Abstract
Malignant brain cancer treatment is limited by a number of barriers, including the blood–brain barrier, transport within the brain interstitium, difficulties in delivering therapeutics specifically to tumor cells, the highly invasive quality of gliomas and drug resistance. As a result, the prognosis for patients with high-grade gliomas is poor and has improved little in recent years. Nanomedicine approaches have been developed in the laboratory, with some technologies being translated to the clinic, in order to address these needs. This review discusses the obstacles to effective treatment that are currently faced in the field, as well as various nanomedicine techniques that have been used or are being explored to overcome them, with a focus on liposomal and polymeric nanoparticles.
Current state of malignant brain cancer
Nearly 25,000 new cases of malignant brain cancer are estimated to be diagnosed in the USA each year, accounting for close to 15,000 deaths [1]. This review focuses on gliomas, the three major classes of which according to WHO classification are astrocytomas, oligodendrogliomas and mixed gliomas (oligoastrocytomas) [2]. The most common of these are astrocytomas, which the WHO separates into various grades of severity based on morphological abnormalities, increased angiogenesis and proliferation, and the presence of necroses. Malignant or high-grade astrocytomas include the grade III anaplastic astrocytomas and grade IV glioblastoma (GB) and are the most common gliomas. Generally, malignant astrocytomas are treated by surgical resection, chemotherapy and radiotherapy, with the goal of prolonging survival time and improving quality of life rather than curing the disease. The median survival for patients with grade III and IV glioma is 2–3 years and 14–15 months [3,4], respectively, prompting the need for novel treatments that can promote a better outcome.
The field of nanomedicine has opened opportunities for improved brain cancer diagnosis and treatment. Nanoparticulate systems are already in use in the clinic for enhancing treatment of various other cancers. Because of the wide variety of biocompatible materials being researched, the range of nanocarrier compositions and design strategies is very wide. While many of these methods are still experimental, nanoparticles have shown great promise for improving delivery of imaging agents and chemotherapeutics. Nanoparticles have been a major focus of research in the drug delivery field because of a wide range of advantages afforded by their small size and other properties. For the purposes of this review, the term ‘nanoparticle’ will be used in reference to any particle of any shape and material below 1 μm in diameter, general types and properties of which will be described briefly. Barriers to effective brain cancer treatment (Table 1; Figure 1) will be discussed, as well as strategies currently under investigation to overcome them.
Table 1.
Strategies for nanoparticle delivery to the brain.
| Approaches to overcome | Examples | Ref. |
|---|---|---|
|
| ||
| Short drug half-life | ||
|
| ||
| Protection of therapeutic from degradation | Condensation of nucleic acid cargo with poly(beta-amino esters) or metallic nanoparticles | [14,124] |
|
| ||
| Increase circulation time | Surface coating with ‘stealth’ molecules such as PEG | [12,18] |
|
| ||
| BBB | ||
|
| ||
| Enhanced permeation and retention effect | Small (<100 nm or <20 nm) inorganic nanoparticles accumulate at the tumor site | [35,131] |
|
| ||
| Global BBB disruption | Use of hypertonic mannitol or vasoactive agents to increase BBB permeability | [29] |
|
| ||
| BBB-penetrating nanoparticles | Coating with ligands for the LDL receptor, such as apoA-I, apoE or angiopep-2 | [43,44,45] |
| Coating with surfactants that increase apolipoprotein deposition, such as poloxamer 188 | [48,49] | |
| Coating with cationized serum albumin | [54] | |
| Conjugation to peptides derived from BBB-penetrating viruses and toxins | [55,57,58] | |
| Conjugation with ligands that bind BBB endothelial cells, such as transferrin and insulin receptors | [61,65,66] | |
|
| ||
| Insufficient transport through brain interstitium | ||
|
| ||
| Increase nanoparticle diffusivity | Decrease nanoparticle size to <70 nm | [75] |
|
| ||
| Surface modification with a dense layer of PEG | [76] | |
|
| ||
| Increase bulk fluid flow for nanoparticle convection | Convection-enhanced delivery of poly(lactic-co-glycolic acid), lipid-based, magnetic, dendrimer and virus nanoparticles | [79–84] |
|
| ||
| Nonspecific delivery of drug to healthy tissue | ||
|
| ||
| Local delivery | Direct injection of particles into tumor or tumor periphery | [79–84] |
|
| ||
| Surface modification with ligands targeting fast-growing cells | Conjugation of nanoparticles to ligands like transferrin or folate | [62,65–67] |
|
| ||
| Surface modification with ligands targeting cancer cells | Conjugation of nanoparticles to chlorotoxin, IL-13, CREKA peptide or glutathione | [87–91,94] |
|
| ||
| Preferential nanoparticle degradation or disassembly in tumor environment | Nanogels containing poly(N-isopropylacrylamide) for preferential release at lowered pH | [92] |
|
| ||
| Engineering of tumor-homing stem cells to act as delivery vehicles | Neutral stem cells and mesenchymal stem cells virally transduced to express cytosine deaminase or HSV-TK to sensitize to prodrugs 5-FC and ganciclovir | [95,97,98] |
| Mesenchymal stem cells carry tumor-killing agent directly to brain tumor via transduction of IFN-β or infection with oncolytic virus | [96,99] | |
| Mesenchymal stem cells carry nanoparticles loaded with imaging contrast agents | [100–102] | |
BBB: Blood–brain barrier.
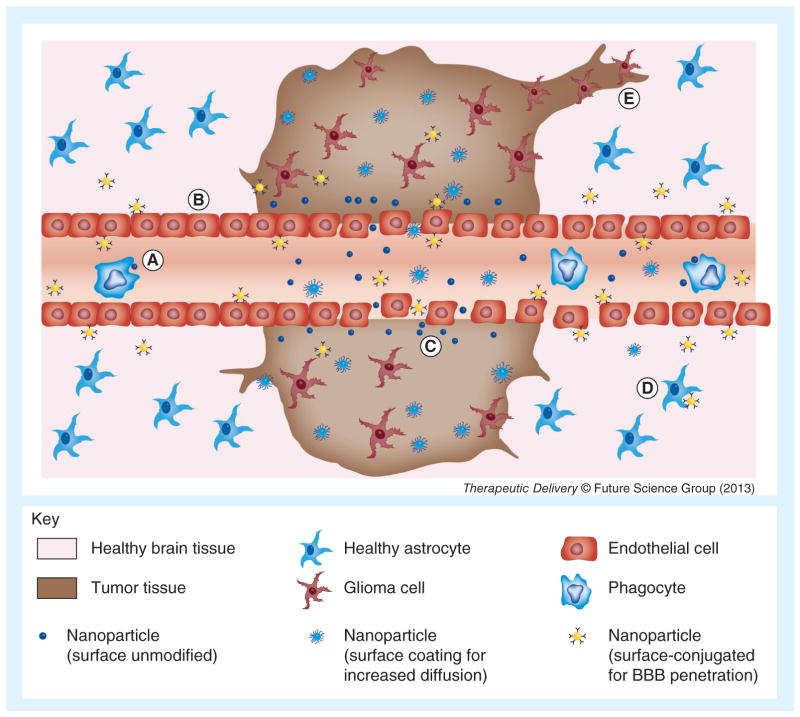
Figure 1. Major barriers in nanoparticle delivery to malignant glioma.
These barriers can be overcome by various particle modifications, such as ‘stealth’ surface coating, exploitation of the enhanced permeation and retention effect, or conjugation with BBB-penetrating or -binding molecules. (A) Clearance by immune cells; short half-life, (B) BBB, (C) insufficient diffusion through tissue, (D) non-specific delivery to healthy cells and (E) tumor invasion. BBB: Blood–brain barrier.
Types of nanoparticles & general properties
Nanoparticles can be fabricated using a wide range of different materials. Synthetic polymers, such as poly(glycolic acid), poly(lactic acid) (PLA), and the copolymer poly(lactic-co-glycolic acid) (PLGA), are biocompatible in a number of systems and are hydrolytically degradable, leading to their use in encapsulating and delivering many types of therapeutic molecules, including conventional small-molecule drugs and large biological macromolecules such as proteins and nucleic acids [5]. Other degradable polymers, including chitosan [6,7], poly(beta-amino esters) [8,9], poly(amidoamines) [10], and many other cationic polymers have also been used to deliver therapeutics to brain cancer models. These positively charged polymers are studied particularly for delivery of nucleic acids, because their cationic character allows them to form nanocomplexes with negatively charged DNA or RNA. While degradable cationic polymers are being studied as an alternative to virus-mediated gene transfer, viruses are also under investigation for their potential to treat brain cancer. Amphiphilic lipids can also self-assemble to form liposomes, artificial vesicles that can be used to encapsulate and deliver either hydrophobic or hydrophilic molecules and whose size can be controlled by the size and properties of the constituent lipids [11]. For example, the chemotherapeutic doxorubicin, with a circulation time of 10–15 min as the free drug, can be encapsulated in liposomes to increase this time to over 2 h [12].
Inorganic nanoparticles can also be used for imaging as well as drug delivery purposes, with advantages of highly tunable and reproducible synthesis processes. Injectable superparamagnetic iron oxide (SPIO) nanoparticles can provide contrast for MRI [13] while other metallic nano-particles, such as materials like gold, can be used to carry a conjugated drug or be used for photo-thermal therapy [14,15]. Mesoporous silicon particles can be loaded with therapeutics or other smaller, drug-loaded nanoparticles for multi-stage delivery or imaging systems [16,17].
The surface of any of these types of nano-particles can be modified to change their transport properties and biological responses to them. One common modification is PEGylation, coating polyethylene glycol onto a surface via physical interaction or covalent conjugation. PEGylation creates a hydrophilic and non-charged surface, which can prevent early clearance of nanoparticles and thereby increase their circulation time [18]. For example, liposomes are often cleared quickly from circulation by the mononuclear phagocyte system [19,20], but their half-life in circulation increases from 2 to 40–71 h when the liposomes are PEGylated [12]. In addition, nanoparticle size, an important property in determining drug delivery efficacy, can often be varied during particle fabrication. A particle’s size affects its non-specific uptake by cells and clearance as well as its ability to deliver intracellular drugs to target cells. Intracellular delivery remains a general challenge in drug delivery because of the plasma membrane that prevents penetration of many drugs and downstream barriers that cause degradation of the drug or prevent it from arriving at the necessary location for activity [21]. The use of a wide variety of drug delivery systems has been explored to increase intracellular drug concentration or to avoid drug efflux as a mechanism of resistance [22]. In particular, the small size of nanoparticles has been demonstrated to allow cellular uptake [1,23,24]. Although uptake is also affected by other factors, including particle composition and surface properties [25,26], particle sizes between 5–500 nm have demonstrated uptake in different cell types and delivery systems, further emphasizing the utility of nanomedicine in cancer treatment.
Another general barrier to effective drug delivery to tumors is the problem of achieving high drug concentration at the site of interest. Concentration of a drug at a tumor site is often limited by short circulation time of the drug due to nonspecific uptake or accumulation in healthy tissue, degradation, and excretion. Nanoparticulate systems have been used to improve the half-life of common tumor drugs like doxorubicin.
Barriers to delivery to the brain
The blood–brain barrier
While radiotherapy has been shown to improve prognosis in malignant glioma, the benefits of chemotherapy are controversial, with clinical trials showing wide variation in whether or not, and to what degree, chemotherapy confers a survival benefit in these patients [27,28]. A challenge specific to delivery of high concentrations of drug to the brain is the presence of the blood–brain barrier (BBB) [29], the arrangement of endothelial cells in capillaries of the brain that ensures particularly tight regulation of transport between the bloodstream and brain tissue. Several methods have been explored to allow transport of a therapeutic across the BBB, each with different advantages and safety concerns [30], some of which will be discussed broadly below. However, as in cancers of other systems, GB tumors are fed by a ‘leaky’ vasculature, an abnormal and disorganized network of blood vessels formed by the high rate of angiogenesis that characterizes high-grade malignant tumors [31]. The discovery that molecules and small particles can more easily extravasate from tumor vasculature and are returned inefficiently to circulation due to poor lymphatic drainage was a major finding in cancer therapy research and was coined the enhanced permeation and retention (EPR) effect [32].
In addition to carrying intracellular drugs and increasing cargo half-life, nanoparticles have been of increasing interest to the cancer drug delivery field for their ability to take advantage of the EPR effect. Particles less than approximately 100 nm in diameter extravasate preferentially from tumor vasculature, due to increased vascular permeability compared with normal tissue, and particles greater than approximately 20 nm are retained in the tumor tissue rather than returning freely to the circulation [33]. Larger particles are unable to leak out of the disorganized blood vessels that supply tumors, while smaller ones are less effectively retained. This effect depends primarily on the physical properties of the nanoparticles – particularly size and the ability to remain in circulation long enough to extravasate into a tumor – but it is limited by a lack of specificity for particular tissues.
The question of whether or not – and to what degree – the BBB is a major obstacle for drug delivery to brain tumors has not seen consensus in the literature over the past decades, although various studies using conventional chemo-therapeutics have found high variability or often only minimal enhanced drug retention at the glioma site over other tissues [34]. Some researchers have found that the EPR effect can allow for accumulation in the tumor via the porous brain tumor–blood barrier (BTBB) and have reported accumulation of imaging agents or particles on the order of 100 nm in diameter [35]. Many others have found that this passive targeting alone cannot overcome the BBB or does so to a degree that is insufficient for effective treatment, showing that only smaller particles (approximately 20 nm) or smaller (<12 nm) [36] are able to cross the BTBB [37,38]. The tumor vasculature is inhomogeneous [39], and some studies show that the BBB remains largely intact at tumor margins despite being more permeable within the bulk of the tumor, which hinders delivery of drugs to the tumor cells most directly responsible for invasion and migration; in addition, increased interstitial pressure within tumors tends to oppose passive diffusion out of the circulation and into the tumor tissue [40]. As a result, although studies have found that small nanoparticles and drugs are able to take advantage of the leaky tumor vas-culature [41], various methods to further improve penetration of the BTBB must also explored.
One method to improve delivery is the use of BBB disruption to further increase the permeability of these vessels by increasing the local osmotic pressure within brain vasculature using hyperosmotic agents [29]. However, this method is known to have significant patient-to-patient variability, which impedes widespread and consistent use [42]. Furthermore, all of these BBB disruption methods cause decreased integrity of the entire BBB, not specifically the vasculature of the tumor, which could adversely affect healthy brain tissue. Aside from non-specific delivery of the chemotherapeutic to healthy tissue, which can lead to unwanted toxicity and dose-limiting side effects, BBB disruption can also allow leakage of unwanted molecules from the circulation into the brain through the globally compromised BBB [37]. To avoid disrupting the entire BBB, various strategies have been employed to penetrate the BBB via the nanoparticle drug carriers themselves.
BBB-penetrating nanoparticles
The LDL receptors on endothelial cells of the BBB can facilitate uptake of nanoparticles coated with ligands for the LDL receptor. Xin et al. showed that Angiopep-coated PEG–poly(ε–caprolactone) nanoparticles could accumulate in the tumor bed in vivo due to the passive EPR effect as well as to the active targeting through Angiopep [43]. In addition, apolipoprotein-coated particles both in vitro [44], using apoA-I-coated protamine/oligonucleotide nanoparticles, and in vivo [45], using apoE-coated serum albumin nanoparticles are taken up by binding to the LDL receptor. By coating the particles with an agent that causes absorptive uptake, this study demonstrated uptake of drug-containing nanoparticles by the BBB endothelium via adsorption by the cells, rather than increased brain penetration via increasing gaps between endothelial cells in the entire brain. Using a similar principle, Kreuter et al. developed poly(butyl cyanoacrylate) nanoparticles as carriers for a peptide that normally could not cross the BBB in any measurable amount. By coating the particles with a surfactant that caused apolipoprotein deposition onto the nanoparticles once in the plasma, they achieved uptake of their particles by absorptive uptake [46]. The use of polysorbate 80 [47] and other surfactant coatings, such as poloxamer 188 [48,49] and Tween® 80 [50] has also been shown to cause high uptake by BBB endothelial cells in models in vitro and led to higher accumulation in the brain in vivo, with varying degrees of toxicity depending on the particular system studied. Liposomes have also been demonstrated to increase BBB penetration [51]. Other lipid-based nanoparticles have been reported to have BBB-penetrating properties as well as the ability to take advantage of the EPR effect in tumor models [52].
By including the BBB-penetrating agent as part of the delivery vehicle itself, inducing endocytosis by endothelial cells rather than physical disruption of the endothelial layer, the use of nanocarriers can reduce the abnormal passage of other molecules into and out of the vasculature of the brain. Although with BBB-penetrating nanoparticles, nanomedicines may no longer be restricted in size by the gaps between endothelial cells, particle size is also an important factor in cellular uptake. Endocytosis, whether clathrin- or caveolae-dependent or -independent, generally involves the formation of vesicles less than 150 nm [53]. In order to either exploit the EPR effect or induce internalization by endothelial cells, particles being studied as potential drug carriers to brain tumors are generally less than 150 nm in diameter.
Surface-modified nanoparticles for BBB penetration
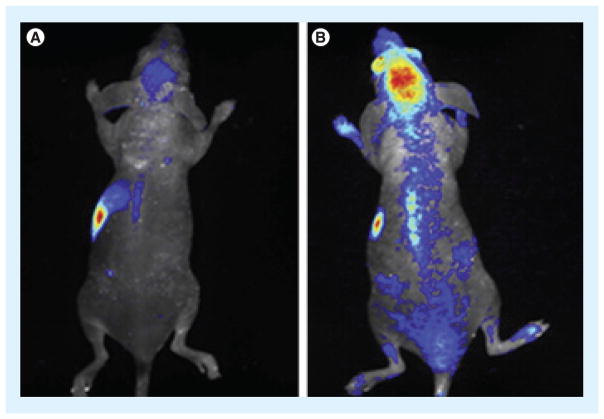
Another advantage of nanoparticle drug carriers in BBB penetration is their chemical versatility. Aside from surface coatings to increase uptake, such as surfactants or stealth coatings such as PEG, ligands and other biological or chemical moieties can be conjugated to the surface to promote active uptake by cells on the luminal side, trafficking of the particle through the endothelial cell, and then exocytosis into the brain tissue. As an example of the various functions that can be designed into nanoparticle systems, PLA nanoparticles were surface-modified with PEG for stability and with cationic serum albumin for increased circulation time. The cationized albumin was able to facilitate interaction of the particles with brain endothelial cells to promote uptake with little to no toxicity observed [54]. Because viruses are also essentially nano-carriers that have evolved an efficient ability to cross cellular barriers, including the BBB, virally derived ligands have also been used to penetrate the BBB, such as the HIV Tat peptide. Polymeric core/shell nanoparticles, made of amphiphilic polymers that form micellar structures, were conjugated to Tat peptide, allowing them to cross the BBB into the brain [55]. Qin et al. used Tat-conjugated cholesterol to formulate liposomes that showed the ability to transcytose through brain capillary endothelial cells and accumulate in the brain [56]. In another study, poly(amidoamine) dendrimers were conjugated to a peptide derived from the rabies virus glycoprotein, RVG29, through a PEG linker and conjugated with DNA to form nanoparticles. The RVG29-modified DNA-containing nano-particles accumulated in the brain significantly more efficiently than unmodified nanoparticles (Figure 2) [57]. Other researchers have taken advantage of toxins that increase vascular permeability, such as the diphtheria toxin. By conjugating a mutated ligand for the diphtheria toxin receptor (CRM197) to liposomes, Van Rooy et al. were able to show in vitro that CRM197-modified particles localized to the endothelium but that other ligands, such as RI7217, an antibody binding to the transferrin receptor (TfR), had the greatest effect in vivo [58].
Figure 2. Targeted nanoparticle delivery to the brain.
(A) Unmodified control nanoparticles demonstrated low accumulation in the brain compared with (B) RVG29-modified particles particles when injected intravenously into a mouse. Reproduced from [57] with permission from Elsevier.
Specificity can also be built into the delivery system by use of specific ligands that promote receptor-mediated endocytosis. Insulin is transported across the BBB from the circulation by receptor-mediated transcytosis [59], and an antibody to the insulin receptor was taken up effectively into the brain in vivo in a primate model [60]. Ulbrich et al. conjugated drug-loaded human serum albumin nanoparticles to antibodies against insulin receptor and were able to achieve uptake into the brain in a mouse model [61]. Another receptor commonly studied for active transport across the BBB is the TfR, which is necessary for transport of iron into the brain and was used to deliver similar serum albumin nanoparticles into the brain [62]. In particular, its expression is high in rapidly dividing cell populations [63], which, while not seen only in malignancies, does have some degree of specificity for rapidly growing tumors. Although TfR is expressed in other tissues such as the liver and bone marrow [64], it is still under active investigation for transport across the BBB. Polyester nanoparticles loaded with anti-cancer drugs like taxols, based on materials such as PEG-conjugated PLA or PLGA [65,66], were able to achieve higher accumulation in brain endothelial cells when conjugated to transferrin. Like transferrin, the folate receptor for transport of folic acid is also upregulated in rapidly dividing cells, including those in malignant brain cancer [67].
Nanoparticle movement within brain tissue
Once a drug passes through the BBTB and into the brain tissue, other barriers still remain. Because surgical resection is normally the initial step in treatment, local delivery of a chemo therapeutic to the site of the tumor is possible and may be a reasonable way to bypass the delivery bottleneck of the BBB. However, high-grade gliomas are highly invasive, and cancer cells are often found extending out of the main tumor bulk [68]. Not only does this complicate surgical resection, as it is difficult or impossible for the surgeon to visualize all tumor cells, let alone remove them without extensive damage to the surrounding brain tissue, but some controversy still exists over the extent of benefit from resection in some cases of malignant glioma [69–71]. As a result, the ability of any drug or drug carrier to diffuse through the brain tissue and affect all relevant cells is imperative for effective treatment. The majority of GB recurrences are seen at or adjacent to the original tumor site [72]. While this indicates that local delivery may be sufficient in many cases as long as the drug or drugs can affect all tumor cells in the area, it also shows that current therapies are thus far unable to kill all tumorigenic cells at the tumor site. A study by Kroin et al., which used local delivery of cisplatin via small cannulas, found that multiple cannulas would be needed to maintain a therapeutic drug concentration for tumors greater than 1 cm; for tumors greater than 2 cm, local delivery would not be sufficient [73]. While this is not the only local delivery system that has been studied, the study’s conclusion raises the concern that most drugs under investigation do not diffuse freely enough through the brain tissue for full efficacy. Furthermore, low diffusion following local delivery can result in high local toxicity at the delivery site. As a result, studies over the last decade have generally used one of the following methods to enhance interstitial movement of therapeutics through the brain.
Nanoparticle diffusion within tissue
Several strategies have been employed to overcome the poor movement of therapeutics within the brain interstitium. Initially, attempts to better understand the dimensions of the extra-cellular space, modeled as pores through which particles can diffuse in the brain, illustrated that particles must be approximately 30–70 nm in diameter or smaller to move through the brain interstitium [74]. In one study, PLA–PEG nanoparticles were loaded with aclarubicin and coated with cationic albumin [75]. By keeping particles within the approximate range stated above, the authors demonstrated improved survival in a rat glioma model using this nano-particle formulation, suggesting that their system was able to effectively deliver particles through the disrupted BBB and move through the tumor interstitium.
However, it has since become clear that, while size is an important factor, nanoparticle surface properties can also alter diffusion through the brain. Nance et al. found that polystyrene nanoparticles (>100 nm) and quantum dots (35 nm) diffused very slowly through brain interstitium when unmodified or coated sparsely with PEG; however, a dense layer of PEG allowed significantly increased movement through the brain with particles of at least 114 nm in hydrodynamic diameter [76]. Therefore, while smaller particles will tend to allow greater diffusion than larger ones, the upper limit of nanoparticle size can be extended with surface modifications that increase the particles’ diffusivity, thereby increasing the capacity of the particles as drug-loaded nanodevices.
Convective transport of drugs & nanoparticles
Another way to increase transport through tissues is to rely on fluid convection during local delivery, or bulk flow, as well as diffusion. A hydrostatic pressure gradient created in the tissue can allow large molecules or particles to be carried much more quickly throughout the tissue. For instance, the well-established local delivery system of the 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU)-loaded Gliadel® wafer (Arbor Pharmaceuticals, LLC, GA, USA) is known to be limited in part because the low diffusion of BCNU through the tumor tissue cannot overcome the rate of drug clearance; however, this system does demonstrate higher initial penetration than in later days, an unexpected finding that was thought to result from convection due to transient local edema following surgery and placement of the wafer [77]. Bobo et al. demonstrated that continual infusion of a drug solution into feline brains via cannulae over the course of approximately 2–3 h, establishing high fluid flow, caused greater penetration of their drug into the brain tissue as well as more homogeneous drug distribution [78]. At the infusion rates used in this study, as well as in other studies using convection-enhanced delivery (CED), the rate of diffusion is very slow or negligible compared with that of convection by bulk flow.
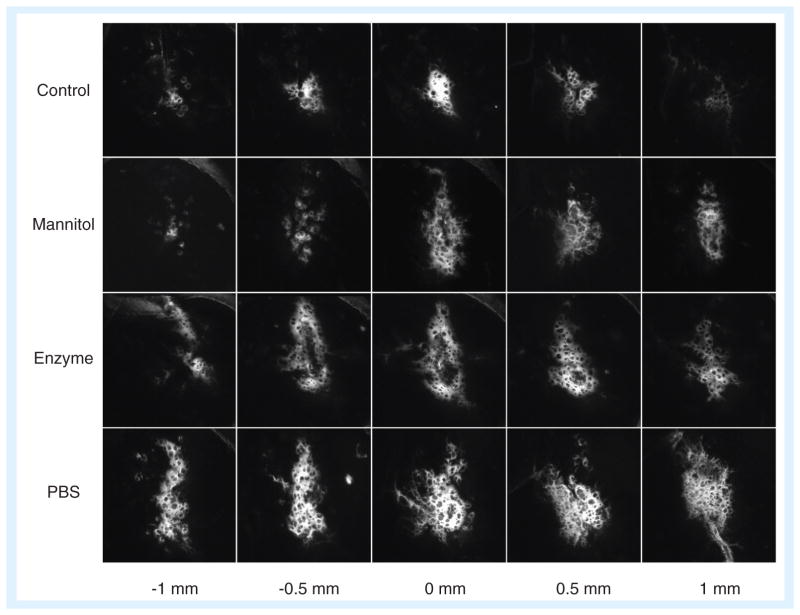
In a study by Sawyer et al., PLGA nano-particles between approximately 90–120 nm mean diameter were loaded with camptothecin and injected intracranially into tumor-bearing rats. The median survival time in rats treated with camptothecin nanoparticles delivered by CED was longer than in rats treated with the same dose of unencapsulated drug; furthermore, drug in nanoparticles could achieve the same effect of twice the concentration of unencapsulated drug [79]. Notably, as discussed above, only nanoparticles below a certain size (approximately 70 nm) are expected to be able to move through the brain tissue, but despite not being PEGylated or otherwise surface-modified, the PLGA nanoparticles in this study were able to move sufficiently through the interstitium with the aid of convective flow. Aside from use in delivering polymeric nanoparticles, CED has also been used to successfully demonstrate improved delivery of chemotherapeutic drug-loaded liposomes [80,81], inorganic nanoparticles for glioma imaging or therapy [82], drug/dendrimer conjugates [83], and even viruses for gene delivery [84]. Moreover, CED can be combined with other strategies mentioned above, such as nanoparticle surface modification, infusion with a hyper osmolar solution and ECM degradation to increase the size of the pores or channels through which nanoparticles can travel (Figure 3) [85]
Figure 3. Nanoparticle transport in the brain.
Nanoparticles 30–40 nm in diameter were infused into the striatum of rats, either on their own in isotonic suspension (control), with hyperosmolar mannitol, or in isotonic suspension after pre-treatment with hyaluronidase or saline. The fluorescence image indicates the distribution of nanoparticles within 1 mm from the injection site. All three treatment conditions served to increase the effective pore size in the brain extracellular space during (mannitol) or prior to (enzyme, PBS) nanoparticle infusion, resulting in increased transport.
PBS: Phosphate-buffered saline.
Reproduced from [85] with permission from Elsevier.
It should be noted, however, that CED is not without risks. A Phase I/II clinical study using CED to enhance paclitaxel transport illustrated high efficacy in tumor reduction or slowed growth; however, complications also arose throughout the study in nine of 15 patients, a high rate that raises concern about translating this technology to the clinic [86]. Along with expected toxic side effects of the drug, there were instances of infection, accidental removal of the catheter, or loss of sufficient convective flux when infused fluid entered a cyst. As with other methods of local delivery, CED is an invasive procedure, and the amount of time that a patient is left exposed is intrinsically long due to the infusion process. Thus, CED has demonstrated promise in improving drug and nanoparticle transport through brain or tumor tissue, but safe ways of implementing the technology are still in development.
Targeting brain tumor tissue over healthy brain tissue
The strategies discussed thus far are generally all methods to cross the major physical barriers specific to brain or CNS delivery. However, as with other cancers, widespread toxic side effects, which may limit the tolerable dose of a drug, are an important problem that must be addressed in brain cancer treatment. Even if limited to the brain alone, minimal damage to healthy brain tissue must be a design goal for any therapy. Local delivery methods sidestep this issue to a large extent, although specificity for cancer cells at the site of the tumor could further ameliorate the problem. For systemic delivery, which has greater potential to reach metastatic cells or tumors that recur distant from the initial tumor site, the ability of a drug or drug carrier to affect the tumor preferentially to healthy cells would be a major advantage.
Nanoparticle–drug formulations are particularly exciting in this regard, as their design parameters can be modified and finely tuned to include one or several layers of delivery specificity. The EPR effect as described allows passive targeting of tumors by preferential extravasation and retention of nanoparticles within a certain size range. Numerous other targeting methods can be used individually or even in combination in the same nanoparticle, such as conjugation of or coating with a targeting ligand to enhance localization at the tumor; introducing degradable units or other properties to promote drug release near the tumor environment, capitalizing on microenvironmental factors such as decreased pH and increased protease expression near tumors; and delivery of cargo that is preferentially or solely active in malignant cells. The last of these will be discussed further below in the context of particular therapeutics currently being studied.
Numerous examples exist of approaches to increase brain cancer specificity of nano-medicines. The folate receptor, as previously mentioned, has some specificity for tumors over healthy tissues, based primarily on the rapid rate of cell growth and division of cancer cells. Chitosan-based nanoparticles were conjugated with fluorescently labeled chlorotoxin, which binds specifically to gliomas and other tumors related in origin [87], and showed preferential accumulation in gliomas in mice [88,89]. Liposomes conjugated with IL-13 were also able to deliver doxorubicin specifically to glioma cell lines in vitro, based on the high expression of IL-13 receptor α2 on glioma cells but not on healthy brain tissue [90]. The CREKA peptide was previously shown to home toward tumor tissue and was coupled to 50-nm SPIO nanoparticles. When injected into a mouse, the particles not only targeted tumor vasculature but also induced clot formation, which in turn allowed binding of more circulating nanoparticles to the site [91]. Although this amplified targeting system was described in a breast cancer model, similar principles, using nanoparticles to incorporate many targeting functionalities, could potentially be applied to brain cancer as well.
Duan et al. developed pH-sensitive nanogels composed primarily of chitosan grafted to poly(N-isopropylacrylamide) and showed that release of the drug oridonin occurred preferentially in the pH range of 5.0–6.5 compared with 7.4 [92]. Because the pH surrounding tumors is typically <7.2, this strategy provides a possible method of targeting tumors [40]. In addition to changes in acidity, tumor cells often differ from healthy cells in that the former have higher intracellular concentrations of glutathione (GSH) [93], which can be used as a method to cause intracellular drug release preferentially in cancer cells. The high expression of GSH transporter on these cells also allows GSH to be used as a targeting ligand conjugated to such nanomedicines as the PEGylated liposomal doxorubicin for increased uptake [94], and this formulation has entered clinical trials [30].
Current & future drugs & experimental therapies in glioma
Nanoparticle-engineered cells as vehicles for glioma treatment
In recent years, some cell types have been found to migrate toward malignant GBs, providing an intriguing and promising option for improving efficacy and specificity of drug delivery to brain tumors. These include some adult stem cells such as neural stem cells (NSCs). It was found in 2000 that NSCs injected into a murine glioma model had high specificity for tumor tissue and the ability to spread throughout the tumor when NSCs were injected intratumorally or distant from the tumor, in either the ipsilateral or contralateral hemisphere, into the ventricles, or via tail-vein injection [95]. In highly invasive models, labeled NSCs were found to ‘track’ infiltrating glioma cells instead of staying merely in the tumor bulk. The authors of this study suggested that genetically engineered NSCs could be used to deliver anti-tumor agents, which they achieved in their study using retroviral gene delivery. Researchers have since discovered that other adult stem cells, such as bone marrow- (BM-MSCs) and adipose-derived mesenchymal stem cells, also display a high degree of tumor specificity in several glioma models, able to migrate across hemispheres and the BBB and toward the tumor [96]. MSCs are more readily available in large numbers than NSCs, potentially from the patient’s own bone marrow, blood or adipose tissue, and they can be expanded in culture.
MSCs have been proposed as a delivery vehicle to brain malignancies, either carrying drug- or imaging agent-loaded nanoparticles or engineered to secrete a therapeutic protein, with the advantage of having active migration mechanisms and not relying solely on diffusive and convective transport through tissue as do nano-particles alone. For the latter, viruses – particles approximately 100 nm in diameter that have evolved for efficient gene transfer – have historically been used as gene delivery vectors because of their high efficiency. For instance, Nakamizo et al. also engineered their MSCs to overexpress interferon-β (IFN-β) using an adenoviral vector before injection, leading to improved survival over controls [96]. A number of groups have used retroviruses to transduce BM-MSCs [97] or adipose-derived mesenchymal stem cells [98] with the HSV-TK gene, which catalyzes a key step in converting the prodrug ganciclovir to a cytotoxic metabolite. These MSCs injected into the ventricles or lateral vein of tumor-bearing mice were shown to localize at the tumor and, upon injection of ganciclovir, reduce tumor growth by the bystander effect and improve survival.
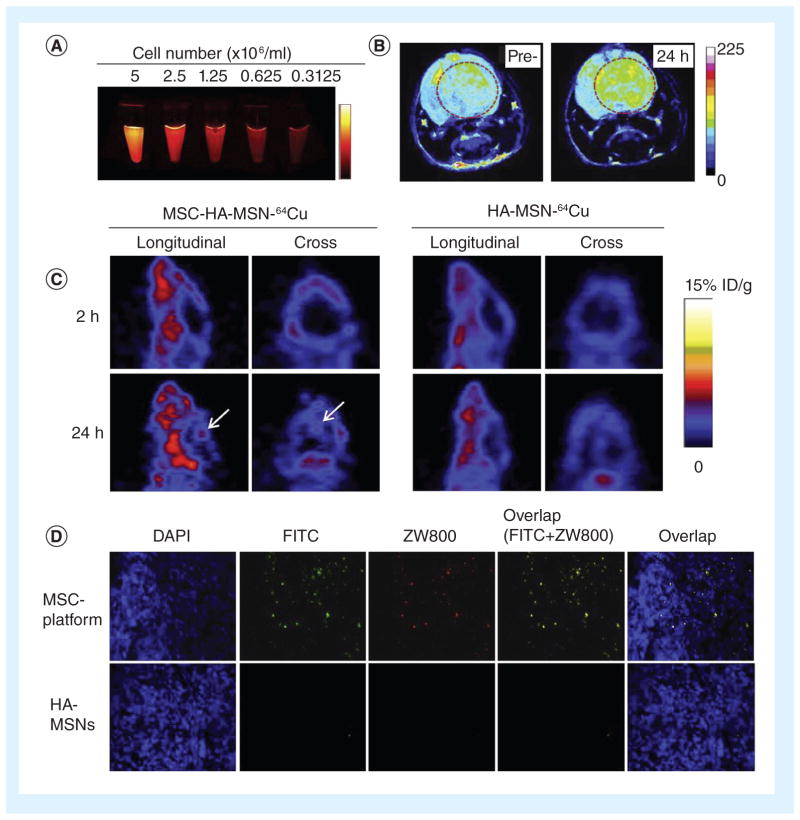
Because nanoparticles are the appropriate size for cellular uptake while also being able to protect or tune the release of drugs encapsulated within, several groups have investigated the ability of MSCs to efficiently take up and carry cargo to brain tumors in the form of intact nanoparticles, which could then release a drug or perform a function at the tumor site. For example, oncolytic viruses can be loaded in vitro into BM-MSCs, which would carry the virus particles to the tumor site [99]. Although virus-mediated toxicity to the MSCs was a limiting factor, using MSCs as a delivery vehicle was superior in efficiency and specificity to delivery of oncolytic viruses alone. PLA nanoparticles loaded with fluorescent coumarin-6 could also be taken up by MSCs without affecting cell viability or phenotype; MSCs loaded with PLA and injected intratumorally in a mouse glioma model tended to stay near the tumor bulk as well as following behind infiltrating glioma cells [100]. SPIO nanoparticles loaded into labeled MSCs showed tumor homing when the loaded stem cells were injected systemically into a rat glioma model, providing a potential method for delivering nanoparticles to enhance MRI contrast [101]. Another group synthesized mesoporous silica nanoparticles (MSNs), loaded the particles with a mixture of different imaging contrast agents, and coated the loaded particles with hyaluronic acid for efficient uptake by BM-MSCs. These MSCs with loaded nanoparticles were injected systemically into mice and showed high specificity for the glioma site, as well as allowing in vivo imaging via near-infrared fluorescence, MRI and PET (Figure 4) [102]. Using adult stem cells as delivery vehicles is therefore a versatile and potentially powerful method for delivery of diagnostic or therapeutic nanoparticles to malignant gliomas.
Figure 4. Mesoporous silica nanoparticles with multimodal imaging capacity demonstrated stem cell accumulation in tumors.
MSNs were synthesized with two fluorescent dyes (FITC and ZW800, blue-green and near-infrared, respectively) and then loaded with 64Cu for MRI or Gd3+ for positron emission tomography imaging. Bone marrow-derived MSCs were then loaded with the labeled mesoporous silica nanoparticles. (A) Diluting the number of cells demonstrated a corresponding decrease in ZW800 signal. (B) After tail-vein injection of loaded MSCs into a mouse orthotopic glioma model, positron emission tomography signal was higher in the tumor 24 h after injection than it was prior to injection. (C) MRI signal was higher in MSC/MSN-treated mice than in MSN-treated mice without MSCs. (D) Fluorescence microscopy on tissue sections also showed higher accumulation at the tumor site by MSC/MSNs than MSNs alone. HA: Hyaluronic acid; MSC; Mesenchymal stem cell; MSN: Mesoporous silica nanoparticles. Reproduced from [102] with permission from Elsevier.
Nanoparticle formulations of conventional drugs
As mentioned above, the drugs currently used for chemotherapy have made significant but small improvements in the prognosis of malignant glioma. Combinations of various therapies can further improve prognosis [103], but there is still a high need for more effective treatment. The current standard of care normally includes surgical resection with subsequent radiotherapy, often supplemented with adjuvant chemotherapy, including the locally implanted Gliadel BCNU wafer and the alkylating agent temozolomide.
Some compounds, including drugs used in the treatment of other cancers, are limited in use for glioma therapy because they cannot cross the BBB or are cleared too quickly for sufficient accumulation, they are not specific enough to prevent widespread toxicity, or tumor cells tend to be resistant to them. As a result, nanoparticles are being used as a method to reduce those problems with conventional drugs as well as to explore the potential use of other, more experimental therapeutic agents. For instance, the drug doxorubicin normally has poor to insignificant penetration of the BBB. By encapsulating doxorubicin into polysorbate 80-coated butylcyanoacrylate nanoparticles, high uptake into the brain was seen in rats, with decreased systemic toxicity, increased survival rate and long-term remission in >20% of animals tested [104]. Efflux of drugs via the P-gp transporter, a mechanism of drug resistance, was decreased by encapsulating paclitaxel in nanoparticles in polysorbate 80-coated nanoparticles [26]. The use of this nanoparticle system simultaneously decreased the toxicity due to Cremophor, which is usually needed for paclitaxel delivery, and also increased BBB penetration and decreased P-gp transporter activity due to the surfactant coating.
Nanoparticles for gene therapy
Nanoparticle-mediated DNA delivery was briefly discussed above in the context of engineering cells for glioma therapy, but biologics like DNA and multiple types of RNA are also under investigation as direct treatments for brain cancer. For nucleic acids, viruses have been the traditional method of delivery, as they have the advantages of high efficacy as well as the potential ability to cross various intra- and extra-cellular barriers. For example, clinical trials have demonstrated the efficacy of gene transfer treatment using an adenoviral vector. The adenovirus carried the HSV-TK gene and was injected into tumor margins following resection [105,106], exploiting the same TK/ganciclovir strategy described above and first proposed in the context of viral gene delivery in 1986 [107]. However, this and other studies have shown that replication-incompetent retroviruses have low efficacy in combating cancer [108]. Adenoviruses that do not express the E1B gene have been found to be selective for p53-deficient cells over the p53-expressing, healthy neural population, successfully targeting the tumor cells for viral infection and death in glioma patients [109]. In a study that integrated aspects of both of these approaches, Tai et al. used a retrovirus that selectively replicated in dividing cells to transduce glioma cells in a mouse with the cytosine deaminase suicide gene [110]. As a result, the transduced glioma cells were able to convert an intravenously injected prodrug, 5-fluorocytosine, to the toxic 5-fluorouracil, thereby causing local tumor death. In addition to conferring sensitivity to a drug or prodrug, viruses have also been used to deliver the p53 gene, a tumor suppressor that is commonly inactivated in gliomas [111]. Phase I clinical trials have used an adenoviral vector delivered intratumorally to transduce tumor cells with p53 [112,113], resulting in common but not prohibitively severe adverse side effects due to viral toxicity or immune response. Furthermore, preliminary results were suggestive of some anti-tumor effect.
It should be noted that, in one of the cases described above, four of 14 patients had an immune response to the adenovirus, and the frequency of seizures increased in two others. A Phase I/II trial using virus-producing cells following the HSV-TK/ganciclovir paradigm reported adverse side effects related to therapy in over 50% of patients [114], and worries about safety have limited the use of viruses in the clinic in general [115]. Aside from safety concerns, however, viruses are limited in their maximum cargo size and ease and consistency of manufacturing [116], leading to interest in the potential of using non-viral gene transfer agents. In a study that took place between 1999 and 2001 HSV-TK was delivered locally to recurrent GB patients using a liposomal formulation and showed increased necrosis to portions of the tumor after ganciclovir infusion, though not all of the patients appeared to have had sufficiently transfected tumor cell [117], and six of eight patients experienced adverse side effects, including fever and neurological deficits. Cationic liposomes carrying the IFN-β gene injected intratumorally into mouse models were also shown to be effective in reducing tumor growth and causing immune response in the tumor, and a pilot clinical trial in five malignant glioma patients based on these results showed >50% tumor reduction or stable disease in four of the patients [118]. In addition to liposomes, other materials have been used to make gene delivery nanoparticles for glioma therapy. PEG conjugated to PLA was used to encapsulate Apo2L/tumor necrosis factor-related apoptosis inducing ligand (TRAIL) plasmid into nano-particles <120 nm in diameter, which were then surface-conjugated to albumin. Because TRAIL is fairly specific for cancer cells over healthy tissue due to overexpression of TRAIL death receptors [119], most resulting cell death was expected to be in the tumors. These nanoparticles were then injected intravenously and caused increased median survival time over controls [120].
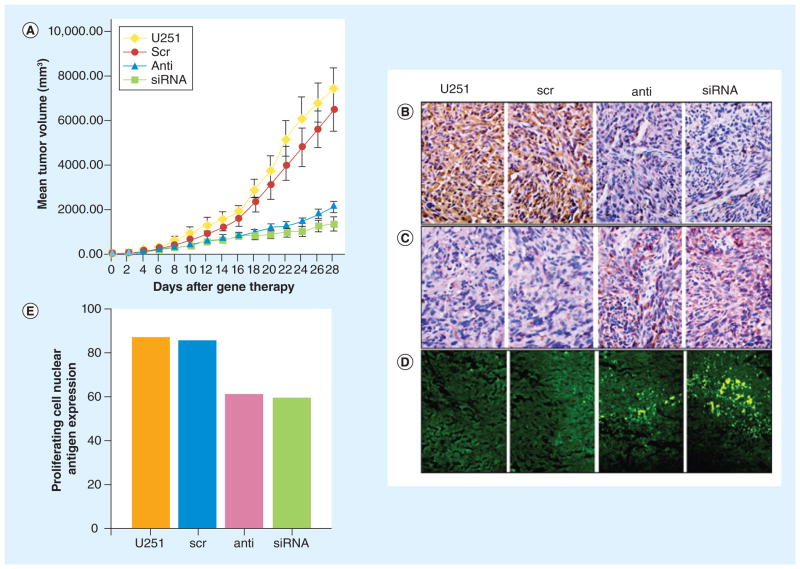
Gene therapy also provides additional ways to introduce specificity into the system. As with the examples of TRAIL and p53 DNA, the protein product of the delivered gene should be designed to be active only in cancer cells. Moreover, the delivered construct can be under the control of a cancer-specific promoter, such as survivin [121] or PEG3 [122], ensuring that, should even healthy cells be transfected or transduced, the therapeutic protein would not be expressed. In addition to DNA delivery for gene overexpression, RNAi to knock down gene expression has in recent years been an active area of investigation. Cationic lipids and synthetic polymers have been used to form nanoparticle complexes with siRNA against a reporter gene as a proof-of-concept, showing effective gene knockdown in vitro in GB cells [123,124]. shRNA can also be expressed from a DNA plasmid, allowing the more extensively studied materials for stable DNA nanoparticles to be used for RNAi. Many potential molecular targets have been explored using nanoscale delivery [125]. A study using the cationic lipid Lipofectamine™ 2000 (Invitrogen, Paisley, UK) to encapsulate plasmids expressing siRNA against EGFR, a gene often mutated or overexpressed in malignant glioma [126], was able to decrease tumor volume a mouse glioma model up to fivefold (Figure 5) [127].
Figure 5. Lipid-based nanoparticle containing plasmid coding for siRNA against EGF receptor demonstrated RNAi delivery as a genetic therapy for glioma.
(A) Mice treated with these liposomes had tumors significantly smaller than those treated with liposomes containing scrambled siRNA sequence or left untreated. The histology of tumors in these mice also indicated (B) downregulated EGF receptor expression, (C) upregulated glial fibrillary acidic protein, indicating a change in phenotype, (D) increased apoptosis by TUNEL staining and (E) decreased proliferating cell nuclear antigen. Adapted with permission from [127].
Inorganic nanoparticles: brain cancer imaging & treatment
Metallic nanoparticles have been studied in the laboratory as well as in the clinic. In one study, direct intratumoral injection of SPIO nanoparticles in a rat glioma model using RG-2 line tumors was followed by application of an alternating magnetic field that caused heat generation by the nanoparticles due to their increased Brownian motion [128]. The authors showed a 4.5-fold improvement in survival over untreated controls, although they did not compare their treatment directly with clinical gold standards like radiotherapy and/or conventional chemotherapy. Nevertheless, thermotherapy using this or similar methods using inorganic nanoparticles has high potential efficacy and has been studied by several groups. The use of super-paramagnetic particles as MRI contrast agents has been studied for over two decades [129], with many important physicochemical parameters for synthesis and imaging purposes already well understood. For example, the properties of these particles can be adjusted to target brain tumors using surface-conjugation of glioma-specific ligands [130], while other researchers use nanoparticles approximately 100 nm in diameter in order to take advantage of the EPR effect from disrupted BBB [35,131], providing MRI contrast of the tumor interstitium as well as providing a potential carrier for drug delivery.
Although inorganic nanoparticles are common in both research and the clinic, some question exists about the safety of their use [132], given that they are not biodegradable as are many nanoparticles currently in development. While several in vivo studies with metallic particles have shown no apparent toxicity, there is still concern about potential adverse side effects, particularly for inorganic particles that nonspecifically accumulate in non-target tissues or in healthy parts of the brain [133].
Brain tumor stem cells as a therapeutic target
Recurrence is an unfortunate certainty for most GB patients. One population of GB cells thought to be responsible in large part for this is the brain tumor stem cells (BTSCs). BTSCs within the bulk tumor have stem-like properties, including the ability to initiate and repopulate a tumor even most of it has been removed, and they have also been found to be refractory to many conventional anticancer treatments like BCNU [134,135]. They are therefore an attractive target for brain cancer therapy research, including experimental techniques that may be able to overcome the drawbacks of conventional chemotherapy and radio-therapy. Aside from inducing cell death in BTSCs, terminal differentiation of BTSCs could prevent surviving stem cells from being able to repopulate a tumor. For example, delivery of certain miRNAs such as miR-124 and miR-137 was found to cause terminal differentiation and cell death of murine BTSC mimics in vitro [136]. In this case, a lipid-based, commonly used commercial gene delivery agent, Lipofectamine formed nanocomplexes with miRNAs for successful in vitro knockdown. Gangemi et al. used shRNA-expressing plasmid in a retroviral vector for in vitro knockdown of SOX2, a transcription factor found in stem cells to be important in self-renewal, and found that this inhibited BTSC proliferation, self-renewal and tumor-initiating capacity [137]. Decreasing the stem-like properties of BTSCs in combination with the anti-tumor treatments described above may be an important and necessary aspect to add to current therapy regimens.
Future perspective
Brain cancer research and medical practice have advanced over the past decades, but progress, while significant, has been incremental and slow. The use of nanoparticles in this field has been fueled by a lack of current solutions to many of the barriers that impede further progress. Clinical translation of these technologies, in particular, has been slow, as few related studies have reached clinical trials and fewer still for applications in the brain [138]. Difficulties in translation of nanomedicine and its use in cancer therapy have been discussed in detail elsewhere [139].
Nanoparticles are, however, inherently well-suited for cancer therapy simply due to their small size and their highly tunable physical, chemical and biological properties. The potential therapeutic value of nanomedicine is huge as several functions for drug delivery and imaging can be simultaneously incorporated. Examples include multistep cancer targeting strategies and multimodal imaging agents as described above. With the additional consideration of using nano-particles to engineer targeting cells like MSCs or using targeting MSCs to deliver nanoparticles, even more potential treatment paradigms are possible. Nanotechnology can allow researchers to develop novel strategies for delivering drug cargos and imaging agents while also allowing the providing chemical flexibility to modify and functionalize nano particles, which may lead to a more comprehensive therapy for brain cancer.
Executive summary.
Shortcomings in current malignant brain cancer treatment
Grade III (anaplastic astrocytoma) and IV (glioblastoma) gliomas have median survivals of 2–3 years and 15 months, respectively. Advances to the field in the past decades have improved prognosis only marginally.
Experimental therapies such as nanotechnology may fill the gaps in the field.
Types of nanoparticles & their properties
Biodegradable polymers (e.g., poly(ester)s, poly(amidoamine)s and chitosan) can form nanoparticles encapsulating small-molecule drugs, proteins, peptides or nucleic acids.
Liposomes are commonly used but are cleared quickly by mononuclear phagocyte system cells. Modification with ‘stealth’ coatings such as polyethylene glycol can increase their half-life.
Inorganic nanoparticles can be used as MRI contrast agents or as composite materials with multimodal function.
Nanoparticles can protect therapeutic cargo from degradation or clearance. Their size and surface properties affect intracellular delivery efficacy (cellular uptake), biodistribution and clearance rates.
Blood–brain barrier
Tight junctions between endothelial cells of capillaries in the brain prevent the exchange of many foreign materials, including systemically administered drugs.
The blood–brain barrier (BBB) is disrupted at tumors, allowing passive nanoparticle accumulation via the enhanced permeability and retention effect.
BBB disruption by hyperosmotic agents (e.g., mannitol) or vasodilators (e.g., bradykinin or histamine) increases the gap between endothelial cells; however, this does not distinguish between BBB at the tumor and the BBB of healthy brain tissue.
Nanoparticles can be coated or conjugated to a ligand that penetrates the BBB.
Nanoparticle transport through brain interstitium
For both systemic and local delivery, extracellular matrix prevents large particles from efficient diffusion through the brain. The maximum particle size can be increased by surface modification (e.g., PEGylation).
Convection-enhanced delivery uses a hydrostatic pressure gradient to drive bulk flow in the tissue interstitium. Although this better distributes the nanoparticles, safety concerns have been seen in clinical trials.
Tumor-specific (targeted) delivery
Ligands can target nanoparticles to fast-growing cells, which overexpress molecules like folate and transferrin receptors. Tumor-specific peptide sequences further increase specificity.
Nanoparticle release can be targeted to the tumor environment (e.g., low pH).
The cargo being delivered can be specific to tumor tissue (e.g., transcriptional targeting with nucleic acids, proteins interacting with molecules overexpressed in tumors).
Strategies under investigation
Common chemotherapy drugs such as doxorubicin have been encapsulated into nanoparticles that can penetrate the BBB.
Viral and non-viral gene carriers can deliver a therapeutic to sensitize tumors to chemotherapy or to directly cause apoptosis.
Neural stem cells and mesenchymal stem cells home toward tumors. Nanoparticles can be used to engineer these cells to express a therapeutic protein at the site of tumor.
Nanoparticle-based therapies are being developed to target brain tumor stem cells to prevent recurrence.
Key Terms
- Glioblastoma
Grade IV malignant glioma with high invasiveness and poor prognosis
- Nanoparticle
Any particle in the size range of 1 nm–1μm. The versatility of nanoparticle composition, structure, and carrying capacity makes them attractive as a potential therapeutic vehicle
- Blood–brain barrier
Endothelial cells of brain vasculature prevent passage of most systemically delivered drugs from the circulation and into the brain
- Enhanced permeability and retention effect
Disrupted, ‘leaky’ vasculature feeding tumors allows preferential accumulation of nanoparticles in the tumor microenvironment
- Convection-enhanced delivery
Use of bulk fluid flow (convection) in addition to diffusion to promote movement of drugs and nanoparticles through the interstitium
- Brain tumor stem cell
One of the population of cells in a tumor with the ability to initiate a new tumor. Brain tumor stem cells have been found to be resistant to many conventional therapies, which may contribute to tumor recurrence
Footnotes
For reprint orders, please contact: reprints@future-science.com
Financial & competing interests disclosure
This work was supported in part by the NIH (1R01EB016721). SY Tzeng thanks the National Science Foundation for fellowship support. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Chithrani BD, Ghazani AA, Chan WCW. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006;6(4):662–668. doi: 10.1021/nl052396o. [DOI] [PubMed] [Google Scholar]
- 2.Behin A, Hoang-Xuan K, Carpentier AF, Delattre JY. Primary brain tumours in adults. Lancet. 2003;361(9354):323–331. doi: 10.1016/S0140-6736(03)12328-8. [DOI] [PubMed] [Google Scholar]
- 3.Palanichamy K, Erkkinen M, Chakravarti A. Predictive and prognostic markers in human glioblastomas. Curr Treat Options Oncol. 2006;7(6):490–504. doi: 10.1007/s11864-006-0024-7. [DOI] [PubMed] [Google Scholar]
- 4.Brandsma D, van den Bent MJ. Molecular targeted therapies and chemotherapy in malignant gliomas. Curr Opin Oncol. 2007;19(6):598–605. doi: 10.1097/CCO.0b013e3282f0313b. [DOI] [PubMed] [Google Scholar]
- 5.Bala I, Hariharan S, Kumar MNVR. PLGA nanoparticles in drug delivery: the state of the art. Crit Rev Ther Drug Carrier Syst. 2004;21(5):387–422. doi: 10.1615/critrevtherdrugcarriersyst.v21.i5.20. [DOI] [PubMed] [Google Scholar]
- 6.Mitra S, Gaur U, Ghosh PC, Maitra AN. Tumour targeted delivery of encapsulated dextran-doxorubicin conjugate using chitosan nanoparticles as carrier. J Control Release. 2001;74(1–3):317–323. doi: 10.1016/s0168-3659(01)00342-x. [DOI] [PubMed] [Google Scholar]
- 7.Park JH, Saravanakumar G, Kim K, Kwon IC. Targeted delivery of low molecular drugs using chitosan and its derivatives. Adv Drug Delivery Rev. 2010;62(1):28–41. doi: 10.1016/j.addr.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Shenoy D, Little S, Langer R, Amiji M. Poly(ethylene oxide)-modified poly(beta-amino ester) nanoparticles as a pH-sensitive system for tumor-targeted delivery of hydrophobic drugs: part 2. In vivo distribution and tumor localization studies. Pharm Res. 2005;22(12):2107–2114. doi: 10.1007/s11095-005-8343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tzeng SY, Guerrero-Cazares H, Martinez EE, Sunshine JC, Quinones-Hinojosa A, Green JJ. Non-viral gene delivery nanoparticles based on poly(beta-amino esters) for treatment of glioblastoma. Biomaterials. 2011;32(23):5402–5410. doi: 10.1016/j.biomaterials.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He H, Li Y, Jia XR, et al. PEGylated Poly(amidoamine) dendrimer-based dual-targeting carrier for treating brain tumors. Biomaterials. 2011;32(2):478–487. doi: 10.1016/j.biomaterials.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Malam Y, Loizidou M, Seifalian AM. Liposomes and nanoparticles: nanosized vehicles for drug delivery in cancer. Trends Pharmacol Sci. 2009;30(11):592–599. doi: 10.1016/j.tips.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Hofheinz RD, Gnad-Vogt SU, Beyer U, Hochhaus A. Liposomal encapsulated anti-cancer drugs. Anti-Cancer Drugs. 2005;16(7):691–707. doi: 10.1097/01.cad.0000167902.53039.5a. [DOI] [PubMed] [Google Scholar]
- 13.Weinstein JS, Varallyay CG, Dosa E, et al. Superparamagnetic iron oxide nanoparticles: diagnostic magnetic resonance imaging and potential therapeutic applications in neurooncology and central nervous system inflammatory pathologies, a review. J Cereb Blood Flow Metab. 2010;30(1):15–35. doi: 10.1038/jcbfm.2009.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawano T, Yamagata M, Takahashi H, et al. Stabilizing of plasmid DNA in vivo by PEG-modified cationic gold nanoparticles and the gene expression assisted with electrical pulses. J Control Release. 2006;111(3):382–389. doi: 10.1016/j.jconrel.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 15.Lee J-S, Green JJ, Love KT, Sunshine J, Langer R, Anderson DG. Gold, poly(B-amino ester) nanoparticles for small interfering RNA delivery. Nano Lett. 2009;9(6):2402–2406. doi: 10.1021/nl9009793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu J, Liong M, Zink JI, Tamanoi F. Mesoporous silica nanoparticles as a delivery system for hydrophobic anticancer drugs. Small. 2007;3(8):1341–1346. doi: 10.1002/smll.200700005. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka T, Mangala LS, Vivas-Mejia PE, et al. Sustained small interfering RNA delivery by mesoporous silicon particles. Cancer Res. 2010;70(9):3687–3696. doi: 10.1158/0008-5472.CAN-09-3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Owens DE, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm. 2006;307(1):93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 19.Awasthi VD, Garcia D, Goins BA, Phillips WT. Circulation and biodistribution profiles of long-circulating PEG-liposomes of various sizes in rabbits. Int J Pharm. 2003;253(1–2):121–132. doi: 10.1016/s0378-5173(02)00703-2. [DOI] [PubMed] [Google Scholar]
- 20.Muthu MS, Kulkarni SA, Xiong JQ, Feng SS. Vitamin E TPGS coated liposomes enhanced cellular uptake and cytotoxicity of docetaxel in brain cancer cells. Int J Pharm. 2011;421(2):332–340. doi: 10.1016/j.ijpharm.2011.09.045. [DOI] [PubMed] [Google Scholar]
- 21.Vasir JK, Labhasetwar V. Biodegradable nanoparticles for cytosolic delivery of therapeutics. Adv Drug Delivery Rev. 2007;59(8):718–728. doi: 10.1016/j.addr.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nori A, Kopecek J. Intracellular targeting of polymer-bound drugs for cancer chemotherapy. Adv Drug Delivery Rev. 2005;57(4):609–636. doi: 10.1016/j.addr.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Win KY, Feng SS. Effects of particle size and surface coating on cellular uptake of polymeric nanoparticles for oral delivery of anticancer drugs. Biomaterials. 2005;26(15):2713–2722. doi: 10.1016/j.biomaterials.2004.07.050. [DOI] [PubMed] [Google Scholar]
- 24.Jiang W, Kim BYS, Rutka JT, Chan WCW. Nanoparticle-mediated cellular response is size-dependent. Nat Nanotechnol. 2008;3(3):145–150. doi: 10.1038/nnano.2008.30. [DOI] [PubMed] [Google Scholar]
- 25.Borchard G, Audus KL, Shi FL, Kreuter J. Uptake of surfactant-coated poly(methyl methacrylate)-nanoparticles by bovine brain microvessel endothelial-cell monolayers. Int J Pharm. 1994;110(1):29–35. [Google Scholar]
- 26.Koziara JM, Lockman PR, Allen DD, Mumper RJ. Paclitaxel nanoparticles for the potential treatment of brain tumors. J Control Release. 2004;99(2):259–269. doi: 10.1016/j.jconrel.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 27.Perry J, Laperriere N, Zuraw L, Chambers A, Spithoff K, Cairncross JG. Adjuvant chemotherapy for adults with malignant glioma: a systematic review. Can J Neurol Sci. 2007;34(4):402–410. doi: 10.1017/s0317167100007265. [DOI] [PubMed] [Google Scholar]
- 28.Adamson C, Kanu OO, Mehta AI, et al. Glioblastoma multiforme: a review of where we have been and where we are going. Expert Opin Inv Drug. 2009;18(8):1061–1083. doi: 10.1517/13543780903052764. [DOI] [PubMed] [Google Scholar]
- 29.Blanchette M, Fortin D. Blood–brain barrier disruption in the treatment of brain tumors. Methods Mol Biol. 2011;686:447–463. doi: 10.1007/978-1-60761-938-3_23. [DOI] [PubMed] [Google Scholar]
- 30.Gaillard PJ, Visser CC, Appeldoorn CCM, Rip J. Enhanced brain drug delivery: safely crossing the blood-brain barrier. Drug Discov Today Technol. 2012;9(2):e155–e160. doi: 10.1016/j.ddtec.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Tate MC, Aghi MK. Biology of angiogenesis and invasion in glioma. Neurotherapeutics. 2009;6(3):447–457. doi: 10.1016/j.nurt.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32▪▪.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer-chemotherapy – mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46(12):6387–6392. Enhanced permeation and retention effect is commonly considered in the design of nanoparticles for cancer applications, including for crossing a compromised blood–brain barrier. [PubMed] [Google Scholar]
- 33.Perrault SD, Walkey C, Jennings T, Fischer HC, Chan WCW. Mediating tumor targeting efficiency of nanoparticles through design. Nano Lett. 2009;9(5):1909–1915. doi: 10.1021/nl900031y. [DOI] [PubMed] [Google Scholar]
- 34.Donelli MG, Zucchetti M, Dincalci M. Do anticancer agents reach the tumor target in the human brain. Cancer Chemother Pharmacol. 1992;30(4):251–260. doi: 10.1007/BF00686291. [DOI] [PubMed] [Google Scholar]
- 35.Chertok B, Moffat BA, David AE, et al. Iron oxide nanoparticles as a drug delivery vehicle for MRI monitored magnetic targeting of brain tumors. Biomaterials. 2008;29(4):487–496. doi: 10.1016/j.biomaterials.2007.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36▪.Sarin H, Kanevsky AS, Wu HT, et al. Physiologic upper limit of pore size in the blood-tumor barrier of malignant solid tumors. J Transl Med. 2009;7:51. doi: 10.1186/1479-5876-7-51. Although vascular abnormalities and the enhanced permeation and retention effect are important considerations in brain cancer therapy, this study emphasizes the strict size requirements of nanoparticles that can use the enhanced permeation and retention effect effect to cross the blood–brain barrier, even when compromised. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muldoon LL, Soussain C, Jahnke K, et al. Chemotherapy delivery issues in central nervous system malignancy: a reality check. J Clin Oncol. 2007;25(16):2295–2305. doi: 10.1200/JCO.2006.09.9861. [DOI] [PubMed] [Google Scholar]
- 38.Groothuis DR. The blood-brain and blood-tumor barriers: A review of strategies for increasing drug delivery. Neuro Oncol. 2000;2(1):45–59. doi: 10.1093/neuonc/2.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nossek E, Ram Z, Bokstein F, Blumenthal D. Antiangiogenesis in recurrent glioblastoma: proof of principle. Neurol Int. 2009;1(1):e21. doi: 10.4081/ni.2009.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fukumura D, Jain RK. Tumor microenvironment abnormalities: causes, consequences, and strategies to normalize. J Cell Biochem. 2007;101(4):937–949. doi: 10.1002/jcb.21187. [DOI] [PubMed] [Google Scholar]
- 41.Miladi I, Le Duc G, Kryza D, et al. Biodistribution of ultra small gadolinium-based nanoparticles as theranostic agent: application to brain tumors. J Biomater Appl. 2012 doi: 10.1177/0885328212454315. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 42.Joshi S, Ergin A, Wang M, et al. Inconsistent blood–brain barrier disruption by intraarterial mannitol in rabbits: implications for chemotherapy. J Neuro Oncol. 2011;104(1):11–19. doi: 10.1007/s11060-010-0466-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xin HL, Jiang XY, Gu JJ, et al. Angiopep-conjugated poly(ethylene glycol)-co-poly(epsilon-caprolactone) nanoparticles as dual-targeting drug delivery system for brain glioma. Biomaterials. 2011;32(18):4293–4305. doi: 10.1016/j.biomaterials.2011.02.044. [DOI] [PubMed] [Google Scholar]
- 44.Kratzer I, Wernig K, Panzenboeck U, et al. Apolipoprotein A-I coating of protamine-oligonucleotide nanoparticles increases particle uptake and transcytosis in an in vitro model of the blood–brain barrier. J Control Release. 2007;117(3):301–311. doi: 10.1016/j.jconrel.2006.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zensi A, Begley D, Pontikis C, et al. Albumin nanoparticles targeted with Apo E enter the CNS by transcytosis and are delivered to neurones. J Control Release. 2009;137(1):78–86. doi: 10.1016/j.jconrel.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 46.Kreuter J, Alyautdin RN, Kharkevich DA, Ivanov AA. Passage of peptides through the blood–brain barrier with colloidal polymer particles (nanoparticles) Brain Res. 1995;674(1):171–174. doi: 10.1016/0006-8993(95)00023-j. [DOI] [PubMed] [Google Scholar]
- 47.Wang CX, Huang LS, Hou LB, et al. Antitumor effects of polysorbate-80 coated gemcitabine polybutylcyanoacrylate nanoparticles in vitro and its pharmacodynamics in vivo on C6 glioma cells of a brain tumor model. Brain Res. 2009;1261:91–99. doi: 10.1016/j.brainres.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 48.Gelperina S, Maksimenko O, Khalansky A, et al. Drug delivery to the brain using surfactant-coated poly(lactide-co-glycolide) nanoparticles: influence of the formulation parameters. Eur J Pharm Biopharm. 2010;74(2):157–163. doi: 10.1016/j.ejpb.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 49.Chen YC, Hsieh WY, Lee WF, Zeng DT. Effects of surface modification of PLGA-PEG-PLGA nanoparticles on loperamide delivery efficiency across the blood–brain barrier. J Biomater Appl. 2011;27(7):909–922. doi: 10.1177/0885328211429495. [DOI] [PubMed] [Google Scholar]
- 50.De Mendoza AEH, Preart V, Mollinedo F, Blanco-Prieto MJ. In vitro and in vivo efficacy of edelfosine-loaded lipid nanoparticles against glioma. J Control Release. 2011;156(3):421–426. doi: 10.1016/j.jconrel.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 51.Xie Y, Ye LY, Zhang XB, et al. Transport of nerve growth factor encapsulated into liposomes across the blood–brain barrier: in vitro and in vivo studies. J Control Release. 2005;105(1–2):106–119. doi: 10.1016/j.jconrel.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 52.Lasa-Saracibar B, De Mendoza AEH, Guada M, Dios-Vieitez C, Blanco-Prieto MJ. Lipid nanoparticles for cancer therapy: state of the art and future prospects. Expert Opin Drug Del. 2012;9(10):1245–1261. doi: 10.1517/17425247.2012.717928. [DOI] [PubMed] [Google Scholar]
- 53.Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422(6927):37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- 54.Lu W, Tan YZ, Hu KL, Jiang XG. Cationic albumin conjugated pegylated nanoparticle with its transcytosis ability and little toxicity against blood–brain barrier. Int J Pharm. 2005;295(1–2):247–260. doi: 10.1016/j.ijpharm.2005.01.043. [DOI] [PubMed] [Google Scholar]
- 55.Liu L, Guo K, Lu J, et al. Biologically active core/shell nanoparticles self-assembled from cholesterol-terminated PEG-TAT for drug delivery across the blood–brain barrier. Biomaterials. 2008;29(10):1509–1517. doi: 10.1016/j.biomaterials.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 56.Qin Y, Chen HL, Yuan WM, et al. Liposome formulated with TAT-modified cholesterol for enhancing the brain delivery. Int J Pharm. 2011;419(1–2):85–95. doi: 10.1016/j.ijpharm.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 57.Liu Y, Huang RQ, Han L, et al. Brain-targeting gene delivery and cellular internalization mechanisms for modified rabies virus glycoprotein RVG29 nanoparticles. Biomaterials. 2009;30(25):4195–4202. doi: 10.1016/j.biomaterials.2009.02.051. [DOI] [PubMed] [Google Scholar]
- 58▪.Van Rooy I, Mastrobattista E, Storm G, Hennink WE, Schiffelers RM. Comparison of five different targeting ligands to enhance accumulation of liposomes into the brain. J Control Release. 2011;150(1):30–36. doi: 10.1016/j.jconrel.2010.11.014. Highlights the advantages and obstacles to using various commonly studied ligands for blood–brain barrier penetration by nanoparticles. [DOI] [PubMed] [Google Scholar]
- 59.Laron Z. Insulin and the brain. Arch Physiol Biochem. 2009;115(2):112–116. doi: 10.1080/13813450902949012. [DOI] [PubMed] [Google Scholar]
- 60.Boado RJ, Zhang YF, Zhang Y, Pardridge WM. Humanization of anti-human insulin receptor antibody for drug targeting across the human blood–brain barrier. Biotechnol Bioeng. 2007;96(2):381–391. doi: 10.1002/bit.21120. [DOI] [PubMed] [Google Scholar]
- 61.Ulbrich K, Knobloch T, Kreuter J. Targeting the insulin receptor: nanoparticles for drug delivery across the blood–brain barrier (BBB) J Drug Target. 2011;19(2):125–132. doi: 10.3109/10611861003734001. [DOI] [PubMed] [Google Scholar]
- 62.Ulbrich K, Hekmatara T, Herbert E, Kreuter J. Transferrin- and transferrin-receptor-antibody-modified nanoparticles enable drug delivery across the blood–brain barrier (BBB) Eur J Pharm Biopharm. 2009;71(2):251–256. doi: 10.1016/j.ejpb.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 63.Daniels TR, Delgado T, Rodriguez JA, Helguera G, Penichet ML. The transferrin receptor part I: biology and targeting with cytotoxic antibodies for the treatment of cancer. Clin Immunol. 2006;121(2):144–158. doi: 10.1016/j.clim.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 64.De Boer AG, Van Der Sandt ICJ, Gaillard PJ. The role of drug transporters at the blood–brain barrier. Annu Rev Pharmacol Toxicol. 2003;43:629–656. doi: 10.1146/annurev.pharmtox.43.100901.140204. [DOI] [PubMed] [Google Scholar]
- 65.Gan CW, Feng SS. Transferrin-conjugated nanoparticles of poly(lactide)-D-alpha-tocopheryl polyethylene glycol succinate diblock copolymer for targeted drug delivery across the blood–brain barrier. Biomaterials. 2010;31(30):7748–7757. doi: 10.1016/j.biomaterials.2010.06.053. [DOI] [PubMed] [Google Scholar]
- 66.Sahoo SK, Labhasetwar V. Enhanced antiproliferative activity of transferrin-conjugated paclitaxel-loaded nanoparticles is mediated via sustained intracellular drug retention. Mol Pharm. 2005;2(5):373–383. doi: 10.1021/mp050032z. [DOI] [PubMed] [Google Scholar]
- 67.Lu YJ, Sega E, Leamon CP, Low PS. Folate receptor-targeted immunotherapy of cancer: mechanism and therapeutic potential. Adv Drug Delivery Rev. 2004;56(8):1161–1176. doi: 10.1016/j.addr.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 68.Lefranc F, Brotchi J, Kiss R. Possible future issues in the treatment of glioblastomas: special emphasis on cell migration and the resistance of migrating glioblastoma cells to apoptosis. J Clin Oncol. 2005;23(10):2411–2422. doi: 10.1200/JCO.2005.03.089. [DOI] [PubMed] [Google Scholar]
- 69.Mitchell P, Ellison DW, Mendelow AD. Surgery for malignant gliomas: mechanistic reasoning and slippery statistics. Lancet Neurol. 2005;4(7):413–422. doi: 10.1016/S1474-4422(05)70118-6. [DOI] [PubMed] [Google Scholar]
- 70.Sanai N, Berger MS. Glioma extent of resection and its impact on patient outcome. Neurosurgery. 2008;62(4):753–764. doi: 10.1227/01.neu.0000318159.21731.cf. [DOI] [PubMed] [Google Scholar]
- 71.Kongkham P, Rutka JT, Piepmeier JM, Kaye AH, Black PM, Barker FG. Glioma extent of resection and its impact on patient outcome – comments. Neurosurgery. 2008;62(4):764–766. doi: 10.1227/01.neu.0000318159.21731.cf. [DOI] [PubMed] [Google Scholar]
- 72.Giese A, Bjerkvig R, Berens ME, Westphal M. Cost of migration: invasion of malignant gliomas and implications for treatment. J Clin Oncol. 2003;21(8):1624–1636. doi: 10.1200/JCO.2003.05.063. [DOI] [PubMed] [Google Scholar]
- 73.Kroin JS, Penn RD. Intracerebral chemotherapy: chronic microinfusion of cisplatin. Neurosurgery. 1982;10(3):349–354. doi: 10.1227/00006123-198203000-00009. [DOI] [PubMed] [Google Scholar]
- 74.Thorne RG, Nicholson C. In vivo diffusion analysis with quantum dots and dextrans predicts the width of brain extracellular space. Proc Natl Acad Sci USA. 2006;103(14):5567–5572. doi: 10.1073/pnas.0509425103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lu W, Wan J, Zhang Q, She Z, Jiang X. Aclarubicin-loaded cationic albumin-conjugated pegylated nanoparticle for glioma chemotherapy in rats. Int J Cancer. 2007;120(2):420–431. doi: 10.1002/ijc.22296. [DOI] [PubMed] [Google Scholar]
- 76▪.Nance EA, Woodworth GF, Sailor KA, et al. A dense poly(ethylene glycol) coating Improves penetration of large polymeric nanoparticles within brain tissue. Sci Transl Med. 2012;4(149):149ra119. doi: 10.1126/scitranslmed.3003594. Emphasizes the insufficient diffusion of nanoparticles often observed after local delivery to the brain and also explains and overcomes physicochemical properties of nanoparticles, other than size, that restrict diffusion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fleming AB, Saltzman WM. Pharmacokinetics of the carmustine implant. Clin Pharmacokinet. 2002;41(6):403–419. doi: 10.2165/00003088-200241060-00002. [DOI] [PubMed] [Google Scholar]
- 78▪▪.Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci USA. 1994;91(6):2076–2080. doi: 10.1073/pnas.91.6.2076. Convection-enhanced delivery, described in this report, is an important technique for local delivery in the brain, transporting therapeutics much more than simple diffusion would normally allow in the brain interstitium. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sawyer AJ, Saucier-Sawyer JK, Booth CJ, et al. Convection-enhanced delivery of camptothecin-loaded polymer nanoparticles for treatment of intracranial tumors. Drug Deliv Transl Res. 2011;1(1):34–42. doi: 10.1007/s13346-010-0001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Krauze MT, Noble CO, Kawaguchi T, et al. Convection-enhanced delivery of nanoliposomal CPT-11 (irinotecan) and PEGylated liposomal doxorubicin (Doxil) in rodent intracranial brain tumor xenografts. Neuro Oncol. 2007;9(4):393–403. doi: 10.1215/15228517-2007-019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Saito R, Bringas JR, Mcknight TR, et al. Distribution of liposomes into brain and rat brain tumor models by convection-enhanced delivery monitored with magnetic resonance imaging. Cancer Res. 2004;64(7):2572–2579. doi: 10.1158/0008-5472.can-03-3631. [DOI] [PubMed] [Google Scholar]
- 82.Perlstein B, Ram Z, Daniels D, et al. Convection-enhanced delivery of maghemite nanoparticles: increased efficacy and MRI monitoring. Neuro Oncol. 2008;10(2):153–161. doi: 10.1215/15228517-2008-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wu G, Barth RF, Yang WL, Kawabata S, Zhang LW, Green-Church K. Targeted delivery of methotrexate to epidermal growth factor receptor-positive brain tumors by means of cetuximab (IMC-C225) dendrimer bioconjugates. Mol Cancer Ther. 2006;5(1):52–59. doi: 10.1158/1535-7163.MCT-05-0325. [DOI] [PubMed] [Google Scholar]
- 84.Hadaczek P, Kohutnicka M, Krauze MT, et al. Convection-enhanced delivery of adeno-associated virus Type 2 (AAV2) into the striatum and transport of AAV2 within monkey brain. Hum Gene Ther. 2006;17(3):291–302. doi: 10.1089/hum.2006.17.291. [DOI] [PubMed] [Google Scholar]
- 85.Neeves KB, Sawyer AJ, Foley CP, Saltzman WM, Olbricht WL. Dilation and degradation of the brain extracellular matrix enhances penetration of infused polymer nanoparticles. Brain Res. 2007;1180:121–132. doi: 10.1016/j.brainres.2007.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lidar Z, Mardor Y, Jonas T, et al. Convection-enhanced delivery of paclitaxel for the treatment of recurrent malignant glioma: a Phase I/II clinical study. J Neurosurg. 2004;100(3):472–479. doi: 10.3171/jns.2004.100.3.0472. [DOI] [PubMed] [Google Scholar]
- 87.Lyons SA, O’Neal J, Sontheimer H. Chlorotoxin, a scorpion-derived peptide, specifically binds to gliomas and tumors of neuroectodermal origin. Glia. 2002;39(2):162–173. doi: 10.1002/glia.10083. [DOI] [PubMed] [Google Scholar]
- 88.Veiseh O, Sun C, Fang C, et al. Specific targeting of brain tumors with an optical/magnetic resonance imaging nanoprobe across the blood–brain barrier. Cancer Res. 2009;69(15):6200–6207. doi: 10.1158/0008-5472.CAN-09-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nam T, Park S, Lee SY, et al. Tumor targeting chitosan nanoparticles for dual-modality optical/MR cancer imaging. Bioconjug Chem. 2010;21(4):578–582. doi: 10.1021/bc900408z. [DOI] [PubMed] [Google Scholar]
- 90.Madhankumar AB, Slagle-Webb B, Mintz A, Sheehan JM, Connor JR. Interleukin-13 receptor-targeted nanovesicles are a potential therapy for glioblastoma multiforme. Mol Cancer Ther. 2006;5(12):3162–3169. doi: 10.1158/1535-7163.MCT-06-0480. [DOI] [PubMed] [Google Scholar]
- 91.Simberg D, Duza T, Park JH, et al. Biomimetic amplification of nanoparticle homing to tumors. Proc Natl Acad Sci USA. 2007;104(3):932–936. doi: 10.1073/pnas.0610298104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Duan CX, Zhang DR, Wang FH, et al. Chitosan-g-poly(N-isopropylacrylamide) based nanogels for tumor extracellular targeting. Int J Pharm. 2011;409(1–2):252–259. doi: 10.1016/j.ijpharm.2011.02.050. [DOI] [PubMed] [Google Scholar]
- 93.Estrela JM, Ortega A, Obrador E. Glutathione in cancer biology and therapy. Crit Rev Clin Lab Sci. 2006;43(2):143–181. doi: 10.1080/10408360500523878. [DOI] [PubMed] [Google Scholar]
- 94.Van Tellingen O, Brandsma D, Boogerd W, et al. Gsh-conjugation improves efficacy of doxil against intracranial xenografts. Neuro Oncol. 2010;12:34–34. [Google Scholar]
- 95▪.Aboody KS, Brown A, Rainov NG, et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci USA. 2000;97(23):12846–12851. doi: 10.1073/pnas.97.23.12846. First report to demonstrate extensive studies on the potential of adult stem cell-based strategies for glioma targeting and delivery of therapeutics, demonstrating both the migratory abilities of injected stem cells and also their potential use as nanoengineered vehicles for drug delivery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nakamizo A, Marini F, Amano T, et al. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65(8):3307–3318. doi: 10.1158/0008-5472.CAN-04-1874. [DOI] [PubMed] [Google Scholar]
- 97.Uchibori R, Okada T, Ito T, et al. Retroviral vector-producing mesenchymal stem cells for targeted suicide cancer gene therapy. J Gene Med. 2009;11(5):373–381. doi: 10.1002/jgm.1313. [DOI] [PubMed] [Google Scholar]
- 98.Matuskova M, Hlubinova K, Pastorakova A, et al. HSV-tk expressing mesenchymal stem cells exert bystander effect on human glioblastoma cells. Cancer Lett. 2010;290(1):58–67. doi: 10.1016/j.canlet.2009.08.028. [DOI] [PubMed] [Google Scholar]
- 99.Sonabend AM, Ulasov IV, Tyler MA, Rivera AA, Mathis JM, Lesniak MS. Mesenchymal stem cells effectively deliver an oncolytic adenovirus to intracranial glioma. Stem Cells. 2008;26(3):831–841. doi: 10.1634/stemcells.2007-0758. [DOI] [PubMed] [Google Scholar]
- 100.Roger M, Clavreul A, Venier-Julienne MC, et al. Mesenchymal stem cells as cellular vehicles for delivery of nanoparticles to brain tumors. Biomaterials. 2010;31(32):8393–8401. doi: 10.1016/j.biomaterials.2010.07.048. [DOI] [PubMed] [Google Scholar]
- 101.Wu X, Hu J, Zhou LF, et al. In vivo tracking of superparamagnetic iron oxide nanoparticle-labeled mesenchymal stem cell tropism to malignant gliomas using magnetic resonance imaging. J Neurosurg. 2008;108(2):320–329. doi: 10.3171/JNS/2008/108/2/0320. [DOI] [PubMed] [Google Scholar]
- 102.Huang X, Zhang F, Wang H, et al. Mesenchymal stem cell-based cell engineering with multifunctional mesoporous silica nanoparticles for tumor delivery. Biomaterials. 2013;34(7):1772–1780. doi: 10.1016/j.biomaterials.2012.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McGirt MJ, Than KD, Weingart JD, et al. Gliadel (BCNU) wafer plus concomitant temozolomide therapy after primary resection of glioblastoma multiforme. J Neurosurg. 2009;110(3):583–588. doi: 10.3171/2008.5.17557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Steiniger SCJ, Kreuter J, Khalansky AS, et al. Chemotherapy of glioblastoma in rats using doxorubicin-loaded nanoparticles. Int J Cancer. 2004;109(5):759–767. doi: 10.1002/ijc.20048. [DOI] [PubMed] [Google Scholar]
- 105.Immonen A, Vapalahti M, Tyynela K, et al. AdvHSV-tk gene therapy with intravenous ganciclovir improves survival in human malignant glioma: a randomised, controlled study. Mol Ther. 2004;10(5):967–972. doi: 10.1016/j.ymthe.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 106.Sandmair AM, Loimas S, Puranen P, et al. Thymidine kinase gene therapy for human malignant glioma, using replication-deficient retroviruses or adenoviruses. Hum Gene Ther. 2000;11(16):2197–2205. doi: 10.1089/104303400750035726. [DOI] [PubMed] [Google Scholar]
- 107.Moolten FL. Tumor chemosensitivity conferred by inserted herpes thymidine kinase genes – paradigm for a prospective cancer control strategy. Cancer Res. 1986;46(10):5276–5281. [PubMed] [Google Scholar]
- 108.Vecil GG, Lang FF. Clinical trials of adenoviruses in brain tumors: a review of Ad-p53 and oncolytic adenoviruses. J Neuro Oncol. 2003;65(3):237–246. doi: 10.1023/b:neon.0000003653.45635.32. [DOI] [PubMed] [Google Scholar]
- 109.Chiocca EA, Abbed KM, Tatter S, et al. A Phase I open-label, dose-escalation, multi-institutional trial of injection with an E1B-attenuated adenovirus, ONYX-015, into the peritumoral region of recurrent malignant gliomas, in the adjuvant setting. Mol Ther. 2004;10(5):958–966. doi: 10.1016/j.ymthe.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 110.Tai CK, Wang WJ, Chen TC, Kasahara N. Single-shot, multicycle suicide gene therapy by replication-competent retrovirus vectors achieves long-term survival benefit in experimental glioma. Mol Ther. 2005;12(5):842–851. doi: 10.1016/j.ymthe.2005.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bogler O, Huang HJS, Kleihues P, Cavenee WK. The p53 gene and its role in human Braintumors. Glia. 1995;15(3):308–327. doi: 10.1002/glia.440150311. [DOI] [PubMed] [Google Scholar]
- 112.Tolcher AW, Hao D, De Bono J, et al. Phase 1, pharmacokinetic, and pharmacodynamic study of intravenously administered Ad5CMV-p53, an adenoviral vector containing the wild-type p53 gene, in patients with advanced cancer. J Clin Oncol. 2006;24(13):2052–2058. doi: 10.1200/JCO.2005.03.6756. [DOI] [PubMed] [Google Scholar]
- 113.Lang FF, Bruner JM, Fuller GN, et al. Phase I trial of adenovirus-mediated p53 gene therapy for recurrent glioma: biological and clinical results. J Clin Oncol. 2003;21(13):2508–2518. doi: 10.1200/JCO.2003.21.13.2508. [DOI] [PubMed] [Google Scholar]
- 114.Prados MD, Mcdermott M, Chang SM, et al. Treatment of progressive or recurrent glioblastoma multiforme in adults with herpes simplex virus thymidine kinase gene vector-producer cells followed by intravenous ganciclovir administration: a Phase I/II multi-institutional trial. J Neuro Oncol. 2003;65(3):269–278. doi: 10.1023/b:neon.0000003588.18644.9c. [DOI] [PubMed] [Google Scholar]
- 115.Pulkkanen KJ, Yla-Herttuala S. Gene therapy for malignant glioma: current clinical status. Mol Ther. 2005;12(4):585–598. doi: 10.1016/j.ymthe.2005.07.357. [DOI] [PubMed] [Google Scholar]
- 116.Putnam D. Polymers for gene delivery across length scales. Nat Mater. 2006;5(6):439–451. doi: 10.1038/nmat1645. [DOI] [PubMed] [Google Scholar]
- 117.Voges J, Reszka R, Gossmann A, et al. Imaging-guided convection-enhanced delivery and gene therapy of glioblastoma. Ann Neurol. 2003;54(4):479–487. doi: 10.1002/ana.10688. [DOI] [PubMed] [Google Scholar]
- 118.Yoshida J, Mizuno M, Fujii M, et al. Human gene therapy for malignant gliomas (glioblastoma multiforme and anaplastic astrocytoma) by in vivo transduction with human interferon beta gene using cationic liposomes. Hum Gene Ther. 2004;15(1):77–86. doi: 10.1089/10430340460732472. [DOI] [PubMed] [Google Scholar]
- 119.Hawkins CJ. Trail and malignant glioma. Vitam Horm. 2004;67:427–452. doi: 10.1016/S0083-6729(04)67022-1. [DOI] [PubMed] [Google Scholar]
- 120.Lu W, Sun Q, Wan J, She ZJ, Jiang XG. Cationic albumin-conjugated pegylated nanoparticles allow gene delivery into brain tumors via intravenous administration. Cancer Res. 2006;66(24):11878–11887. doi: 10.1158/0008-5472.CAN-06-2354. [DOI] [PubMed] [Google Scholar]
- 121.Van Houdt WJ, Haviv YS, Lu BG, et al. The human survivin promoter: a novel transcriptional targeting strategy for treatment of glioma. J Neurosurg. 2006;104(4):583–592. doi: 10.3171/jns.2006.104.4.583. [DOI] [PubMed] [Google Scholar]
- 122.Su ZZ, Sarkar D, Emdad L, et al. Targeting gene expression selectively in cancer cells by using the progression-elevated gene-3 promoter. Proc Natl Acad Sci USA. 2005;102(4):1059–1064. doi: 10.1073/pnas.0409141102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cardoso ALC, Simoes S, De Almeida LP, et al. SiRNA detivery by a transferrin-associated lipid-based vector: a non-viral strategy to mediate gene silencing. J Gene Med. 2007;9(3):170–183. doi: 10.1002/jgm.1006. [DOI] [PubMed] [Google Scholar]
- 124.Tzeng SY, Green JJ. Subtle changes to polymer structure and degradation mechanism enable highly effective nanoparticles for siRNA and DNA delivery to human brain cancer. Adv Healthcare Mater. 2012;2(3):468–480. doi: 10.1002/adhm.201200257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Caruso G, Caffo M, Alafaci C, et al. Could nanoparticle systems have a role in the treatment of cerebral gliomas? Nanomedicine. 2011;7(6):744–752. doi: 10.1016/j.nano.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 126.Furnari FB, Fenton T, Bachoo RM, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21(21):2683–2710. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- 127.Kang CS, Zhang ZY, Jia ZF, et al. Suppression of EGFR expression by antisense or small interference RNA inhibits U251 glioma cell growth in vitro and in vivo. Cancer Gene Ther. 2006;13(5):530–538. doi: 10.1038/sj.cgt.7700932. [DOI] [PubMed] [Google Scholar]
- 128.Jordan A, Scholz R, Maier-Hauff K, et al. The effect of thermotherapy using magnetic nanoparticles on rat malignant glioma. J Neuro Oncol. 2006;78(1):7–14. doi: 10.1007/s11060-005-9059-z. [DOI] [PubMed] [Google Scholar]
- 129.Bachgansmo T. Ferrimagnetic susceptibility contrast agents. Acta Radiol. 1993;34(387):1–30. [PubMed] [Google Scholar]
- 130.Xie H, Zhu YH, Jiang WL, et al. Lactoferrin-conjugated superparamagnetic iron oxide nanoparticles as a specific MRI contrast agent for detection of brain glioma in vivo. Biomaterials. 2011;32(2):495–502. doi: 10.1016/j.biomaterials.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 131.Neuwelt EA, Varallyay P, Bago AG, Muldoon LL, Nesbit G, Nixon R. Imaging of iron oxide nanoparticles by MR and light microscopy in patients with malignant brain tumours. Neuropathol Appl Neurobiol. 2004;30(5):456–471. doi: 10.1111/j.1365-2990.2004.00557.x. [DOI] [PubMed] [Google Scholar]
- 132.Soenen SJH, Himmelreich U, Nuytten N, De Cuyper M. Cytotoxic effects of iron oxide nanoparticles and implications for safety in cell labelling. Biomaterials. 2011;32(1):195–205. doi: 10.1016/j.biomaterials.2010.08.075. [DOI] [PubMed] [Google Scholar]
- 133.Huang HC, Barua S, Sharma G, Dey SK, Rege K. Inorganic nanoparticles for cancer imaging and therapy. J Control Release. 2011;155(3):344–357. doi: 10.1016/j.jconrel.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 134.Kang MK, Kang SK. Tumorigenesis of chemotherapeutic drug-resistant cancer stem-like cells in brain glioma. Stem Cells Dev. 2007;16(5):837–847. doi: 10.1089/scd.2007.0006. [DOI] [PubMed] [Google Scholar]
- 135.Clarke MF, Dick JE, Dirks PB, et al. Cancer stem cells – perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66(19):9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 136.Silber J, Lim DA, Petritsch C, et al. miR-124 and miR-137 inhibit proliferation of glioblastoma multiforme cells and induce differentiation of brain tumor stem cells. BMC Med. 2008;6:14. doi: 10.1186/1741-7015-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gangemi RMR, Griffero F, Marubbi D, et al. SOX2 silencing in glioblastoma tumor-initiating cells causes stop of proliferation and loss of tumorigenicity. Stem Cells. 2009;27(1):40–48. doi: 10.1634/stemcells.2008-0493. [DOI] [PubMed] [Google Scholar]
- 138.Costantino L, Boraschi D. Is there a clinical future for polymeric nanoparticles as brain-targeting drug delivery agents? Drug Discov Today. 2012;17(7–8):367–378. doi: 10.1016/j.drudis.2011.10.028. [DOI] [PubMed] [Google Scholar]
- 139.Stern ST, Hall JB, Yu LL, et al. Translational considerations for cancer nanomedicine. J Control Release. 2010;146(2):164–174. doi: 10.1016/j.jconrel.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]