Abstract
Objectives
Unhealthy lifestyle factors may contribute to apparent treatment resistant hypertension (aTRH). We examined associations of unhealthy lifestyle factors with aTRH in individuals taking antihypertensive medications from three or more classes.
Methods
Participants (n=2,602) taking three or more antihypertensive medication classes were identified from the population-based REasons for Geographic And Racial Differences in Stroke (REGARDS) study. aTRH was defined as having systolic/diastolic blood pressure ≥140/90 mmHg despite the use of three or more antihypertensive medication classes or the use of four or more classes to achieve blood pressure control. Lifestyle factors included obesity, physical inactivity, current smoking, heavy alcohol consumption, a low DASH diet score and high sodium-to-potassium (Na/K) intake.
Results
Among participants taking three or more antihypertensive medication classes, 1,293 (49.7%) participants had aTRH. The prevalence of unhealthy lifestyle factors in participants with and without aTRH was 55.2% and 51.7% respectively for obesity, 42.2% and 40.5% for physical inactivity, 11.3% and 11.5% for current smoking, 3.1% and 4.0% for heavy alcohol consumption, 23.1% and 21.5% for low DASH diet score, and 25.4% and 24.4% for high Na/K intake. After adjustment for age, sex, race, and geographic region of residence, none of the unhealthy lifestyle factors was associated with aTRH. The associations between each unhealthy lifestyle factor and aTRH remained non-significant after additional adjustment for education, income, depressive symptoms, total calorie intake, and co-morbidities.
Conclusions
Unhealthy lifestyle factors did not have independent associations with aTRH among individuals taking three or more antihypertensive medication classes.
Keywords: Hypertension, blood pressure, antihypertensive agents, epidemiology
Introduction
A large proportion of hypertensive patients require antihypertensive medications from three or more classes to control their blood pressure [1–3]. Cushman et al. [1] reported that approximately one third of hypertensive participants enrolled in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) were taking three or more antihypertensive medications after 5 years of follow-up. Using data from the National Health and Nutrition Examination Surveys (NHANES), Egan et al. [2] demonstrated that the percentage of US adults taking multiple antihypertensive medications has steadily increased from 1988–1994 through 2005–2008. In 2005–2008, 20% of US adults with hypertension were taking antihypertensive medications from three or more classes [2]. Recently, using data from the Kaiser Permanente Colorado and Northern California healthcare systems, Daugherty et al. [3] reported that approximately 21% of patients who were newly diagnosed with and treated for hypertension were taking antihypertensive medications from three or more classes within three years.
Apparent treatment resistant hypertension (aTRH) is defined as blood pressure that remains above goal despite the use of antihypertensive medications from three or more classes, or the use of four or more antihypertensive medication classes to achieve blood pressure control [4–7]. It has been suggested that unhealthy lifestyle factors such as obesity, physical inactivity, smoking, heavy alcohol use, and a high salt diet contribute to aTRH [7, 8]. However, this hypothesis has not been tested in population-based studies and the prevalence and contributions of these factors in aTRH remain uncertain, particularly among those taking antihypertensive medications from three or more classes [8]. If unhealthy lifestyle factors are common and independently associated with aTRH, it will highlight the need to invest more resources in testing behavioral interventions to improve blood pressure control in these individuals. This is particularly relevant, as the prevalence of aTRH is high and the efficacy of lifestyle modification has not been specifically evaluated in this population [7, 8]. We, therefore, sought to evaluate the association between unhealthy lifestyle factors and aTRH among individuals taking antihypertensive medications from three or more classes, in the REasons for Geographic And Racial Differences in Stroke (REGARDS) study.
Methods
Study Population
The REGARDS study has been described in detail previously [9]. In brief, REGARDS is a population-based cohort study of 30,239 adults ≥ 45 years of age from across the continental United States. To examine the association between unhealthy lifestyle factors and aTRH, the study population was limited to individuals who reported taking medications to lower their blood pressure, and were on three or more classes of antihypertensive medications (n=4,128). Participants missing blood pressure data (n=17) or information on lifestyle behaviors (n=1,509) were excluded. Missing data on dietary intake accounted for most of the missing information on lifestyle behaviors (n=1,385). After these exclusions, 2,602 participants were included in these analyses. The REGARDS study protocol was approved by the Institutional Review Boards governing research in human subjects at the participating centers and all participants provided informed consent.
Data Collection
REGARDS study data were collected via a telephone interview, a self-administered questionnaire, and in-home examination. Trained interviewers collected information on participants’ age, education, smoking status, physical activity, alcohol consumption, symptoms of depression and self-reports of prior physician diagnosed co-morbid conditions (e.g., diabetes, hypertension, myocardial infarction and coronary revascularization procedures). Elevated symptoms of depression were defined as being present for participants with scores ≥ 4 on the 4-item Centers for Epidemiologic Studies of Depression scale [10]. Trained health professionals conducted in-home examinations which included weight, height and blood pressure measurements, an electrocardiogram (ECG), collection of blood and spot urine samples, and a review of medication pill bottles. Coronary heart disease (CHD) was defined by a self-reported history or ECG evidence of myocardial infarction or a self-reported history of a revascularization procedure. Diabetes was defined as a serum glucose ≥ 126 mg/dL for participants who had fasted ≥ 8 hours prior to their blood draw, a serum glucose ≥ 200 mg/dL for those who had not fasted, or a self-report of a prior diagnosis of diabetes (excluding pregnancy induced) with the current use of insulin or oral hypoglycemic medications. Dyslipidemia was defined as an LDL-cholesterol ≥ 160 mg/dL, total cholesterol ≥ 240 mg/dL, HDL-cholesterol < 40 mg/dL or the self-reported use of lipid-lowering medication. Using isotope-dilution mass spectrometry (IDMS)–traceable serum creatinine, estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [11]. Reduced eGFR was defined as levels < 60 ml/min/1.73 m2. Albuminuria was defined as a urinary albumin to urinary creatinine ratio ≥ 30 mg/g. Chronic kidney disease was defined by the presence of reduced eGFR and/or albuminuria [12]. Medication adherence was assessed using the 4-item Morisky Medication Adherence Scale (MMAS) [13]. Each item has a yes/no response option. One point is assigned to each “yes” response, and points are summed (totaling 0–4) with a higher score indicating worse adherence [14].
Blood Pressure Measurement, Medication Use and aTRH
During the in-home examination, blood pressure was measured two times by trained examiners using aneroid sphygmomanometers following a standardized protocol. Participants were asked to sit for three minutes with both feet on the floor prior to having their blood pressure measured; the two blood pressure measurements were averaged. Hypertension was defined as systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, and/or the self-reported use of antihypertensive medications. Also, during the in-home visit, participants were asked to provide all medications they had taken in the past two weeks, and medication names were recorded and subsequently coded into drug classes. Antihypertensive medication classes were defined using categories described in the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) [15]. Single-pill combinations were classified as individual components. Information on medication dose was not recorded. Uncontrolled hypertension was defined as a systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg. aTRH was defined as having uncontrolled hypertension despite the use of antihypertensive medications from three or more classes or the use of four or more antihypertensive medication classes to achieve blood pressure control [4–7].
Unhealthy Lifestyle Factors
Six lifestyle factors were evaluated in the current analysis: obesity, physical inactivity, cigarette smoking, heavy alcohol consumption, a low Dietary Approaches to Stop Hypertension (DASH) diet score and a high sodium-to-potassium (Na/K) intake. Height and weight were measured during the in-home study visit using calibrated equipment. Body mass index (BMI) was defined as weight in kilograms (kg) divided by height in meters (m) squared and obesity was defined as levels ≥ 30 kg/m2. Current smoking was determined by affirmative responses to the following two questions “Have you smoked at least 100 cigarettes in your lifetime?” and “Do you smoke cigarettes now, even occasionally?” Physical activity was assessed through a single question “How many times per week do you engage in intense physical activity, enough to work up a sweat?” with response options of “none”, “1 to 3 times per week” and “four or more times per week”. Participants who answered “none” were considered to be physically inactive. Men and women who reported consuming > 14 and > 7 alcoholic beverages per week, respectively, were considered to have heavy alcohol consumption. Participants were asked to complete a self-administered Block 98 Food Frequency Questionnaire (FFQ) following the in-home study visit [16–18]. The FFQ was used to estimate average dietary intake for one year prior to their in-home visit. Nutrient analysis was conducted by NutritionQuest. A DASH dietary score was created using methods similar to those described by Fung et al. [19] Using the distribution of DASH dietary scores from participants included in the current study, a low DASH diet score was defined by being in the lowest quartile (DASH scores <20). High Na/K intake was defined by being in the upper quartile (Na/K ≥ 1.08). The cut-points for the lowest quartile of the DASH diet score and highest quartile of Na/K intake were similar for men and women and, therefore, gender specific cut-points were not used. Finally, total caloric intake was derived by using algorithms based on the FFQ [20].
Statistical Analysis
The prevalence of aTRH was calculated for all REGARDS participants with hypertension and for those with hypertension taking three or more antihypertensive medication classes. The characteristics of REGARDS study participants taking three or more antihypertensive medication classes were calculated by aTRH status with the statistical significance of differences determined using chi square and t-tests, as appropriate. The prevalence of each unhealthy lifestyle factor was calculated by aTRH status with the statistical significance of differences calculated using chi-square tests. Next, using Poisson regression, we calculated the prevalence ratio for aTRH associated with each unhealthy lifestyle behavior [21, 22]. Robust standard errors were calculated using sandwich estimators. Prevalence ratios are recommended instead of odds ratios for cross sectional studies with common outcomes [21, 22]. Initial models included adjustment for age, sex, race, and geographic region of residence. Subsequent models included additional adjustment for education level (less than high school education), annual household income (<$20,000), elevated depressive symptoms, reduced eGFR, albuminuria, diabetes, dyslipidemia, history of CHD, and total caloric intake. Next, we calculated the distribution of the number of unhealthy lifestyle behaviors for participants with and without aTRH. Additionally, we calculated the prevalence ratio for aTRH associated with 1, 2, 3, and ≥4 versus no unhealthy lifestyle behaviors. Prevalence ratios were calculated with the two levels of adjustment described above. Three sensitivity analyses were conducted. First, as some investigators [7] have suggested that a diuretic be one of the antihypertensive classes to meet the definition for aTRH, we repeated the analyses excluding individuals not on a diuretic (n=385). Second, we conducted analyses defining uncontrolled hypertension as a systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg except for individuals with diabetes or chronic kidney disease in whom uncontrolled hypertension was defined as systolic blood pressure ≥ 130 mmHg or diastolic blood pressure ≥ 80 mmHg. Third, as aTRH may be partially explained by reduced medication adherence [8], we restricted the study population to participants who reported a high level of medication adherence, defined as a MMAS score of 0 (n=1,771). Data analysis was conducted using SAS version 9.3 (SAS Institute, Cary, NC).
Results
Prevalence of aTRH
Of REGARDS participants with complete data for lifestyle behaviors and systolic and diastolic blood pressure, 11,742 had hypertension. Of this group, 2,602 (22.2%) were taking three or more antihypertensive medication classes, and 1,293 had aTRH (11.0% of all participants with hypertension and 49.7% of those taking 3 or more antihypertensive medication classes). Of participants with aTRH, 589 (45.6%) had uncontrolled blood pressure despite the use of antihypertensive medications from three classes, and 704 (54.4%) were taking antihypertensive medications from four or more classes.
Participant Characteristics
Table 1 shows the characteristics of the 2,602 participants who were taking three or more antihypertensive medication classes. Those with aTRH were more likely to be black, male, have less than a high school education, reduced eGFR, albuminuria, diabetes, and a history of CHD. Those with aTRH also had a higher mean BMI (mean ± standard deviation, 31.9 ± 6.8 kg/m2 for participants with aTRH vs. 31.4 ± 6.6 kg/m2 for participants with controlled blood pressure on three medication classes). There were no significant differences in other participant characteristics.
Table 1.
Characteristics* of REGARDS study participants by aTRH status
| Controlled† on three medication classes (n=1,309) |
aTRH‡ (n=1,293) |
P value | |
|---|---|---|---|
| Age, years | 67.3 (8.6) | 67.8 (8.5) | 0.182 |
| Male, % | 548 (41.9) | 625 (48.3) | <0.001 |
| Black, % | 546 (41.7) | 669 (51.7) | <0.001 |
| Residence in stroke belt, % | 458 (35.0) | 460 (35.6) | 0.754 |
| Residence in stroke buckle, % | 283 (21.6) | 289 (22.4) | 0.652 |
| Less than high school education, % | 161 (12.3) | 195 (15.1) | 0.039 |
| Annual household income <$20,000, % | 253 (21.9) | 276 (24.3) | 0.171 |
| Elevated depressive symptoms, % | 153 (11.7) | 156 (12.1) | 0.767 |
| eGFR <60 ml/min/1.73 m2, % | 337 (25.7) | 401 (31.0) | 0.003 |
| Albuminuria, % | 239 (18.3) | 383 (29.6) | <0.001 |
| Diabetes, % | 447 (35.2) | 529 (42.2) | <0.001 |
| Dyslipidemia, % | 911 (72.1) | 937 (74.8) | 0.124 |
| History of CHD, % | 370 (28.7) | 458 (36.0) | <0.001 |
| BMI, kg/m2 | 31.4 (6.6) | 31.9 (6.8) | 0.026 |
| Total caloric intake, g/day | 1541 (1506, 1576) | 1495 (1461, 1530) | 0.057 |
Data are expressed as number (percentage) or mean value (SD) except total caloric intake which is expressed as geometric mean (95% confidence interval).
Controlled hypertension is defined as blood pressure < 140/90 mmHg.
aTRH was defined as having uncontrolled hypertension despite the use of antihypertensive medications from three or more classes or the use of four or more antihypertensive medication classes to achieve blood pressure control.
aTRH = apparent treatment resistant hypertension, BMI = body mass index, CHD = coronary heart disease, eGFR = estimated glomerular filtration rate.
Relation Between Unhealthy Lifestyle Factors and aTRH
Table 2 shows the prevalence of each unhealthy lifestyle factor (obesity, physical inactivity, current smoking, heavy alcohol consumption, low DASH diet score, and high Na/K intake) in participants with aTRH and participants whose blood pressure was controlled on three antihypertensive medication classes. Participants with aTRH were more likely to be obese, compared to participants whose blood pressure was controlled on three medication classes, however, this difference was not statistically significant (P=0.07). There was also no significant difference in the prevalence of physical inactivity (P=0.37), current smoking (P=0.89), heavy alcohol use (P=0.22), low DASH diet score (P=0.33), and high Na/K intake (P=0.56) between participants with and without aTRH. After adjustment for age, sex, race, and geographic region of residence (Table 2, Model 1), none of the unhealthy lifestyle factors was significantly associated with aTRH. The association between each unhealthy lifestyle factor and aTRH remained non-significant after further multivariable adjustment (Table 2, Model 2).
Table 2.
Prevalence ratios for aTRH* associated with individual unhealthy lifestyle factors
| Number (%) | Prevalence ratio (95% CI) | |||
|---|---|---|---|---|
| Controlled on three medication classes (n=1,309) |
aTRH (n=1,293) |
Model 1† | Model 2‡ | |
| Obesity | 677 (51.7) | 714 (55.2) | 1.08 (1.00 – 1.17) | 1.05 (0.97 – 1.15) |
| Physical inactivity | 530 (40.5) | 546 (42.2) | 1.05 (0.97 – 1.13) | 1.03 (0.95 – 1.12) |
| Current smoking | 150 (11.5) | 146 (11.3) | 0.99 (0.88 – 1.12) | 0.97 (0.86 – 1.10) |
| Heavy alcohol consumption | 52 (4.0) | 40 (3.1) | 0.89 (0.70 – 1.12) | 0.93 (0.73 – 1.18) |
| Low DASH diet score | 281 (21.5) | 298 (23.1) | 1.03 (0.94 – 1.13) | 1.03 (0.94 – 1.13) |
| High Na/K intake | 319 (24.4) | 328 (25.4) | 1.00 (0.91 – 1.09) | 1.01 (0.92 – 1.11) |
aTRH was defined as having uncontrolled hypertension despite the use of antihypertensive medications from three or more classes or the use of four or more antihypertensive medication classes to achieve blood pressure control.
Model 1 adjusted for age, sex, race, and geographic region of residence.
Model 2 adjusted for covariables in Model 1 + education level (less than high school education), annual household income (<$20,000), elevated depressive symptoms, reduced estimated glomerular filtration rate (<60 ml/min/1.73 m2), albuminuria (≥ 30 mg/g), diabetes, dyslipidemia, history of coronary heart disease, and total caloric intake.
aTRH = apparent treatment resistant hypertension, CI = confidence interval, DASH = Dietary Approaches to Stop Hypertension, Na/K = sodium-to-potassium.
Clustering of Unhealthy Lifestyle Factors and aTRH
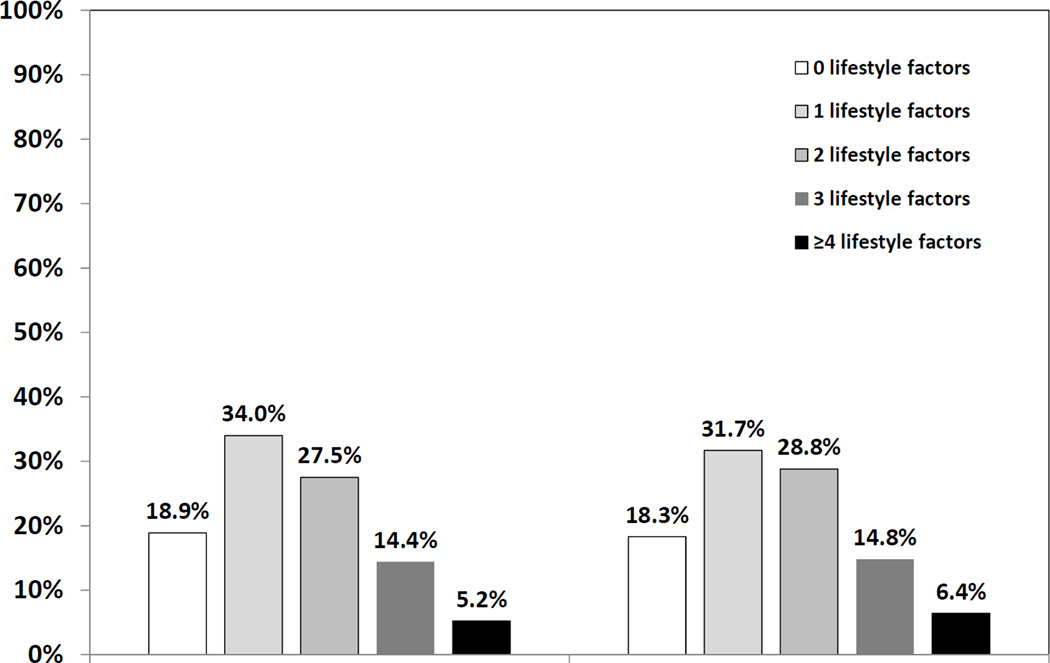
As shown in Figure 1, participants whose blood pressure was controlled on three medication classes did not differ in the number of unhealthy lifestyle factors, compared with participants with aTRH (P=0.50). After adjustment for age, sex, race, and geographic region of residence, the association between the number of lifestyle factors and aTRH was not statistically significant (Table 3, Model 1). The association between the number of lifestyle factors and aTRH did not change in a fully adjusted model (Table 3, Model 2).
Figure 1. Number of unhealthy lifestyle factors by aTRH status.
Lifestyle factors include obesity, physical inactivity, current smoking, heavy alcohol consumption, a low DASH diet score, and a high Na/K intake. Controlled hypertension was defined as blood pressure < 140/90 mmHg. aTRH was defined as having uncontrolled hypertension despite the use of antihypertensive medications from three or more classes or the use of four or more antihypertensive medication classes to achieve blood pressure control. P=0.50 comparing the distribution of the number of unhealthy lifestyle factors for participants with and without aTRH. See Table 2 for abbreviations.
Table 3.
| Prevalence ratio (95% CI) | ||
|---|---|---|
| Number of unhealthy lifestyle factors | Model 1‡ | Model 2§ |
| 0 | 1.00 (ref) | 1.00 (ref) |
| 1 | 0.97 (0.86 – 1.09) | 0.96 (0.85 – 1.08) |
| 2 | 1.03 (0.92 – 1.16) | 1.02 (0.90 – 1.15) |
| 3 | 1.02 (0.89 – 1.18) | 1.01 (0.88 – 1.17) |
| ≥ 4 | 1.11 (0.93 – 1.32) | 1.07 (0.89 – 1.28) |
aTRH was defined as having uncontrolled hypertension despite the use of antihypertensive medications from three or more classes or the use of four or more antihypertensive medication classes to achieve blood pressure control.
Lifestyle factors include obesity, physical inactivity, current smoking, heavy alcohol consumption, a low DASH diet score, and a high Na/K intake.
Model 1 adjusted for age, sex, race, and geographic region of residence.
Model 2 adjusted for covariables in Model 1 + education level (less than high school education), annual household income (<$20,000), elevated depressive symptoms, reduced estimated glomerular filtration rate (<60 ml/min/1.73 m2), albuminuria (≥ 30 mg/g), diabetes, dyslipidemia, history of coronary heart disease, and total caloric intake.
See Table 2 for abbreviations.
Sensitivity Analyses
The results were similar when we repeated the analyses restricting the sample to participants taking three or more antihypertensive medication classes with one class being a diuretic (Supplementary Table 1, Supplemental Digital Content 1). When defining uncontrolled blood pressure as systolic blood pressure ≥ 130 mmHg or diastolic blood pressure ≥ 80 mmHg for people with diabetes or chronic kidney disease, the prevalence of unhealthy lifestyle factors with aTRH was similar to those in the primary analyses (Supplementary Table 2, Supplemental Digital Content 1). In this analysis, there was a modest association between obesity and aTRH in a fully adjusted model (prevalence ratio 1.09, 95% CI 1.02 – 1.16, P=0.009). Finally, after restricting the population to participants with high medication adherence, the results were also similar to the primary analyses (Supplementary Table 3, Supplemental Digital Content 1).
Discussion
This study adds important information to the existing literature on aTRH. First, among participants taking antihypertensive medications from three or more classes, the prevalence of unhealthy lifestyle factors, particularly obesity, physical inactivity, low DASH diet score and high Na/K intake was high regardless of whether or not participants had aTRH. Second, none of the lifestyle factors was significantly associated with aTRH. There was a significant association between obesity and aTRH in an analysis that defined uncontrolled hypertension using lower cutpoints for participants with diabetes or chronic kidney disease. However, the magnitude of this association was small. Third, the clustering of unhealthy lifestyle factors was common in both aTRH and in those whose blood pressure was controlled on three antihypertensive medications; and the clustering of unhealthy lifestyle factors was not associated with aTRH.
Unhealthy lifestyle factors such as obesity, physical inactivity, smoking, heavy alcohol use, and a high salt diet have been identified as factors underlying difficult-to-control blood pressure [6, 8]. Obesity is associated with hypertension, higher blood pressure levels and a greater use of multiple antihypertensive medications [1, 23–27]. Similar associations have been reported for physical inactivity, smoking, heavy alcohol use, and a high salt diet.[28] Thus, our finding that unhealthy lifestyle factors are common among participants taking antihypertensive medications from three or more classes is consistent with this prior literature.
It is unknown why unhealthy lifestyle factors was not associated with aTRH in our study. One explanation is that the prevalence of unhealthy lifestyle factors was also relatively high in participants whose blood pressure was controlled with antihypertensive medications from three classes, making it difficult to ascertain differences in unhealthy lifestyle factors between these participants and those with aTRH. For example, in our study, the prevalence of obesity was high not only in participants with aTRH but also in participants whose blood pressure was controlled on three antihypertensive medication classes. Similarly, in NHANES [2], among participants on 3 or more antihypertensive medication classes, BMI was only modestly higher in participants with uncontrolled hypertension compared to participants with controlled hypertension in 2005–2008 (mean BMI was 32.3 kg/m2 and 31.8 kg/m2 respectively). Although obesity was higher among patients with aTRH compared to patients who had controlled hypertension while taking antihypertensive medications from 3 classes or less in a recent analysis of the Spanish Ambulatory Blood Pressure Monitoring Registry which included hypertensive clinic patients [29], the difference in BMI between the two groups was modest (mean BMI was 30.8 kg/m2 and 29.3 kg/m2 respectively).
Another explanation why unhealthy lifestyle factors did not have strong associations with aTRH is the relative contributions of other determinants of aTRH. We observed substantial differences in race, sex, and prevalence of comorbidities, particularly measures of chronic kidney disease and diabetes, between participants with aTRH and those with controlled hypertension. These factors may have played an important role in the development of aTRH, thereby diminishing the association between unhealthy lifestyle factors and aTRH. Unhealthy lifestyle factors could also have contributed indirectly to aTRH through the development of these comorbidities.
JNC 7 [15] recommends that hypertensive patients undertake lifestyle modifications such as weight reduction, increasing physical activity, moderation of alcohol consumption, adoption of the DASH diet, dietary salt reduction, and the cessation of smoking. Most of these interventions have been demonstrated to lower blood pressure in unselected patients with hypertension or in those with mild to moderate hypertension [7, 8]. In contrast, the efficacy of lifestyle modification interventions for the treatment of aTRH has been largely untested [7, 8]. Pimenta et al. [30] showed in a small crossover study of 12 participants with aTRH that a low salt diet reduced systolic and diastolic blood pressure by 22.7 and 9.1 mm Hg, respectively. Therefore, salt restriction may improve blood pressure control for aTRH participants. Future randomized controlled trials should examine whether individual or multi-faceted lifestyle interventions can facilitate blood pressure control for patients with aTRH, particularly those with comorbidities such as chronic kidney disease and diabetes.
This study has a number of important strengths. The REGARDS study is one of the largest population-based cohort studies conducted in the United States. Black and white study participants were recruited from across the continental United States, and therefore, the findings are likely highly generalizable. Blood pressure was measured by trained technicians following a standardized protocol and medications being taken were recorded through direct inspection. Finally, since available data on the contribution of unhealthy lifestyle factors to aTRH are very limited, the current study provides new data on the etiology and pathogenesis of difficult-to-control blood pressure.
The current study also has several limitations. Many participants with aTRH may have been on suboptimal medication doses [8]. However, medication dosing is not available in the REGARDS study database. In addition, blood pressure levels and control were defined by readings on a single visit. Ambulatory blood pressure monitoring was also not performed. Therefore, it is unknown how many participants had white coat and masked resistant hypertension. Finally, dietary measures (DASH diet and Na/K ratio) were obtained by the FFQ, and a limitation of dietary assessment instruments is measurement error.
In conclusion, in a bi-racial, population-based sample, unhealthy lifestyle factors did not demonstrate a strong association with aTRH among participants taking antihypertensive medications from three or more classes. The prevalence of unhealthy lifestyle factors, particularly obesity, physical inactivity, low DASH diet score and high Na/K intake were high regardless of whether participants had aTRH or not. Given the increasing prevalence of aTRH among US adults, studies are needed to assess whether lifestyle modification interventions are effective in facilitating blood pressure control in patients with aTRH.
Supplementary Material
Acknowledgements
The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org/
Funding: This study was supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis or interpretation of the data.
Footnotes
Conflicts of Interest: The authors have no financial conflicts of interest.
List of Supplemental Digital Content
Supplemental Digital Content 1 (which contains Supplementary Tables 1, 2 and 3). Microsoft Word document (doc)
References
- 1.Cushman WC, Ford CE, Cutler JA, Margolis KL, Davis BR, Grimm RH, et al. Success and predictors of blood pressure control in diverse North American settings: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT) J Clin Hypertens. 2002;4(6):393–404. doi: 10.1111/j.1524-6175.2002.02045.x. [DOI] [PubMed] [Google Scholar]
- 2.Egan BM, Zhao Y, Axon RN, Brzezinski WA, Ferdinand KC. Uncontrolled and Apparent Treatment Resistant Hypertension in the United States, 1988 to 2008. Circulation. 2011;124(9):1046–1058. doi: 10.1161/CIRCULATIONAHA.111.030189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daugherty SL, Powers JD, Magid DJ, Tavel HM, Masoudi FA, Margolis KL, et al. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation. 2012;125(13):1635–1642. doi: 10.1161/CIRCULATIONAHA.111.068064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Persell SD. Prevalence of Resistant Hypertension in the United States, 2003–2008. Hypertension. 2011;57(6):1076–1080. doi: 10.1161/HYPERTENSIONAHA.111.170308. [DOI] [PubMed] [Google Scholar]
- 5.Sarafidis PA. Epidemiology of Resistant Hypertension. J Clin Hypertens. 2011;13(7):523–528. doi: 10.1111/j.1751-7176.2011.00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarafidis PA, Bakris GL. Resistant hypertension: an overview of evaluation and treatment. J Am Coll Cardiol. 2008;52(22):1749–1757. doi: 10.1016/j.jacc.2008.08.036. [DOI] [PubMed] [Google Scholar]
- 7.Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, et al. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008;51(6):1403–1419. doi: 10.1161/HYPERTENSIONAHA.108.189141. [DOI] [PubMed] [Google Scholar]
- 8.Fagard RH. Resistant hypertension. Heart. 2012;98(3):254–261. doi: 10.1136/heartjnl-2011-300741. [DOI] [PubMed] [Google Scholar]
- 9.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, et al. The Reasons for Geographic and Racial Differences in Stroke Study: Objectives and Design. Neuroepidemiology. 2005;25(3):135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 10.Radloff LS. The CES-D Scale : A Self-Report Depression Scale for Research in the General Population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 11.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2) Suppl 1:S1–S266. [PubMed] [Google Scholar]
- 13.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Shalansky SJ, Levy AR, Ignaszewski AP. Self-reported Morisky score for identifying nonadherence with cardiovascular medications. Ann Pharmacother. 2004;38(9):1363–1368. doi: 10.1345/aph.1E071. [DOI] [PubMed] [Google Scholar]
- 15.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 16.Block G, Thompson FE, Hartman AM, Larkin FA, Guire KE. Comparison of two dietary questionnaires validated against multiple dietary records collected during a 1-year period. J Am Diet Assoc. 1992;92(6):686–693. [PubMed] [Google Scholar]
- 17.Block G, Woods M, Potosky A, Clifford C. Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol. 1990;43(12):1327–1335. doi: 10.1016/0895-4356(90)90099-b. [DOI] [PubMed] [Google Scholar]
- 18.Mares-Perlman JA, Klein BE, Klein R, Ritter LL, Fisher MR, Freudenheim JL. A diet history questionnaire ranks nutrient intakes in middle-aged and older men and women similarly to multiple food records. J Nutr. 1993;123(3):489–501. doi: 10.1093/jn/123.3.489. [DOI] [PubMed] [Google Scholar]
- 19.Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med. 2008;168(7):713–720. doi: 10.1001/archinte.168.7.713. [DOI] [PubMed] [Google Scholar]
- 20.Lin J, Judd S, Le A, Ard J, Newsome BB, Howard G, et al. Associations of dietary fat with albuminuria and kidney dysfunction. Am J Clin Nutr. 2010;92(4):897–904. doi: 10.3945/ajcn.2010.29479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barros AJ, Hirakata VN. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol. 2003;3:21. doi: 10.1186/1471-2288-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162(3):199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 23.Jordan J, Grassi G. Belly fat and resistant hypertension. J Hypertens. 2010;28(6):1131–1133. doi: 10.1097/HJH.0b013e328339b8d9. [DOI] [PubMed] [Google Scholar]
- 24.Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999;282(16):1523–1529. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- 25.Lloyd-Jones DM, Evans JC, Larson MG, O'Donnell CJ, Roccella EJ, Levy D. Differential control of systolic and diastolic blood pressure : factors associated with lack of blood pressure control in the community. Hypertension. 2000;36(4):594–599. doi: 10.1161/01.hyp.36.4.594. [DOI] [PubMed] [Google Scholar]
- 26.Wilson PW, D'Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med. 2002;162(16):1867–1872. doi: 10.1001/archinte.162.16.1867. [DOI] [PubMed] [Google Scholar]
- 27.Jordan J, Yumuk V, Schlaich M, Nilsson PM, Zahorska-Markiewicz B, Grassi G, et al. Joint statement of the European Association for the Study of Obesity and the European Society of Hypertension: obesity and difficult to treat arterial hypertension. J Hypertens. 2012;30(6):1047–1055. doi: 10.1097/HJH.0b013e3283537347. [DOI] [PubMed] [Google Scholar]
- 28.Moser M, Setaro JF. Clinical practice. Resistant or difficult-to-control hypertension. N Engl J Med. 2006;355(4):385–392. doi: 10.1056/NEJMcp041698. [DOI] [PubMed] [Google Scholar]
- 29.de la Sierra A, Banegas JR, Oliveras A, Gorostidi M, Segura J, de la Cruz JJ, et al. Clinical differences between resistant hypertensives and patients treated and controlled with three or less drugs. J Hypertens. 2012;30(6):1211–1216. doi: 10.1097/HJH.0b013e328353634e. [DOI] [PubMed] [Google Scholar]
- 30.Pimenta E, Gaddam KK, Oparil S, Aban I, Husain S, Dell'Italia LJ, Calhoun DA. Effects of Dietary Sodium Reduction on Blood Pressure in Subjects With Resistant Hypertension: Results From a Randomized Trial. Hypertension. 2009;54(3):475–481. doi: 10.1161/HYPERTENSIONAHA.109.131235. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.