Abstract
Graft-versus-host disease (GVHD) remains the major complication of allogeneic bone marrow transplantation (allo-BMT). GVHD fundamentally depends upon the activation of donor T cells by host antigen-presenting cells (APCs), but the precise location of these interactions remains uncertain. We examined the role of secondary lymphoid organs (SLO) in the induction of GVHD by using homozygous aly/aly mice that are deficient in lymph nodes (LNs) and Peyer’s patches (PPs). Lethally irradiated, splenectomized, aly/ aly (LN/PP/Sp−/−) mice and sham-splenectomized, aly/+ (LN/PP/Sp+/+) mice received BMT from either syngeneic (aly/aly) or allogeneic, major histocompatibility complex (MHC) disparate donors. Surprisingly, although LN/PP/Sp−/− allo-BMT recipients experience a survival advantage, they developed significant systemic and target organ GVHD that is comparable to LN/PP/Sp+/+ controls. Early after allo-BMT, the activation and proliferation of donor T cells was significantly greater in the BM cavity of LN/PP/Sp−/− mice compared to LN/PP/Sp+/+ controls. Donor T cells in LN/PP/Sp−/− mice demonstrated cytolytic activity in vitro, but Graft vs Leukemia (GVL) activity could be overcome by increasing the tumor burden. These data suggest that SLO contribute to, but are not required for, allogeneic T cell responses, and suggest that the BM may represent an alternative, albeit less efficient site for T cell activation following allo-BMT.
Keywords: Inflammation, Cytotoxicity, Immunity, Graft-versus-leukemia, Antigen presentation
INTRODUCTION
Allogeneic bone marrow transplantation (allo-BMT) is the only curative treatment for a number of malignant and nonmalignant hematologic diseases. Immunologic dysregulation occurring after allo-BMT represents a double-edged sword to the clinical BMT specialist. On one hand, T cells and cytokines responsible for the toxicity of graft-versus-host disease (GVHD) limit the use of this form of therapy, but on the other, they enhance the therapeutic potential of BMT through the graft-versus-leukemia (GVL) effect. Unfortunately, beneficial GVL effects are closely linked to the deleterious consequences of GVHD. GVHD is the most significant contributor to nonrelapse mortality after allo-BMT, and remains the primary barrier to broadening the scope of this treatment option; outcomes have not improved over the past 2 decades despite our enhanced knowledge of the biology of GVHD.
Although interactions between donor T cells and antigen-presenting cells (APCs) are fundamental to the development of both GVHD and GVL, the exact location of these encounters remains speculative. The prevailing theory is that secondary lymphoid organs (SLO) are necessary for activation of naïve donor T cells following allo-BMT, but published data on this topic are conflicting. Previous work showed that Peyer’s patches (PPs) are critical for the generation of donor-derived, anti-host cytotoxic T lymphocytes (CTLs) that cause acute GVHD (aGVHD) after non-myeloblative (NMA) conditioning [1]. Moreover, tracking of cell migration early after BMT, using bio-luminescent imaging, revealed increased numbers of T cells in a variety of secondary lymphoid tissues including the spleen, PP and mesenteric lymph nodes (LNs) [2]. By contrast, a recent study demonstrated that PPs are not required for aGVHD after myeloablative (MA) conditioning [3].
We set out to examine the role of SLO in GVHD induction and hypothesized that if SLO, including LN, PP, and spleen, are essential for donor T cell activation, then the absence of these tissues in the BMT recipients should completely abrogate GVHD. We designed experiments using unique mutant mice (alymphoplasia; aly/aly) as BMT recipients in well described major histocompability complex (MHC)-disparate BMT systems. Aly/aly mice were originally described following a spontaneous autosomal mutation in the gene encoding NF-κB-inducing kinase [4]. This mutation results in a systemic absence of LN, PP [4–6], and recently discovered isolated lymphoid follicles (ILF) [7,8], as well as structural defects of the spleen and thymus. The aly mutation is also associated with reduced levels of serum IgG and complete absence of IgA, the latter serving as an effective marker to phenotypically distinguish between homozygous and heterozygous littermates [5,6,9,10]. Although deficiencies in cell-mediated immune function, such as impaired intestinal humoral immune responses, are observed in aly/aly mice, normal dendritic cell (DC) function is preserved [5]. Thus, we reasoned that following splenectomy, aly/ aly mice would lack all traditional SLO (LN/PP/ Sp−/−), and therefore serve as suitable BMT recipients to test our central hypothesis.
We found that LN/PP/Sp−/− recipients developed clinical and target organ GVHD that was comparable, if not greater than, both LN/PP/Sp+/+ littermate and wild-type, C57BL/6 controls. Nearly identical results were also found in a second and more robust strain combination wherein MHC-disparate B10.BR (H-2k) mice were used as allogeneic BMT donors. Although present, the severity of GVHD was less severe and the generation of GVL responses could be overcome when tumor burden was increased. Collectively, our data challenge the paradigm that secondary lymphoid tissues are required for GVHD induction, but suggest they may be required for the generation of optimal GVL responses.
MATERIALS AND METHODS
Mice and BMT
Female C57BL/6 (H-2b) and male and female BALB/c (H-2d), and B10.BR (H-2k) mice were purchased from the Jackson Laboratory (JAX; Bar Harbor, ME). Homozygous aly/aly and heterozygous aly/+ (H-2b) mice were originally purchased from Japan and bred at the University of Michigan [4]. C57BL/6 mice deficient in alpha(1,3)-fucosyltransferase VII and alpha(2,3)-sialyltransferase IV (FucT dko) were kindly provided by Dr. John Lowe. Synthesis of fucosylated glycans required for E-, P-, and L-selectin ligand activity is catalyzed by an ordered series of reactions that are ultimately controlled by specific α(1,3) fucosyltransferases, including Fuc-TIV and Fuc-TVII [11,12]. FucT dko mice lack P- and E-selectin ligands on leukocyte surfaces and L-selectin ligands on high endothelial venules of SLO where it is constitutively expressed. Thus, lymphocyte homing to LNs is completely abrogated in FucT dko mice, whereas migration to PP is reduced by approximately 50% [13].
Mice received BMT according to a standard protocol [14]. Briefly, lethally irradiated (13 Gy; 137Cs source), splenectomized aly/aly (LN/PP/Sp−/−) or sham-splenectomized aly/+ littermates (LN/PP/ Sp+/+) received 5 × 106 BM cells supplemented with 2.0 or 5.0 × 106 AutoMacs (Miltenyi Biotech, Auburn, CA) purified splenic T cells (CD90-conjugated magnetic beads; Miltenyi Biotech) from syngeneic (aly/aly) or allogeneic BALB/c (H-2d), or B10.BR (H-2k) donors. T cell (TCRαβ+) purity was routinely between 80% and 90%. In some experiments, wild-type C57BL/6 or C57BL/6 FucT dko recipients of syngeneic (C57BL/6) or allogeneic (Balb/c or B10.BR) BMT served as additional controls. Donor and recipient mice were between 8 to 20 weeks of age at the time of use. Mice were housed in microisolator cages and received normal chow and autoclaved hyperchlorinated drinking water for the first 3 weeks after BMT, and filtered water thereafter. All procedures were approved by and conducted in accordance with regulations of the University of Michigan Committee on the Use and Care of Animals, and the Case Western Reserve University Institutional Animal Care and Use Committee.
Splenectomy and Sham Procedure
Splenectomies were performed under anesthesia in a Unit for Laboratory Animal Medicine (ULAM) designated surgical room at the University of Michigan. Briefly, mice received the inhaled anesthetic AErrane (isoflurane, USP, Baxter, IL) using a device that controls the amount of isoflurane and O2 released inside a restraining container. Once adequately anesthetized, the spleen was isolated and removed using a sterile hemostat and an electrocautery tool. The peritoneal cavity and skin were closed with surgical staples. Mice were observed until they fully recovered from anesthesia. Heterozygous aly/+ littermates underwent a sham procedure wherein the spleen was lifted and gently placed back in the peritoneal cavity before applying surgical staples. Greater than 90% of the animals survived each procedure and were ultimately able to undergo BMT after a recovery period of 2 weeks.
Evaluation of GVHD Severity
Survival was monitored daily and GVHD clinical scores were assessed weekly for all BMT recipient mice using a previously described system [15]. Acute GVHD was also assessed in the small (ileum) and large (ascending) intestine, liver, and skin. Tissues were harvested from animals at defined time points after BMT and prepared for histologic examination. Slides were coded without reference to mouse type or prior treatment status and examined by a single pathologist (C.L.) using semiquantitative scoring systems previously described. Seven parameters known to be consistent with GVHD were scored for small bowel and large bowel, and 10 parameters were evaluated for liver. Skin GVHD was evaluated by using sections of tongue as described [16].
Mixed Lymphocyte Reactions (MLR) and Cytotoxic T Lymphocyte (CTL) Activity
Ninety-six-well plates (Sarstedt, NC) were seeded with 2.0 × 106 AutoMACS purified (CD90 mAbs) BALB/c or B10.BR T cells in 10% fetal calf serum (FCS) DMEM and cocultured for 72 hours with 2 × 104 BM-derived DCs (see below) or AutoMACS purified (CD11c+) splenic DCs from either aly/aly, aly/+, C57BL/6, or FucT dko mice [17]. At 48 hours, 100 μL of supernatant were collected for cytokine analysis. Cell cultures were subsequently pulsed with 50 μL of 3H-thymidine (1 μCi/well [0.037 MBq]) and proliferation was determined 12–20 hours later on a 1205 Betaplate reader (Wallac, Turku, Filand).
For CTL assays, allogeneic (B10.BR or Balb/c) T cells were cultured for 5 days in a bulk-MLR with irradiated (2000 rad) whole splenocytes from aly/aly, aly/+, and C57BL/6 mice. Cells were harvested, washed, resuspended in warm DMEM medium, and then slowly transferred to the wall of 15 mL tubes with 3 mL of Ficoll-Paque Plus (Amersham Bioscience, Piscataway, NJ). Following centrifugation, leukocytes were isolated, washed twice, incubated with antibodies to CD4 (PE) and CD8 (FITC) (BD Bioscience, San Jose, CA), and analyzed by flow cytometry. CTL activity was analyzed by 51Cr release assays as described previously [18,19]. First, 2 × 106 allogeneic EL4 or MBL-2 (H-2b) and syngeneic P815 (H2d) tumor targets were labeled with 10 μL of 100 μCi (3.7 MBq) of 51Cr sodium salt (NEN Life Sciences Products, Frederick, CO) after incubation at 37°C for 1.5 hours. Labeled targets were resuspended in 10% FCS RPMI and plated at 104 cells per well in U-bottom plates (Corning-Costar, Cambridge, MA). Activated CD8+ cells collected following bulk MLR and normalized by flow cytometry were added to quadruplicate wells at varying effector (CD8+)-to-target ratios (starting at 50:1 trough 1.5:1) and incubated at 37°C for 4 hours. Maximal and background release were determined by the addition of Triton-X (Sigma Chemical, St. Louis, MO) or media alone to targets, respectively. 51Cr activity in supernatants taken at hour 4 was determined in a top count liquid microplate scintillation Counter (Perkin-Elmer, Shelton, CT), and lysis was expressed as a percentage of maximum. In some experiments, donor-derived B10.BR T cells that were collected from the BM cavity of LN/PP/ Sp−/− BMT recipients served as the effector cell source in CTL experiments using the JAM assay as described [20].
Flow Cytometric Analysis
Single-cell suspensions collected from the liver and bone marrow (BM) on day 3 and 7 after BMT were counted and subsequently stained with allophycocyanin (APC)-conjugated monoclonal antibodies (mAbs) to CD3 and phycoerythrin (PE)-conjugated mAbs to either H-2kd, H-2kk, or CD69 (BD Bioscience, Pharmingen, San Diego, CA). Two-color flow cytometric analysis of 1 × 104 cells was performed using a FACS-Calibur (BD Biosciences, San Jose, CA). In some experiments, intracytoplasmic cytokine production was measured in donor-derived B10.BR T cells collected from the BM cavity of LN/PP/Sp−/− BMT recipients between days 3 and 5. Following cell surface staining for CD4 and CD8, cells were fixed, permeabilized, stained with PE-conjugated mAbs for TNFα, and IFNγ (eBioscience, San Diego, CA), and ultimately analyzed by 3-color flow cytometric analysis as above.
Donor T Cell Divisions In Vivo
In carboxyfluorescein diacetate, succinimidyl ester (CFDA SE) studies, donor T cells were stained with Vibrant CFDA SE (Invitrogen, Carlsbad, CA) prior to injection into recipient mice. CFDA SE was reconstituted with dimethyl sulfoxide (DMSO) to a final concentration of 5 μM. Cells were resuspended in phosphate-buffered saline (PBS) + 5% FCS (Gibco, Gaithersburg, MD) at 10 × 106/mL and were incubated with CFDA SE for 5 minutes at room temperature. Incubation was stopped by placing cells on ice for 5 minutes, and cells were washed twice in ice-cold 5% FCS-RPMI and resuspended in medium with final concentration 5 × 106 cells/mL prior to injection into irradiated BMT recipients.
Cytokine Enzyme-Linked Immunosorbent Assay (ELISA)
We have established an ELISA protocol to phenotype as homozygous (aly/aly) and heterozygous (aly/+) by screening the sera obtained from 6- to 7-week-old mice for the absence of IgA (homozygous) or the presence of IgA (heterozygous) (data not shown). Antibodies used were purchased from R&D Systems (Minneapolis, MN) and assays were performed according to the manufacturer’s protocol. Rat antimouse capture and detection mAbs were diluted 1:100 and 1:20,000 (respectively) in Coating Buffer (Sigma), whereas serum samples were diluted 1:100. IFNγ ELISAs were performed according to the manufacturer’s protocol (OptEIA, BD Biosciences Pharmingen, San Diego, CA) and assay sensitivity was <7.5 pg/mL. Plates were ultimately read at 450 nm using a microplate reader (Bio-Rad Laboratories, Hercules, CA). Samples and standards were duplicated.
Real-Time Quantitative RT-PCR Analysis
T cells were obtained from whole BM collected between 3 and 5 days after transplantation using Ficoll-Paque separation. mRNA was extracted from whole T cells using TRIzol following the manufacturer’s protocol (Invitrogen), quantified by specrophotometry, and stored at −80°C. TNFα, IFNγ, perforin, and GAPDH (an internal control) expression were analyzed by real-time RT-PCR procedure using an ABI 7300 Real-time PCR system (Applied Biosystems, Foster City, CA). All primers and probes used were purchased from Applied Biosystems. Target gene expression was normalized to GAPDH before the fold change in expression was calculated by comparing values obtained from individual allo-SCT recipients to the average values measured in syngeneic controls.
Leukemia Induction
GVL activity was assessed using a MBL-2 leukemia model as previously described [21,22]. MBL-2 is a Moloney virus-induced T cell lymphoma of C57BL/6 origin (H-2b) and is syngeneic to aly/aly, aly/+ littermates, and C57BL/6 hosts and allogeneic to B10.BR (H-2k) donors. On day 0, 1000, 2000, or 5000 BML-2 cells were injected i.v. into subsets of BMT recipients along with syngeneic (aly/aly) or allogeneic (B10.BR) BM and splenic T cells. Survival was monitored daily and the cause of each death after BMT was determined by postmortem examination. Death from MBL-2 was attributable to tumor expansion as measured by enlargement of the liver and spleen with macroscopic tumor nodules evident upon necropsy or evidence of hind leg paralysis prior to sacrifice. By contrast, GVHD death was indicated with the absence of tumor and the presence of clinical and target organ GVHD.
In Vivo Treatment with Anti-MadCAM-1
In experiments using C57BL/6 wild-type and FucT dko mice as BMT recipients, we used the mAb anti-MadCAM-1 (mucosal address in cell adhesion molecule; eBioscience) to block trafficking of T cells to PPs [23,24]. C57BL/6 wild-type and FucT dko mice were treated with 2 mg/kg of anti-MadCAM given intraperitoneally on days −1, 0, 1, 3, 5, 7, and 10 [24–27]. Mice in control groups received injections of control hamster immunoglobulin.
Statistical Considerations
All values are expressed as the mean ± standard error margin (SEM). Statistical comparisons between groups were completed using the parametric, independent sample, T test, or the nonparametric Mann- Whitney test when sample numbers are 5 or less. Survival curves were generated using Kaplain-Meier estimates and the Wilcoxon rank-test was used for analyzing survival data.
RESULTS
Characterization of aly/aly Mice Prior to BMT
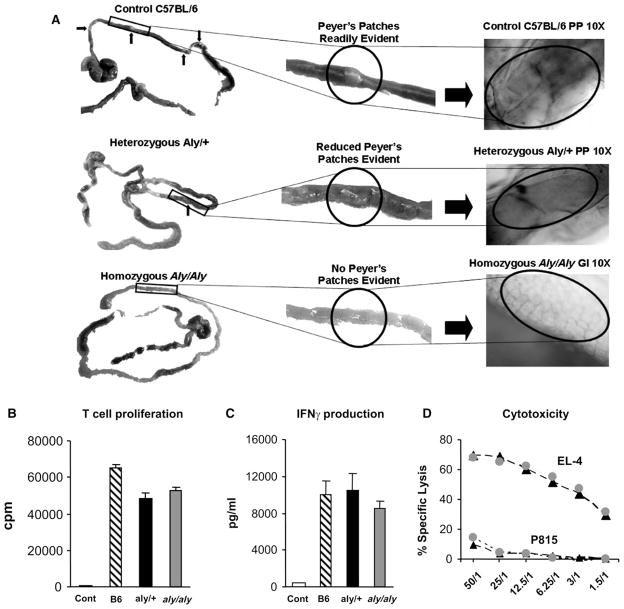
Homozygous aly/aly and heterozygous aly/+ (H-2b) mice were bred at the University of Michigan; progeny were distinguished phenotypically by measuring serum IgA levels as described in the Materials and Methods section (data not shown). Prior to BMT studies, surrogate, age-matched, littermates were examined for the presence or absence of LNs and PPs. Consistent with the previously reported phenotype, gross and microscopic inspection of aly/aly mice demonstrated the absence of PPs (Figure 1A) and lymph nodes in traditional anatomic sites (not shown) [4–6,8,9]. As expected, these structures were present in both wild-type C57BL/6 mice and heterozygous (aly/+) littermates (Figure 1A). We next determined the ability of host aly/aly and aly/+ splenic DCs to stimulate donor Balb/c T cells in vitro. These studies were deemed essential prior to completing and interpreting BMT experiments to ensure that differences in the ability of aly/aly APCs to stimulate donor type T cells would not account for any observed differences in GVH or GVL responses that could be otherwise attributed to the lack of SLOs alone. As shown in Figure 1B–D, no differences in proliferation, IFNγ production, or CTL generation were noted between groups. Similar results were obtained when either peritoneal cells or BM-derived DCs from aly/aly and aly/+ littermates were used as allogeneic stimulator cells and when purified, MHC disparate B10.BR T cells were used as responders (data not shown).
Figure 1.
Aly/aly mice lack Peyer’s patches, but the allo-stimulatory capacity of their DCs is comparable to littermate controls. The small intestines from age-matched naïve wild-type C57BL/6, aly/+, and aly/aly mice were harvested, examined, and photographed using a Nikon digital camera alone or attached to a microscope using 10× magnification lens. Although readily apparent in both wild-type C57BL/6 and aly/+ heterozygote littermate controls, aly/aly mice lacked visible Peyer’s patches. (A) Splenic DCs were purified from wild-type C57BL/6, aly/+littermate controls, and aly/aly mice and used to stimulate purified, MHC disparate Balb/c T cells in an MLR as described in the Materials and Methods section. No differences in the ability to stimulate T cell proliferation (B) IFNγ production (C) or the generation of CTL effectors (D) were observed among groups. Data are expressed as mean±SEM and are representative of 1 of 3 comparable experiments. Wild-type C57BL/6 ▨ aly/+ ■ aly/aly
 compared to T cells only
compared to T cells only
 .
.
MICE LACKING SECONDARY LYMPHOID STRUCTURES DEVELOP aGVHD
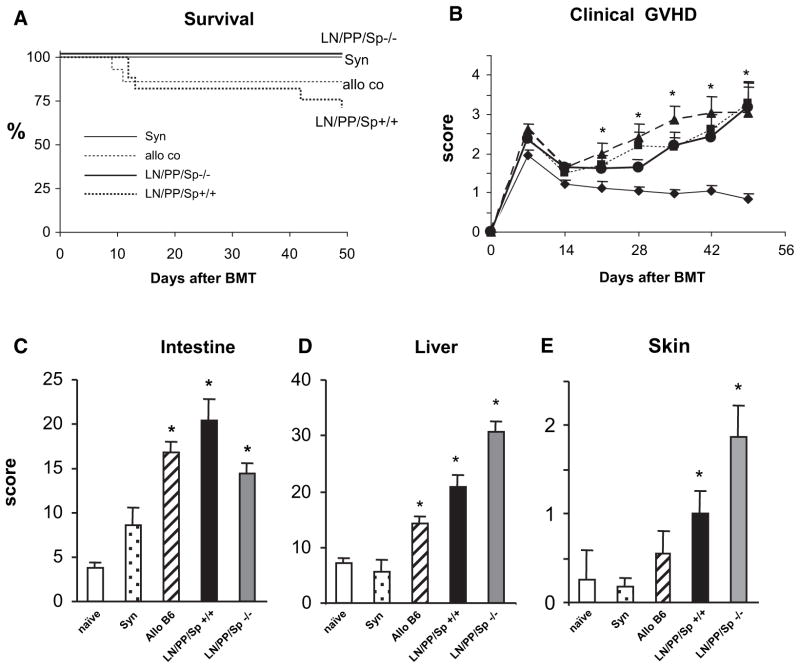
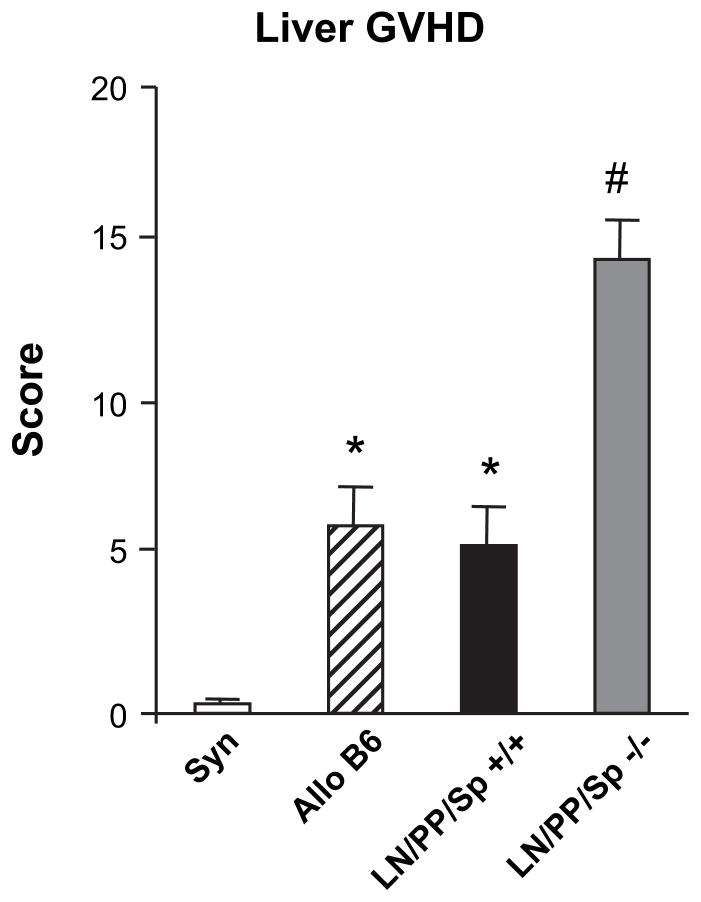
In our next set of experiments, lethally irradiated, splenectomized aly/aly mice (LN/PP/Sp−/−) and sham-splenectomized, aly/+ mice (LN/PP/Sp+/+) received BMT from either syngeneic (aly/aly) or allogeneic, MHC-disparate (Balb/c) donors and were subsequently followed for signs of GVHD. In some experiments, wild-type C57BL/6 BMT recipients were also included as additional allogeneic controls. As expected, all syngeneic BMT recipients survived and ultimately were indistinguishable from naïve, untransplanted animals. By contrast, LN/PP/Sp+/+ mice receiving allo-BMT developed significant GVHD with approximately 30% mortality by day 49. All LN/PP/Sp−/− allo-BMT recipients also survived, but surprisingly, they too developed significant clinical GVHD compared to syngeneic controls (Figure 2A, B). Histopathologic analysis demonstrated that enhanced GVHD clinical scores correlated with significantly greater GVHD target tissue damage in the liver, intestinal tract, and skin compared to syngeneic controls at day 49 (Figure 2C-E). When compared to allogeneic, LN/PP/Sp+/+ littermates, LN/PP/ Sp−/− allo-BMT recipients developed significantly greater hepatic GVHD (30.8 ± 1.9 versus 20.7 ± 2.2; P < .01), whereas intestinal GVHD was less severe. We also examined organs of age-matched naïve aly/aly mice, and these data in combination with those obtained from syngeneic controls confirm that pathology seen after allo-BMT is secondary to GVHD (Figure 2C-E). As shown in Figure 3, similar findings were observed using a second, MHC-disparate mouse model where in B10.BR mice were used as allo-BMT donors and GVHD severity is more robust [28,29].
Figure 2.
Splenectomized, aly/aly (LN/PP/Sp−/−) mice develop significant clinical and target organ GVHD following allo-BMT from MHC-mismatched Balb/c donors. Lethally irradiated, splenectomized aly/aly mice (LN/PP/Sp−/−) received BMT from syngeneic aly/aly or allogeneic Balb/c donors as described in the Materials and Methods section. Littermate, sham splenectomized aly/+mice (LN/PP/Sp+/+) served as allo-BMT controls. In some experiments, wild-type C57BL/6 recipients of C57BL/6 or Balb/c BMT served as additional negative and positive GVHD controls, respectively. The severity of GVHD was subsequently assessed by survival (A) and clinical score (B).◆ syngeneic, ■ C57BL/6, ▲ LN/PP/Sp+/+, ● LN/PP/Sp−/−. Target organ histopathology in the intestinal tract (C), liver (D), and skin (E) was also examined in surviving mice 6 to 7 weeks after BMT. Histology scores from naïve, untransplanted aly/aly mice are also shown. Data are expressed as mean±SEM from of 4 (survival and clinical score) or 3 (target organ pathology) different experiments. n = 12 to 18, (survival and clinical score) or 6 to 12 (pathology) per group; *P<.01 compared to syn controls. naïve aly/aly □; syn
 ; allo-C57BL/6 cont ▨; LN/PP/Sp+ ■; LN/PP/Sp−/−
; allo-C57BL/6 cont ▨; LN/PP/Sp+ ■; LN/PP/Sp−/−
 .
.
Figure 3.
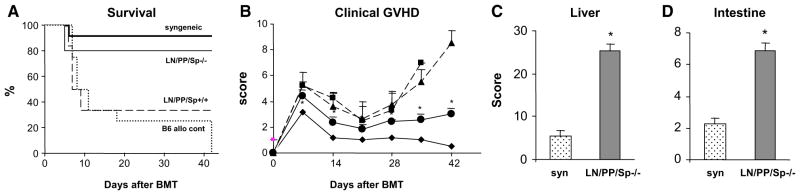
Splenectomized, aly/aly (LN/PP/Sp−/−) mice develop significant clinical and target organ GVHD following allo-BMT from MHC mismatched B10.BR donors. Lethally irradiated, splenectomized aly/aly mice (LN/PP/Sp−/−) received BMT from syngeneic aly/aly or allogeneic B10.BR donors as described in the Materials and Methods section. Littermate, sham splenectomized aly/+ mice (LN/PP/Sp+/+) served as allo-BMT controls. Wildt-ype C57BL/6 recipients of C57BL/6 of B10.BR BMT served as additional positive GVHD controls. The severity of GVHD was subsequently assessed by survival (A) and clinical score (B). ◆ syngeneic, ■ C57BL/6, ▲ LN/PP/Sp+/+ ● LN/PP/Sp−/−. Target organ histopathology in the liver (C) and intestinal tract (D) was also examined in surviving LN/PP/SP−/− allogeneic mice at 6 to 7 weeks after BMT. Data are expressed as mean ± SEM from of 3 (survival and clinical score) or 2 (target organ pathology) different experiments. n=12 to 18 (survival and clinical score) or 8 to 12 (pathology) per group; *p<0.01 compared to syngeneic controls. syn
 LN/PP/Sp−/− ■.
LN/PP/Sp−/− ■.
ESTABLISHMENT OF AN ALTERNATIVE SYSTEM TO ABROGATE LEUKOCYTE MIGRATION TO SLO
To ensure that experimental differences seen with aly/aly animals in the MHC-disparate system did not simply represent a phenomenon related to the aly/aly mouse strain, we conducted similar experiments in a second and distinct GVHD model. In these studies we utilized C57BL/6 FucT dko mice as recipients of BMT from either syngeneic (C57BL/6 FucTdko) or allogeneic (B10.BR) donors. The ultimate absence of E, P, and L selectin ligands in FucT dko mice results in a distinct phenotype that includes the inability of lymphocytes to traffic to lymph nodes, whereas lymphocyte homing to the spleen is not affected [12]. Moreover, we found that DCs from C57BL/6 FucT dko mice are functional and capable of stimulating allogeneic T cell responses to a degree comparable to wild-type C57BL/6 mice (data not shown).
To recapitulate our efforts to completely abrogate leukocyte migration to all SLO, subsets of FucT dko mice were splenectomized and ultimately treated with mAbs to MAdCAM-1 at indicated time points. Wild-type C57BL/6 and FucT dko mice undergoing sham surgery and treatment with isotype Ig antibodies served as allogeneic controls. As shown in Table 1, sham splenectomized, FucT dko syngeneic controls all survived after BMT, whereas 90% to 100% of allogeneic control mice died by day 30. Splenectomized FucT dko mice treated with anti-MAdCAM-1 antibodies experienced a significant survival advantage, but they still demonstrated evidence of clinical and target tissue GVHD. These finding are consistent with results using aly/aly mice as allo-BMT recipients and demonstrate that our observations are independent of strain specificity.
Table 1.
Survival and GVHD after allo-BMT using FucT dko recipients
| Recipients | SLO status | Day 28 survival | day 28 Clinical Score | Intestinal GVHD |
|---|---|---|---|---|
| syn: FucT dko sham + Ig | LN− / Sp+ / PP+ | 100 % | 1.5 ± 0 | 4.6 ± 0.2 |
| allo: wild-type B6 | LN+ / Sp+/ PP+ | 0 % | NA | 10.4 ± 1.2* |
| allo: FucT dko + sham + Ig | LN− / Sp+/ PP+ | 10 % | 6.5 ± 1.5 | 11.6 ± 0.9 |
| allo: FucT dko + surg+ Ig | LN−/ Sp− / PP+ | 0% | NA | 8.3 ± 1.0* |
| allo: FucT dko + surg + anti-Mad | LN− / Sp− / PP− | 50%* | 5 ± 0 | 11.6 ± 0.4* |
BMT indicates bone marrow transplantation; GVHD, graft-versus-host disease.
Lethally irradiated, splenectomized, FucT dko (LN/Sp−/−), sham-splenectomized, FucT dko and C57BL/6 (LN/PP/Sp+/+) mice received cell mixtures of 5 × 106 BM cells and 2.0 × 106 splenic T cells from allogeneic B10.BR donors. FucT dko recipients of syngeneic BMT from FucT dko donors severed as negative controls. To neutralize migration of naïve T cells to PPs following BMT, some FucT dko mice were also treated i.p. with monoclonal anti- MadCAM-1 antibodies (whereas control animals received control hamster immunoglobulin) at the dose and schedule described in the Materials and Methods section. BMT recipients were analyzed daily for survival and weekly through week 5 for the development of clinical GVHD. In addition, intestinal tissue collected at the time of death, or at the end of the evaluation period was analyzed for evidence of target tissue injury. Data were combined from 2 independent experiments (n = 9–15 per group).
P < .05 compared with syngeneic group.
Donor T cell expansion in the Liver and BM Cavity of LN/PP/Sp−/− Mice 7 Days after Allo-BMT
To begin to ascertain what extra-lymphoid host tissues might serve as initial sites for allo-antigen exposure, we next examined donor T cell expansion in the liver and BM cavity 7 days after BMT. As shown in Table 2, LN/PP/Sp−/− allo-BMT recipients had significantly higher numbers of donor, CD3+ T cells in the liver and BM compared to both LN/PP/Sp+/ + littermate and wild-type C57BL/6 allogeneic groups and syngeneic controls. As shown in Figure 4, enhanced trafficking of donor T cells to the liver correlated with a significant increase in hepatic GVHD at day 7 in LN/PP/Sp−/− mice compared to syngeneic and both LN/PP/Sp+/+ allogeneic controls (16.0 ± 2.2 versus 7.3 ± 0.9; P<.01).
Table 2.
Donor T cell numbers in the BM and liver 7 days after BMT
| bone marrow (×106)
|
liver cells (×106)
|
|||||
|---|---|---|---|---|---|---|
| CD3+ | H2kd+ | CD3+H2Kd+ | CD3+ | H2kd+ | CD3+H2Kd+ | |
| Syn | 0.40 ± 0.07 | N/A | N/A | 0.59 ± 0.12 | N/A | N/A |
| Allo Co | 0.47 ± 0.01 | 3.45 ± 0.52 | 0.41 ± 0.07 | 0.23 ± 0.51 | 0.99 ± 0.68 | 1.59 ± 0.39 |
| LN/PP/Sp+/+ | 1.05 ± 0.28 | 1.92 ± 0.97 | 0.41 ± 0.19 | 1.07 ± 0.31 | 0.90 ± 0.44 | 0.78 ± 0.42 |
| LN/PP/Sp−/− | 1.12 ± 0.12*,‡ | 8.85 ± 1.5†,‡ | 1.17 ± 0.18†,‡ | 3.57 ± 0.68*,†,‡ | 4.75 ± 1.10†,‡ | 3.10 ± 0.54†,‡ |
Lethally irradiated, LN/PP/Sp−/−, LN/PP/Sp+/+, and wild-type C57BL/6 recipients received BMT as in Figure 2. Seven days after BMT, single cell suspensions were prepared from the bone marrow cavity or liver tissue, and cells from individual mice were labeled and analyzed by flow cytometry. Increased numbers of donor T cells are present in the BM and liver of LN/PP/Sp−/− mice compared to allogeneic controls. Data are presented as mean ± SEM and represent 1 of 3 similar experiments.
p< 0.05 compared to syn;
p< 0.05 compared to Allo Co;
p< 0.05 compared to LN/PP/Sp +/+
Figure 4.
Early infiltration of donor T cells into the livers of LN/PP/ Sp−/− mice correlates with the severity of hepatic GVHD on day 7 after BMT. Lethally irradiated LN/PP/Sp−/− and LN/PP/Sp+/+ and wild-type C57BL/6 recipients received BMT as in Figure 2. Liver tissue was collected on day 7, and the severity of GVHD was determined as described in the Materials and Methods section. Data are presented as mean ± SEM, and were combined from 2 similar experiments. n = 6 to 10 per group. *P<.05, allo C57BL/6 and LN/PP/Sp+/+ versus syn; #P<.01, LN/PP/Sp−/− versus all groups. syn
 ; allo C57BL/6 ▨; LN/ PP/Sp+/+ ■; LN/PP/Sp−/−
; allo C57BL/6 ▨; LN/ PP/Sp+/+ ■; LN/PP/Sp−/−
 .
.
Donor T Cells Are Activated and Proliferate in the BM Cavity of Mice Lacking Secondary Lymphoid Organs Early after allo-BMT
The elevated numbers of allogeneic donor T cells in the BM cavity and liver observed on day 7 was intriguing, and suggested that either of these 2 organs might serve as an alternative site for T cell activation early after allo-BMT. We therefore examined donor T cell expansion (CD3+), activation (CD69+), and proliferation (CFDA-SE) in both anatomic sites 3 days after allo-BMT. In these studies, B10.BR mice were used as BMT donors to take advantage of more robust T cell activation observed in this model. The total number (Table 3) and percentage (Figure 5a) of donor, CD3+ cells present in the BM cavity of LN/ PP/Sp−/− allo-BMT recipients was 10-fold higher than that observed in allogeneic LN/PP/Sp+/+littermate controls. As expected, these cells expressed CFDA-SE, and flow cytometric analysis revealed that they had undergone a significant number of cell divisions (Figure 5a). By contrast very few CFDA-SE expressing donor T cells were present in the BM of LN/PP/Sp+/+ controls. Moreover, the number of donor T cells in the BM of LN/PP/Sp−/− allo- BMT recipients coexpressing the activation marker CD69 was also significantly increased (Table 3). When we specifically evaluated donor CD4+ and CD8+ T cell subsets in the BM of LN/PP/Sp−/− allo-BMT recipients, we found that in each case, donor T cells expressed activation markers and produced both IFNγ and TNFα upon restimulation (Figure 6a and b). This modest increase in cytokine production was confirmed in additional experiments showing that mRNA expression of IFNγ and TNFα was increased compared to cells collected from syngeneic controls (Figure 6c). Importantly, mRNA expression of perforin was also increased (1.43-fold compared to syngeneic), and these T cells also possessed cytolytic activity against allogeneic tumor targets in vitro (Figure 6c).
Table 3.
Donor T cell expansion and activation in the bone marrow and liver 3 days after BMT
| Bone Marrow (cells × 105)
|
Liver (cells × 105)
|
|||
|---|---|---|---|---|
| CD3+CFSE+ | CD3+CFSE+CD69+ | CD3+CFSE+ | CD3+CFSE+CD69+ | |
| Syn | 0.39 ± 0.17 | 0.26 ± 0.07 | 0.14 ± 0.15 | 0.10 ± 0.22 |
| LN/PP/Sp+/+ | 0.07 ± 0.00 | 0.04 ± 0.00 | 0.43 ± 0.08 | 0.27 ± 0.07 |
| LN/PP/Sp−/− | 0.87 ± 0.00# | 0.72 ± 0.00# | 0.75 ± 0.01* | 0.29 ± 0.08 |
BMT indicates bone marrow transplantation. T cells (10 × 106) from B10.BR donors were stained with vibrant CFDA SE and infused into lethally irradiated, LN/PP/Sp−/− or LN/PP/Sp+/+ recipients as described in Figure 5. Three days after transfer, single cell suspensions were prepared from the bone marrow cavity (A) or liver tissue (B) and cells from mice in each group were pooled (BM) or processed individually (liver) and subsequently counted, labeled, and analyzed by flow cytometry. Increased numbers of activated and dividing donor T cells are present in the BM of LN/PP/SP−/− mice compared to allogeneic, LN/PP/Sp+/+ littermate controls. Data are presented as mean ± SEM, and represent 1 of 3 similar experiments, n = 4 mice per group per experiment.
P < .05,
P =.06.
Figure 5.
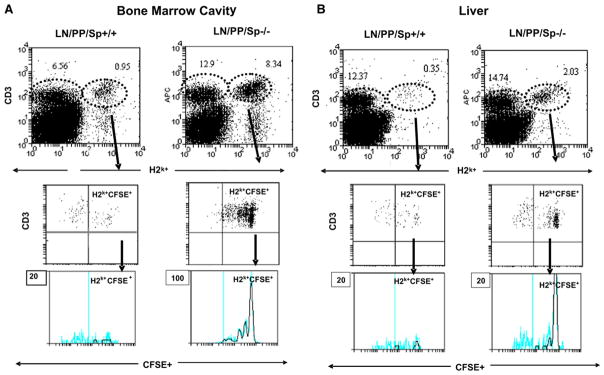
Division of donor T cells in bone marrow cavity and liver 3 days following BMT. T cells (10 × 106) from B10.BR donors were stained with Vibrant CFDA SE and infused into lethally irradiated, LN/PP/Sp−/− or LN/PP/Sp+/+ recipients as described in the Materials and Methods section. Three days after transfer, single cell suspensions were prepared from the bone marrow cavity (A) or liver tissue (B), and cells were pooled, labeled, and analyzed by flow cytometry. Data are presented as percent of total cells collected from each tissue and are shown from 1 of 3 similar experiments.
Figure 6.
Donor CD4+ and CD8+ T cells present in the bone marrow of LN/PP/Sp−/− BMT recipients are activated, produce cytokines, and are capable of killing allogeneic targets in vitro. T cells (10 × 106) from B10.BR donors were infused into lethally irradiated, LN/PP/Sp−/− recipients as described in the Materials and Methods section. Three to 5 days after transfer, single cell suspensions were prepared from the bone marrow cavity and cells were pooled, labeled, and analyzed by flow cytometry for cell surface (A), intracytoplasmic cytokine production (B), mRNA expression (C) and cytolytic function against allogeneic (solid square) or syngeneic (solid circle) targets (D). Data shown are representative from 1 of 3 (A, B, D) or 2 (C) similar experiments (n = 3 to 4 animals per experiment).
Although a greater number of donor T cells were also found in the liver of LN/PP/Sp−/− mice on day 3, these cells were not significantly activated or dividing (Figure 5b and Table 3). Of note, we found that the absolute numbers of BM cells from naïve aly/aly, aly/+ and C57BL/6 mice are comparable suggesting that the increased numbers of donor T cells present in LN/PP/Sp−/− allo-BMT recipients was not because of an intrinsic alteration in cellular migration to the BM cavity in these mice (data not shown).
EVALUATION OF GVL EFFECTS IN LN/PP/ Sp−/− MICE FOLLOWING ALLO-BMT
We next determined whether the activation of donor T cells observed in the in the BM cavity of LN/PP/ Sp−/− mice early after BMT and prior to the development of clinical and target organ GVHD was also associated with preservation of GVL effects. In these experiments, irradiated LN/PP/Sp−/− and LN/PP/ Sp+/+ mice received allo-BMT from B10.BR donors as in Figure 3, and LN/PP/Sp−/− recipients of syngeneic BMT served as controls. In separate experiments, increasing numbers (1000, 2000, and 5000) of host-type, (H-2b) MBL-2 tumor cells were added to the BM inoculum in some allogeneic and syngeneic groups. BMT recipients were subsequently monitored for survival, and cause of death was recorded as GVHD or leukemia as described in the Materials and Methods section. Recipients of syngeneic BMT that did not receive tumor cells all survived, whereas all LN/PP/ Sp+/+ recipients of allogeneic BMT died of GVHD prior to day 20 regardless of whether MBL-2 cells were added at day 0 (data not shown). As expected, syngeneic BMT recipients uniformly died of disseminated MBL-2 tumor cell infiltration regardless of tumor dose. Although the majority of LN/PP/Sp−/− allo- BMT recipients effectively rejected their tumor and survived at the lowest tumor dose, this effect was lost when we increased the tumor burden at the time of BMT by 2- and 5-fold in subsequent experiments (Figure 7a–c).
Figure 7.
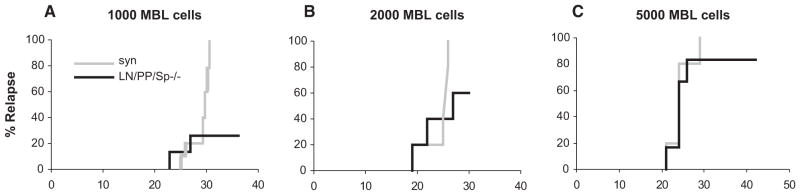
Evaluation of GVL responses in LN/PP/Sp−/− following allo-BMT. Lethally irradiated, LN/PP/Sp−/− and LN/PP/Sp+/+ and wild-type C57BL/6 recipients received BMT from syngeneic or allogeneic, B10.BR donors as in Figure 3. On day 0, either 1000 (A) or 2000 (B) or 5000 (C) MBL-2 cells were added to the donor stem cell inoculum. Survival was monitored daily and the cause of each death after BMT was determined by postmortem examination and ascribed to either GVHD or leukemia as described in the Materials and Methods section. In each case, data are combined from 2 different experiments (n = 8 to 16 per group).
DISCUSSION
GVHD remains the major complication of allo- BMT. Unfortunately, GVHD is tightly linked, both clinically and biologically, to potent and desirable GVL effects. GVHD and GVL responses are dependent upon the activation of donor T cells by APCs [30,31], but the precise location of these interactions remains uncertain. Our results demonstrate that SLO contribute to, but are not required for the development of GVHD using MHC disparate mouse strains following MA conditioning. In this context, we identified the BM as a possible alternative, nonlymphoid organ where T cell activation can occur early after BMT. However, our data show that the allo-stimulatory capacity of the BM cavity is less efficient than that of traditional SLOs; GVHD, although clearly present, is less severe, and associated GVL responses, although present, could be overcome when the tumor burden was increased.
Several groups have studied the role of various SLOs in the generation of allo-immune responses following BMT, and published data on this topic are conflicting. Murai and colleagues [1] were the first to demonstrate that a role for PPs in the development of GVHD; disrupting the recruitment of donor T cells into PP using several strategies effectively prevented GVHD induction following NMA conditioning. This model is characterized by the adoptive transfer of donor T cells (without BM rescue) and the ultimate reliance on a “graft-versus-host hematopoietic cell” response, wherein allo-BMT recipients die of BM aplasia. By contrast, subsequent data by Welniak et al. [3] showed that PPs are not required for GVHD following MA conditioning. These differences might be explained in part by the effects that dose intense regimens have on the host environment, because the proinflammatory milieu created by MA conditioning is known to have a significant impact on the trafficking of alloreactive T cells into target tissues [3,32,33].
PPs have also been found to contribute to the homing and priming of T cell effectors responsible for intestinal aGVHD in some models [2,34]. In the early phase of allo-BMT, circulating naïve donor T cells rapidly enter into the host SLO by using homing molecules such as L-selectin, α4β7, MAdCAM-1, and the chemokine receptor CCR7. In particular, α4β7 has been shown to be an important intestinal homing receptor for both the naïve and effector/memory T cells. During GVHD, donor T cells gradually upregulate the adhesion molecule α4β7 as they differentiate into effector cells [26], and subsequently interact with MAdCAM-1-bearing high endothelial venules in PPs patches, mesenteric LNs, and lamina propria venules in the intestine [1,26,34]. Preventive measures targeting these molecules inhibit the development of antihost CTL and ameliorate GVHD [1,26,34,35].
Experiments using in vivo fluorescein imaging to track cell migration have also supported a role for SLO in the activation of donor T cells following allogeneic BMT and have revealed increased numbers of T cells in the spleen and peripheral LN, such as PP and mesenteric LN (mLNs) at early time points after BMT and prior to infiltration into the small and large bowel [2]. These findings were recently expanded upon by work showing that strategies blocking T cell trafficking to all SLO completely abrogates bioluminescent signals and cellular infiltration into the intestinal track early after BMT and results in long-term survival [36]. Our results confirm and extend these findings; LN/PP/Sp−/− aly/aly allo-BMT recipients all survive, but closer examination of surviving mice at later time points after BMT clearly show that they ultimately develop significant clinical and histopathologic evidence for GVHD, similar to finding reported by Anderson and colleagues [37]. When compared to LN/PP/Sp+/+ controls, hepatic GVHD was more robust in LN/PP/Sp−/− allo-BMT recipients, whereas GI GVHD was less severe. This observation supports a particularly key role for PPs and mesenteric lymph nodes in the recruitment of donor cells and the development of allo-immune inflammation in intestinal track as suggested by others [2,34].
Results included herein also suggest that the bone marrow space may serve as an alternative, albeit less efficient site of donor T cell activation following MA conditioning. The BM cavity displays structural and functional features of a SLO; it contains follicle-like structures similar to LNs or spleen, can initiate primary T cell responses, and represents a major part of the lymphocyte recirculation network [38,39]. The frequency of lymphoid follicles in BM can increase during infection, inflammation, and autoimmunity [38,40]. In this context, the BM seems to be unique among lymphoid organs in performing primary and secondary immune functions, hemato and lymphopoiesis, and systemic immune control of blood-borne antigen. We found that donor T cell (CD3+) expansion, activation (CD69+) and proliferation (CFDA-SE) was significantly higher in the BM of aly/aly LN/PP/Sp−/− recipients 3 days after BMT compared to allogeneic LN/PP/Sp+/+ littermate and wild-type C57BL/6 controls. Following activation, increased numbers of donor T cells were observed in the liver by day 7 and correlated with significant hepatic GVHD at that time. Our findings are in accord with recent studies showing that antigen presentation to T cells could be mediated by DCs in the BM space of aly/aly (Sp+) mice.
Recently, BM-resident DCs have been shown to be critical for maintenance of recirculating B cells and capable of T cell activation [38,41] Following the adoptive transfer of CD4+ and CD8+ T cells, the formation of multicellular clusters of T cells and BM-resident DCs was associated with the subsequent activation and proliferation of the transferred T lymphocytes. T cell activation was not tolerogenic, and resulted in generation of CTL activity in situ within the BM, protective antitumor immunity, and immunologic memory [38]. Moreover, work by Beilhack and colleagues [36] showed that bioluminescent signals in splenectomized mice receiving antibodies to CD62L and MAdCAM-1 projected predominantly to the BM compartments in mice receiving allo-BMT.
In our final set of experiments, we determined whether the graft-versus-host activity that resulted in significant systemic and target tissue GVHD was robust enough to generate protective GVL effects. Although LN/PP/Sp−/− allogeneic BMT recipients were capable of generating CTL activity to allogeneic tumor targets in vitro, GVL responses in vivo were overcome when the tumor burden was increased, albeit to a level 4-fold less than that effectively rejected in other BMT strain combinations [22]. These findings diverge from those of reported by Feuerer and colleagues [39]. One explanation for these differences is that aly/aly mice were splenectomized and lethally irradiated prior to the infusion of tumor cells in our experiments, whereas nonirradiated mice with a full complement of immune cells were used in previous studies. Indeed, we found that nonirradiated, naïve aly/aly mice were able to reject and thereby survive an allogeneic tumor challenge (data not shown). It is also possible that the generation of significant CTL responses is slower in the BM, and as such, challenging mice at later time points after BMT could reveal the presence of more robust antitumor activity. Results more consistent with ours were observed in a report examining immunologic responses to lymphocytic choriomeningitis virus (LCMV) in unirradiated aly/ aly (Sp+). In these studies, CTL induction was found to be present early after infection, but LCMV-specific CTL were rapidly exhausted and remained suboptimal even when aly/aly mice were reconstituted with wildtype BM [10,42].
Although the BM may function as an alternative, nonlymphoid site for T cell activation and the development of GVHD in animals devoid of traditional SLOs, other explanations for this surprising finding were considered. Prior to BMT experiments, we found that the ability of splenic and BM-derived DCs, from aly/aly mice to stimulate allogeneic T cell proliferation, IFNγ secretion, and CTL generation was comparable to naïve, aly/+ littermate, and wild-type C57BL/6 mice, thus ruling out enhanced allo-stimulatory capacity as a cause for persistent GVHD in splenectomized, aly/alyBMT recipients. Work done by Matsumoto and colleagues [43] found that defective lymphoid organ development in aly/aly mice is partially restored by the generation of chimeras with normal donors early in development, suggesting that agenesis of LN and PP in aly/aly mice is primarily dependent upon stromal, rather than hematopoietic elements. This is in contrast to that observed in LTα−/− mice, where similar chimera experiments resulted in complete restoration of SLO architecture in LTα−/− mice. Although partial restoration of PPs was noted in some recipients of syngeneic BMT, similar observations were not observed in allo-BMT recipients (data not shown). Similarly, it is possible that the presence of other nontraditional, SLO-like nasal-associated lymphoid tissue could contribute to Tcell activation, but these sites were not fully evaluated in this study. Finally, although we and others have found that normal DC function, particularly with respect to stimulating allo-antigen responses, is preserved in aly/aly mice [5,6,9,10], recent data have demonstrated that the capacity for DCs to expand CD25+ CD4+ regulatory T cells in vitro, is impaired, and contributes to decreased numbers of regulatory T cells in the periphery of aly/aly mice [44]. It is therefore possible that this effect contributes, at least in part, to the persistence of significant, albeit attenuated GVH responses seen in splenectomized aly/aly allo-BMT recipients.
Collectively, our data generated using mice anatomically or functionally devoid of SLOs challenge the paradigm that these structures are required for the induction of GVH responses. Because our studies were conducted with fully MHC disparate mouse strains, our results do not discount that blocking entrance into SLO could be a clinically viable option to reduce GVHD in the context of less severe MHC disparity. Rather, we believe these findings enhance our understanding of where T cell-APC interactions occur during the development of GVHD, and anticipate that insights derived from this work will ultimately improve current clinical strategies to optimize the impact graft-versus- host responses have on our patients receiving allo-BMT.
Footnotes
Authorship Statement
I.A.S. and K.R.C. participated in designing and writing the manuscript; I.A.S., K.O., D.A., and E.P. performed the research; S.G.C. contributed with developing splenectomy and sham surgeries; I.A.S., K.O., M.N.C., D.D., J.M.F., K.V., and S.W.C. collected the data; I.A.S. and K.R.C. analyzed the data and C.L. analyzed the pathology data; I.A.S. and K.R.C. wrote the article; and all authors checked the final version of the manuscript.
Financial disclosure: Dr. Cooke is an Amy Strelzer- Manasevit Scholar of the National Marrow Program, a Clinical Scholar of the Leukemia and Lymphoma Society, and the recipient of a Clinical Scientist in Translational Research Award from the Burroughs Well come Fund. This work was supported in part by the Walther Cancer Institute.
References
- 1.Murai M, Yoneyama H, Ezaki T, et al. Peyer’s patch is the essential site in initiating murine acute and lethal graft-versus-host reaction. Nat Immunol. 2003;4:154–160. doi: 10.1038/ni879. [DOI] [PubMed] [Google Scholar]
- 2.Beilhack A, Schulz S, Baker J, et al. In vivo analyses of early events in acute graft-versus-host disease reveal sequential infiltration of T-cell subsets. Blood. 2005;106:1113–1122. doi: 10.1182/blood-2005-02-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Welniak LA, Kuprash DV, Tumanov AV, et al. Peyer’s patches are not required for acute graft-versus-host disease after myeloablative conditioning and murine allogeneic bone marrow transplantation. Blood. 2006;107:410–412. doi: 10.1182/blood-2004-11-4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miyawaki S, Nakamura Y, Suzuka H, et al. A new mutation, aly, that induces a generalized lack of lymph nodes accompanied by immunodeficiency in mice. Eur J Immunol. 1994;24:429–434. doi: 10.1002/eji.1830240224. [DOI] [PubMed] [Google Scholar]
- 5.Yamada T, Mitani T, Yorita K, et al. Abnormal immune function of hemopoietic cells from alymphoplasia (aly) mice, a natural strain with mutant NF-kappa B-inducing kinase. J Immunol. 2000;165:804–812. doi: 10.4049/jimmunol.165.2.804. [DOI] [PubMed] [Google Scholar]
- 6.Shinkura R, Kitada K, Matsuda F, et al. Alymphoplasia is caused by a point mutation in the mouse gene encoding Nf-kappa b-inducing kinase. Nat Genet. 1999;22:74–77. doi: 10.1038/8780. [DOI] [PubMed] [Google Scholar]
- 7.Nonaka S, Naito T, Chen H, et al. Intestinal gamma delta T cells develop in mice lacking thymus, all lymph nodes, Peyer’s patches, and isolated lymphoid follicles. J Immunol. 2005;174:1906–1912. doi: 10.4049/jimmunol.174.4.1906. [DOI] [PubMed] [Google Scholar]
- 8.Nanno M, Matsumoto S, Koike R, et al. Development of intestinal intraepithelial T lymphocytes is independent of Peyer’s patches and lymph nodes in aly mutant mice. J Immunol. 1994;153:2014–2020. [PubMed] [Google Scholar]
- 9.Shinkura R, Matsuda F, Sakiyama T, et al. Defects of somatic hypermutation and class switching in alymphoplasia (aly) mutant mice. Int Immunol. 1996;8:1067–1075. doi: 10.1093/intimm/8.7.1067. [DOI] [PubMed] [Google Scholar]
- 10.Karrer U, Althage A, Odermatt B, Hengartner H, Zinkernagel RM. Immunodeficiency of alymphoplasia mice (aly/aly) in vivo: structural defect of secondary lymphoid organs and functional B cell defect. Eur J Immunol. 2000;30:2799–2807. doi: 10.1002/1521-4141(200010)30:10<2799::AID-IMMU2799>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 11.Maly P, Thall A, Petryniak B, et al. The alpha(1,3)fucosyltransferase Fuc-TVII controls leukocyte trafficking through an essential role in L-, E-, and P-selectin ligand biosynthesis. Cell. 1996;86:643–653. doi: 10.1016/s0092-8674(00)80137-3. [DOI] [PubMed] [Google Scholar]
- 12.Homeister JW, Thall AD, Petryniak B, et al. The alpha(1,3)fucosyltransferases FucT-IV and FucT-VII exert collaborative control over selectin-dependent leukocyte recruitment and lymphocyte homing. Immunity. 2001;15:115–126. doi: 10.1016/s1074-7613(01)00166-2. [DOI] [PubMed] [Google Scholar]
- 13.Smithson G, Rogers CE, Smith PL, et al. Fuc-TVII is required for T helper 1 and T cytotoxic 1 lymphocyte selectin ligand expression and recruitment in inflammation, and together with Fuc-TIV regulates naive T cell trafficking to lymph nodes. J Exp Med. 2001;194:601–614. doi: 10.1084/jem.194.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooke KR, Hill GR, Crawford JM, et al. Tumor necrosis factoralpha production to lipopolysaccharide stimulation by donor cells predicts the severity of experimental acute graft-versus-host disease. J Clin Invest. 1998;102:1882–1891. doi: 10.1172/JCI4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooke KR, Kobzik L, Martin TR, et al. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation. I. The roles of minor H antigens and endotoxin. Blood. 1996;88:3230–3239. [PubMed] [Google Scholar]
- 16.Whitaker-Menezes D, Jones SC, Friedman TM, Korngold R, Murphy GF. An epithelial target site in experimental graftversus- host disease and cytokine-mediated cytotoxicity is defined by cytokeratin 15 expression. Biol Blood Marrow Transplant. 2003;9:559–570. doi: 10.1016/s1083-8791(03)00288-x. [DOI] [PubMed] [Google Scholar]
- 17.Cooke KR, Hill GR, Gerbitz A, et al. Hyporesponsiveness of donor cells to lipopolysaccharide stimulation reduces the severity of experimental idiopathic pneumonia syndrome: potential role for a gut-lung axis of inflammation. J Immunol. 2000;165:6612–6619. doi: 10.4049/jimmunol.165.11.6612. [DOI] [PubMed] [Google Scholar]
- 18.Cooke KR, Gerbitz A, Crawford JM, et al. LPS antagonism reduces graft-versus-host disease and preserves graft-versus-leukemia activity after experimental bone marrow transplantation. J Clin Invest. 2001;107:1581–1589. doi: 10.1172/JCI12156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teshima T, Hill GR, Pan L, et al. IL-11 separates graft-versus-leukemia effects from graft-versus-host disease after bone marrow transplantation. J Clin Invest. 1999;104:317–325. doi: 10.1172/JCI7111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matzinger P. The JAM test. A simple assay for DNA fragmentation and cell death. J Immunol Methods. 1991;145:185–192. doi: 10.1016/0022-1759(91)90325-a. [DOI] [PubMed] [Google Scholar]
- 21.Gatza E, Okada CY. Tumor cell lysate-pulsed dendritic cells are more effective than TCR Id protein vaccines for active immunotherapy of T cell lymphoma. J Immunol. 2002;169:5227–5235. doi: 10.4049/jimmunol.169.9.5227. [DOI] [PubMed] [Google Scholar]
- 22.Reddy P, Maeda Y, Liu C, Krijanovski OI, Korngold R, Ferrara JL. A crucial role for antigen-presenting cells and alloantigen expression in graft-versus-leukemia responses. Nat Med. 2005;11:1244–1249. doi: 10.1038/nm1309. [DOI] [PubMed] [Google Scholar]
- 23.Nagamatsu H, Tsuzuki Y, Matsuzaki K, et al. Regulation of T-lymphocyte trafficking by ICAM-1, MAdCAM-1, and CCR7 in microcirculation of appendicular and intestinal lymphoid tissues. Microcirculation. 2004;11:493–502. doi: 10.1080/10739680490476079. [DOI] [PubMed] [Google Scholar]
- 24.Teramoto K, Miura S, Tsuzuki Y, et al. Increased lymphocyte trafficking to colonic microvessels is dependent on MAdCAM-1 and C-C chemokine mLARC/CCL20 in DSS-induced mice colitis. Clin Exp Immunol. 2005;139:421–428. doi: 10.1111/j.1365-2249.2004.02716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watanabe C, Miura S, Hokari R, et al. Spatial heterogeneity of TNF-alpha-induced T cell migration to colonic mucosa is mediated by MAdCAM-1 and VCAM-1. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1379–G1387. doi: 10.1152/ajpgi.00026.2002. [DOI] [PubMed] [Google Scholar]
- 26.Ueha S, Murai M, Yoneyama H, et al. Intervention of MAdCAM-1 or fractalkine alleviates graft-versus-host reaction associated intestinal injury while preserving graft-versus-tumor effects. J Leukoc Biol. 2007;81:176–185. doi: 10.1189/jlb.0306231. [DOI] [PubMed] [Google Scholar]
- 27.Matsuzaki K, Tsuzuki Y, Matsunaga H, et al. In vivo demonstration of T lymphocyte migration and amelioration of ileitis in intestinal mucosa of SAMP1/Yit mice by the inhibition of MAdCAM-1. Clin Exp Immunol. 2005;140:22–31. doi: 10.1111/j.1365-2249.2005.02742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giver CR, Montes RO, Mittelstaedt S, et al. Ex vivo fludarabine exposure inhibits graft-versus-host activity of allogeneic T cells while preserving graft-versus-leukemia effects. Biol Blood Marrow Transplant. 2003;9:616–632. doi: 10.1016/s1083-8791(03)00229-5. [DOI] [PubMed] [Google Scholar]
- 29.Ziegler TR, Panoskaltsus-Mortari A, Gu LH, et al. Regulation of glutathione redox status in lung and liver by conditioning regimens and keratinocyte growth factor in murine allogeneic bone marrow transplantation. Transplantation. 2001;72:1354–1362. doi: 10.1097/00007890-200110270-00004. [DOI] [PubMed] [Google Scholar]
- 30.Tewari A, Buhles WC, Starnes HF. Preliminary report: Effects of interleukin-1 on platelet counts. Lancet. 1990;336:712. doi: 10.1016/0140-6736(90)92206-w. [DOI] [PubMed] [Google Scholar]
- 31.Teshima T, Ordemann R, Reddy P, et al. Acute graft-versus-host disease does not require alloantigen expression on host epithelium. Nat Med. 2002;8:575–581. doi: 10.1038/nm0602-575. [DOI] [PubMed] [Google Scholar]
- 32.Welniak LA, Wang Z, Sun K, et al. An absence of CCR5 on donor cells results in acceleration of acute graft-vs-host disease. Exp Hematol. 2004;32:318–324. doi: 10.1016/j.exphem.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 33.Wysocki CA, Burkett SB, Panoskaltsis-Mortari A, et al. Differential roles for CCR5 expression on donor T cells during graftversus- host disease based on pretransplant conditioning. J Immunol. 2004;173:845–854. doi: 10.4049/jimmunol.173.2.845. [DOI] [PubMed] [Google Scholar]
- 34.Petrovic A, Alpdogan O, Willis LM, et al. LPAM (alpha 4 beta 7 integrin) is an important homing integrin on alloreactive T cells in the development of intestinal graft-versus-host disease. Blood. 2004;103:1542–1547. doi: 10.1182/blood-2003-03-0957. [DOI] [PubMed] [Google Scholar]
- 35.Li B, New JY, Yap EH, Lu J, Chan SH, Hu H. Blocking L-selectin and alpha4-integrin changes donor cell homing pattern and ameliorates murine acute graft versus host disease. Eur J Immunol. 2001;31:617–624. [PubMed] [Google Scholar]
- 36.Beilhack A, Schulz S, Baker J, et al. Prevention of acute graft-versus- host disease by blocking T-cell entry to secondary lymphoid organs. Blood. 2008;111:2919–2928. doi: 10.1182/blood-2007-09-112789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anderson BE, Taylor PA, McNiff JM, et al. Effects of donor T-cell trafficking and priming site on graft-versus-host disease induction by naive and memory phenotype CD4 T cells. Blood. 2008;111:5242–5251. doi: 10.1182/blood-2007-09-107953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feuerer M, Beckhove P, Garbi N, et al. Bone marrow as a priming site for T-cell responses to blood-borne antigen. Nat Med. 2003;9:1151–1157. doi: 10.1038/nm914. [DOI] [PubMed] [Google Scholar]
- 39.Muller M, Gounari F, Prifti S, Hacker HJ, Schirrmacher V, Khazaie K. EblacZ tumor dormancy in bone marrow and lymph nodes: active control of proliferating tumor cells by CD8+ immune T cells. Cancer Res. 1998;58:5439–5446. [PubMed] [Google Scholar]
- 40.Schirrmacher V, Feuerer M, Fournier P, Ahlert T, Umansky V, Beckhove P. T-cell priming in bone marrow: the potential for long-lasting protective anti-tumor immunity. Trends Mol Med. 2003;9:526–534. doi: 10.1016/j.molmed.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 41.Sapoznikov A, Pewzner-Jung Y, Kalchenko V, Krauthgamer R, Shachar I, Jung S. Perivascular clusters of dendritic cells provide critical survival signals to B cells in bone marrow niches. Nat Immunol. 2008;9:388–395. doi: 10.1038/ni1571. [DOI] [PubMed] [Google Scholar]
- 42.Karrer U, Althage A, Odermatt B, et al. On the key role of secondary lymphoid organs in antiviral immune responses studied in alymphoplastic (aly/aly) and spleenless (Hox11(−)/−) mutant mice. J Exp Med. 1997;185:2157–2170. doi: 10.1084/jem.185.12.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsumoto M, Iwamasa K, Rennert PD, et al. Involvement of distinct cellular compartments in the abnormal lymphoid organogenesis in lymphotoxin-alpha-deficient mice and alymphoplasia (aly) mice defined by the chimeric analysis. J Immunol. 1999;163:1584–1591. [PubMed] [Google Scholar]
- 44.Tamura C, Nakazawa M, Kasahara M, et al. Impaired function of dendritic cells in alymphoplasia (aly/aly) mice for expansion of CD25+ regulatory T cells. Autoimmunity. 2006:39. doi: 10.1080/08916930600833390. [DOI] [PubMed] [Google Scholar]