrial cells. J Clin Endocrinol Metab. 2006;91:1296–304. doi: 10.1210/jc.2005-2379. [DOI] [PubMed] [Google Scholar]
- 134.Wang J, Ohara N, Wang Z, Chen W, Morikawa A, Sasaki H, et al. A novel selective progesterone receptor modulator asoprisnil (J867) down-regulates the expression of EGF, IGF-I, TGFbeta3 and their receptors in cultured uterine leiomyoma cells. Hum Reprod. 2006;21:1869–77. doi: 10.1093/humrep/del035. [DOI] [PubMed] [Google Scholar]
- 135.Sasaki H, Ohara N, Xu Q, Wang J, DeManno DA, Chwalisz K, et al. A novel selective progesterone receptor modulator asoprisnil activates tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated signaling pathway in cultured human uterine leiomyoma cells in the absence of comparable effects on myometrial cells. J Clin Endocrinol Metab. 2007;92:616–23. doi: 10.1210/jc.2006-0898. [DOI] [PubMed] [Google Scholar]
- 136.Stewart EA, Austin DJ, Jain P, Penglase MD, Nowak RA. RU486 suppresses prolactin production in explant cultures of leiomyoma and myometrium. Fertil Steril. 1996;65:1119–24. [PubMed] [Google Scholar]
- 137.Hyder SM, Huang JC, Nawaz Z, Boettger-Tong H, Makela S, Chiappetta C, et al. Regulation of vascular endothelial growth factor expression by estrogens and progestins. Environ Health Perspect. 2000;108(Suppl 5):785–90. doi: 10.1289/ehp.00108s5785. [DOI] [PubMed] [Google Scholar]
- 138.Wang H, Jin J. Effects of mifepristone on estrogen and progestin receptors in human uterine leiomyoma. Zhonghua Fu Chan Ke Za Zhi. 2000;35:79–81. [PubMed] [Google Scholar]
- 139.Chegini N, Ma C, Tang XM, Williams RS. Effects of GnRH analogues, ‘add-back’ steroid therapy, antiestrogen and antiprogestins on leiomyoma and myometrial smooth muscle cell growth and transforming growth factor-beta expression. Mol Hum Reprod. 2002;8:1071–8. doi: 10.1093/molehr/8.12.1071. [DOI] [PubMed] [Google Scholar]
- 140.Jeng YJ, Suarez VR, Izban MG, Wang HQ, Soloff MS. Progesterone-induced sphingosine kinase-1 expression in the rat uterus during pregnancy and signaling consequences. Am J Physiol Endocrinol Metab. 2007;292:E1110–21. doi: 10.1152/ajpendo.00373.2006. [DOI] [PubMed] [Google Scholar]
- 141.Kettel LM, Murphy AA, Morales AJ, Yen SS. Clinical efficacy of the antiprogesterone RU486 in the treatment of endometriosis and uterine fibroids. Hum Reprod. 1994;9(Suppl 1):116–20. doi: 10.1093/humrep/9.suppl_1.116. [DOI] [PubMed] [Google Scholar]
- 142.Murphy AA, Castellano PZ. RU486: pharmacology and potential use in the treatment of endometriosis and leiomyomata uteri. Curr Opin Obstet Gynecol. 1994;6:269–78. [PubMed] [Google Scholar]
- 143.Ramachandran S, Song MQ, Lowe E, Dominguez CE, Parthasarathy S, Murphy AA. RU486 inhibits expression of lysophosphatidic acid induced glycodelin. Am J Obstet Gynecol. 2005;192:1285–93. doi: 10.1016/j.ajog.2004.12.084. discussion 1293–84. [DOI] [PubMed] [Google Scholar]
- 144.Kayisli UA, Berkkanoglu M, Kizilay G, Senturk L, Arici A. Expression of proliferative and preapoptotic molecules in human myometrium and leiomyoma throughout the menstrual cycle. Reprod Sci. 2007;14:678–86. doi: 10.1177/1933719107305866. [DOI] [PubMed] [Google Scholar]
- 145.Shimomura Y, Matsuo H, Samoto T, Maruo T. Up-regulation by progesterone of proliferating cell nuclear antigen and epidermal growth factor expression in human uterine leiomyoma. J Clin Endocrinol Metab. 1998;83:2192–8. doi: 10.1210/jcem.83.6.4879. [DOI] [PubMed] [Google Scholar]
- 146.Ohara N, Morikawa A, Chen W, Wang J, DeManno DA, Chwalisz K, et al. Comparative effects of SPRM asoprisnil (J867) on proliferation, apoptosis, and the expression of growth factors in cultured uterine leiomyoma cells and normal myometrial cells. Reprod Sci. 2007;14:20–7. doi: 10.1177/1933719107311464. [DOI] [PubMed] [Google Scholar]
- 147.Kovacs KA, Lengyel F, Kornyei JL, Vertes Z, Szabo I, Sumegi B, et al. Differential expression of Akt/protein kinase B, Bcl-2 and Bax proteins in human leiomyoma and myometrium. J Steroid Biochem Mol Biol. 2003;87:233–40. doi: 10.1016/j.jsbmb.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 148.Wu X, Blanck A, Olovsson M, Henriksen R, Lindblom B. Expression of Bcl-2, Bcl-x, Mcl-1, Bax and Bak in human uterine leiomyomas and myometrium during the menstrual cycle and after menopause. J Steroid Biochem Mol Biol. 2002;80:77–83. doi: 10.1016/s0960-0760(01)00177-7. [DOI] [PubMed] [Google Scholar]
- 149.Yin P, Lin Z, Cheng YH, Marsh EE, Utsunomiya H, Ishikawa H, et al. Progesterone receptor regulates Bcl-2 gene expression through direct binding to its promoter region in uterine leiomyoma cells. J Clin Endocrinol Metab. 2007;92:4459–66. doi: 10.1210/jc.2007-0725. [DOI] [PubMed] [Google Scholar]
- 150.Hassan MH, Salama SA, Arafa HM, Hamada FM, Al-Hendy A. Adenovirus-mediated delivery of a dominant-negative estrogen receptor gene in uterine leiomyoma cells abrogates estrogen- and progesterone-regulated gene expression. J Clin Endocrinol Metab. 2007;92:3949–57. doi: 10.1210/jc.2007-0823. [DOI] [PubMed] [Google Scholar]
- 151.Stewart EA, Friedman AJ, Peck K, Nowak RA. Relative overexpression of collagen type I and collagen type III messenger ribonucleic acids by uterine leiomyomas during the proliferative phase of the menstrual cycle. J Clin Endocrinol Metab. 1994;79:900–6. doi: 10.1210/jcem.79.3.8077380. [DOI] [PubMed] [Google Scholar]
- 152.Dou Q, Tarnuzzer RW, Williams RS, Schultz GS, Chegini N. Differential expression of matrix metalloproteinases and their tissue inhibitors in leiomyomata: a mechanism for gonadotrophin releasing hormone agonist-induced tumour regression. Mol Hum Reprod. 1997;3:1005–14. doi: 10.1093/molehr/3.11.1005. [DOI] [PubMed] [Google Scholar]
- 153.Levens E, Luo X, Ding L, Williams RS, Chegini N. Fibromodulin is expressed in leiomyoma and myometrium and regulated by gonadotropin-releasing hormone analogue therapy and TGF-beta through Smad and MAPK-mediated signalling. Mol Hum Reprod. 2005;11:489–94. doi: 10.1093/molehr/gah187. [DOI] [PubMed] [Google Scholar]
- 154.Morikawa A, Ohara N, Xu Q, Nakabayashi K, DeManno DA, Chwalisz K, et al. Selective progesterone receptor modulator asoprisnil down-regulates collagen synthesis in cultured human uterine leiomyoma cells through up-regulating extracellular matrix metalloproteinase inducer. Hum Reprod. 2008;23:944–51. doi: 10.1093/humrep/den025. [DOI] [PubMed] [Google Scholar]
- 155.Marsh EE, Lin Z, Yin P, Milad M, Chakravarti D, Bulun SE. Differential expression of microRNA species in human uterine leiomyoma versus normal myometrium. Fertil Steril. 2008;89:1771–6. doi: 10.1016/j.fertnstert.2007.05.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wang T, Zhang X, Obijuru L, Laser J, Aris V, Lee P, et al. A micro-RNA signature associated with race, tumor size, and target gene activity in human uterine leiomyomas. Genes Chromosomes Cancer. 2007;46:336–47. doi: 10.1002/gcc.20415. [DOI] [PubMed] [Google Scholar]
- 157.Pan Q, Luo X, Chegini N. Differential expression of microRNAs in myometrium and leiomyomas and regulation by ovarian steroids. J Cell Mol Med. 2008;12:227–40. doi: 10.1111/j.1582-4934.2007.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 158.Rossi MJ, Chegini N, Masterson BJ. Presence of epidermal growth factor, platelet-derived growth factor, and their receptors in human myometrial tissue and smooth muscle cells: their action in smooth muscle cells in vitro. Endocrinology. 1992;130:1716–27. doi: 10.1210/endo.130.3.1311246. [DOI] [PubMed] [Google Scholar]
- 159.Yeh J, Rein M, Nowak R. Presence of messenger ribonucleic acid for epidermal growth factor (EGF) and EGF receptor demonstrable in monolayer cell cultures of myometria and leiomyomata. Fertil Steril. 1991;56:997–1000. doi: 10.1016/s0015-0282(16)54681-0. [DOI] [PubMed] [Google Scholar]
- 160.Chandrasekhar Y, Heiner J, Osuamkpe C, Nagamani M. Insulin-like growth factor I and II binding in human myometrium and leiomyomas. Am J Obstet Gynecol. 1992;166:64–9. doi: 10.1016/0002-9378(92)91831-t. [DOI] [PubMed] [Google Scholar]
- 161.Yu L, Saile K, Swartz CD, He H, Zheng X, Kissling GE, et al. Differential expression of receptor tyrosine kinases (RTKs) and IGF-I pathway activation in human uterine leiomyomas. Mol Med. 2008;14:264–75. doi: 10.2119/2007-00101.Yu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gao Z, Matsuo H, Wang Y, Nakago S, Maruo T. Up-regulation by IGF-I of proliferating cell nuclear antigen and Bcl-2 protein expression in human uterine leiomyoma cells. J Clin Endocrinol Metab. 2001;86:5593–9. doi: 10.1210/jcem.86.11.8008. [DOI] [PubMed] [Google Scholar]
- 163.Strawn EY, Jr, Novy MJ, Burry KA, Bethea CL. Insulin-like growth factor I promotes leiomyoma cell growth in vitro. Am J Obstet Gynecol. 1995;172:1837–43. doi: 10.1016/0002-9378(95)91420-x. discussion 1843–4. [DOI] [PubMed] [Google Scholar]
- 164.van der Ven LT, Gloudemans T, Roholl PJ, van Buul-Offers SC, Bladergroen BA, Welters MJ, et al. Growth advantage of human leiomyoma cells compared to normal smooth-muscle cells due to enhanced sensitivity toward insulin-like growth factor I. Int J Cancer. 1994;59:427–34. doi: 10.1002/ijc.2910590323. [DOI] [PubMed] [Google Scholar]
- 165.Giudice LC, Irwin JC, Dsupin BA, Pannier EM, Jin IH, Vu TH, et al. Insulin-like growth factor (IGF), IGF binding protein (IGFBP), and IGF receptor gene expression and IGFBP synthesis in human uterine leiomyomata. Hum Reprod. 1993;8:1796–806. doi: 10.1093/oxfordjournals.humrep.a137937. [DOI] [PubMed] [Google Scholar]
- 166.Yamada T, Nakago S, Kurachi O, Wang J, Takekida S, Matsuo H, et al. Progesterone down-regulates insulin-like growth factor-I expression in cultured human uterine leiomyoma cells. Hum Reprod. 2004;19:815–21. doi: 10.1093/humrep/deh146. [DOI] [PubMed] [Google Scholar]
- 167.Palman C, Bowen-Pope DF, Brooks JJ. Platelet-derived growth factor receptor (beta-subunit) immunoreactivity in soft tissue tumors. Lab Invest. 1992;66:108–15. [PubMed] [Google Scholar]
- 168.Arici A, Sozen I. Expression, menstrual cycle-dependent activation, and bimodal mitogenic effect of transforming growth factor-beta1 in human myometrium and leiomyoma. Am J Obstet Gynecol. 2003;188:76–83. doi: 10.1067/mob.2003.118. [DOI] [PubMed] [Google Scholar]
- 169.Fayed YM, Tsibris JC, Langenberg PW, Robertson AL., Jr Human uterine leiomyoma cells: binding and growth responses to epidermal growth factor, platelet-derived growth factor, and insulin. Lab Invest. 1989;60:30–7. [PubMed] [Google Scholar]
- 170.Ross R, Bowen-Pope DF, Raines EW. Platelet-derived growth factor: its potential roles in wound healing, atherosclerosis, neoplasia, and growth and development. Ciba Found Symp. 1985;116:98–112. doi: 10.1002/9780470720974.ch7. [DOI] [PubMed] [Google Scholar]
- 171.Liang M, Wang H, Zhang Y, Lu S, Wang Z. Expression and functional analysis of platelet-derived growth factor in uterine leiomyomata. Cancer Biol Ther. 2006;5:28–33. doi: 10.4161/cbt.5.1.2234. [DOI] [PubMed] [Google Scholar]
- 172.Boehm KD, Daimon M, Gorodeski IG, Sheean LA, Utian WH, Ilan J. Expression of the insulin-like and platelet-derived growth factor genes in human uterine tissues. Mol Reprod Dev. 1990;27:93–101. doi: 10.1002/mrd.1080270203. [DOI] [PubMed] [Google Scholar]
- 173.Mangrulkar RS, Ono M, Ishikawa M, Takashima S, Klagsbrun M, Nowak RA. Isolation and characterization of heparin-binding growth factors in human leiomyomas and normal myometrium. Biol Reprod. 1995;53:636–46. doi: 10.1095/biolreprod53.3.636. [DOI] [PubMed] [Google Scholar]
- 174.Hwu YM, Li SH, Lee RK, Tsai YH, Yeh TS, Lin SY. Increased expression of platelet-derived growth factor C messenger ribonucleic acid in uterine leiomyomata. Fertil Steril. 2008;89:468–71. doi: 10.1016/j.fertnstert.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 175.Ingman WV, Robertson SA. Defining the actions of transforming growth factor beta in reproduction. Bioessays. 2002;24:904–14. doi: 10.1002/bies.10155. [DOI] [PubMed] [Google Scholar]
- 176.Verrecchia F, Mauviel A. Transforming growth factor-beta and fibrosis. World J Gastroenterol. 2007;13:3056–62. doi: 10.3748/wjg.v13.i22.3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Dou Q, Zhao Y, Tarnuzzer RW, Rong H, Williams RS, Schultz GS, et al. Suppression of transforming growth factor-beta (TGF beta) and TGF beta receptor messenger ribonucleic acid and protein expression in leiomyomata in women receiving gonadotropin-releasing hormone agonist therapy. J Clin Endocrinol Metab. 1996;81:3222–30. doi: 10.1210/jcem.81.9.8784073. [DOI] [PubMed] [Google Scholar]
- 178.Tang XM, Dou Q, Zhao Y, McLean F, Davis J, Chegini N. The expression of transforming growth factor-beta s and TGF-beta receptor mRNA and protein and the effect of TGF-beta s on human myometrial smooth muscle cells in vitro. Mol Hum Reprod. 1997;3:233–40. doi: 10.1093/molehr/3.3.233. [DOI] [PubMed] [Google Scholar]
- 179.Chegini N, Tang XM, Ma C. Regulation of transforming growth factor-beta1 expression by granulocyte macrophage-colony-stimulating factor in leiomyoma and myometrial smooth muscle cells. J Clin Endocrinol Metab. 1999;84:4138–43. doi: 10.1210/jcem.84.11.6147. [DOI] [PubMed] [Google Scholar]
- 180.De Falco M, Staibano S, D’Armiento FP, Mascolo M, Salvatore G, Busiello A, et al. Preoperative treatment of uterine leiomyomas: clinical findings and expression of transforming growth factor-beta3 and connective tissue growth factor. J Soc Gynecol Investig. 2006;13:297–303. doi: 10.1016/j.jsgi.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 181.Lee BS, Nowak RA. Human leiomyoma smooth muscle cells show increased expression of transforming growth factor-beta 3 (TGF beta 3) and altered responses to the antiproliferative effects of TGF beta. J Clin Endocrinol Metab. 2001;86:913–20. doi: 10.1210/jcem.86.2.7237. [DOI] [PubMed] [Google Scholar]
- 182.McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007;1773:1263–84. doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Barbarisi A, Petillo O, Di Lieto A, Melone MA, Margarucci S, Cannas M, et al. 17-Beta estradiol elicits an autocrine leiomyoma cell proliferation: evidence for a stimulation of protein kinase-dependent pathway. J Cell Physiol. 2001;186:414–24. doi: 10.1002/1097-4652(2000)9999:999<000::AID-JCP1040>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 184.Hermon TL, Moore AB, Yu L, Kissling GE, Castora FJ, Dixon D. Estrogen receptor alpha (ERalpha) phosphor-serine-118 is highly expressed in human uterine leiomyomas compared to matched myometrium. Virchows Arch. 2008;453:557–69. doi: 10.1007/s00428-008-0679-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Simoncini T, Hafezi-Moghadam A, Brazil DP, Ley K, Chin WW, Liao JK. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature. 2000;407:538–41. doi: 10.1038/35035131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Carnevale RP, Proietti CJ, Salatino M, Urtreger A, Peluffo G, Edwards DP, et al. Progestin effects on breast cancer cell proliferation, proteases activation, and in vivo development of metastatic phenotype all depend on progesterone receptor capacity to activate cytoplasmic signaling pathways. Mol Endocrinol. 2007;21:1335–58. doi: 10.1210/me.2006-0304. [DOI] [PubMed] [Google Scholar]
- 187.Boonyaratanakornkit V, Scott MP, Ribon V, Sherman L, Anderson SM, Maller JL, et al. Progesterone receptor contains a proline-rich motif that directly interacts with SH3 domains and activates c-Src family tyrosine kinases. Mol Cell. 2001;8:269–80. doi: 10.1016/s1097-2765(01)00304-5. [DOI] [PubMed] [Google Scholar]
- 188.Ballare C, Vallejo G, Vicent GP, Saragueta P, Beato M. Progesterone signaling in breast and endometrium. J Steroid Biochem Mol Biol. 2006;102:2–10. doi: 10.1016/j.jsbmb.2006.09.030. [DOI] [PubMed] [Google Scholar]
- 189.Lengyel F, Vertes Z, Kovacs KA, Kornyei JL, Sumegi B, Vertes M. Effect of estrogen and inhibition of phosphatidylinositol-3 kinase on Akt and FOXO1 in rat uterus. Steroids. 2007;72:422–8. doi: 10.1016/j.steroids.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 190.Vallejo G, Ballare C, Baranao JL, Beato M, Saragueta P. Progestin activation of nongenomic pathways via cross talk of progesterone receptor with estrogen receptor beta induces proliferation of endometrial stromal cells. Mol Endocrinol. 2005;19:3023–37. doi: 10.1210/me.2005-0016. [DOI] [PubMed] [Google Scholar]
- 191.Hoekstra AV, Sefton EC, Berry E, Lu Z, Hardt J, Marsh E, et al. Progestins activate the AKT pathway in leiomyoma cells and promote survival. J Clin Endocrinol Metab. 2009 doi: 10.1210/jc.2008-2093. in review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kovacs KA, Lengyel F, Vertes Z, Kornyei JL, Gocze PM, Sumegi B, et al. Phosphorylation of PTEN (phosphatase and tensin homologue deleted on chromosome ten) protein is enhanced in human fibromyomatous uteri. J Steroid Biochem Mol Biol. 2007;103:196–9. doi: 10.1016/j.jsbmb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 193.Bifulco G, Miele C, Pellicano M, Trencia A, Ferraioli M, Paturzo F, et al. Molecular mechanisms involved in GnRH analogue-related apoptosis for uterine leiomyomas. Mol Hum Reprod. 2004;10:43–8. doi: 10.1093/molehr/gah002. [DOI] [PubMed] [Google Scholar]
- 194.Hodges LC, Houston KD, Hunter DS, Fuchs-Young R, Zhang Z, Wineker RC, et al. Transdominant suppression of estrogen receptor signaling by progesterone receptor ligands in uterine leiomyoma cells. Mol Cell Endocrinol. 2002;196:11–20. doi: 10.1016/s0303-7207(02)00230-7. [DOI] [PubMed] [Google Scholar]


