Abstract
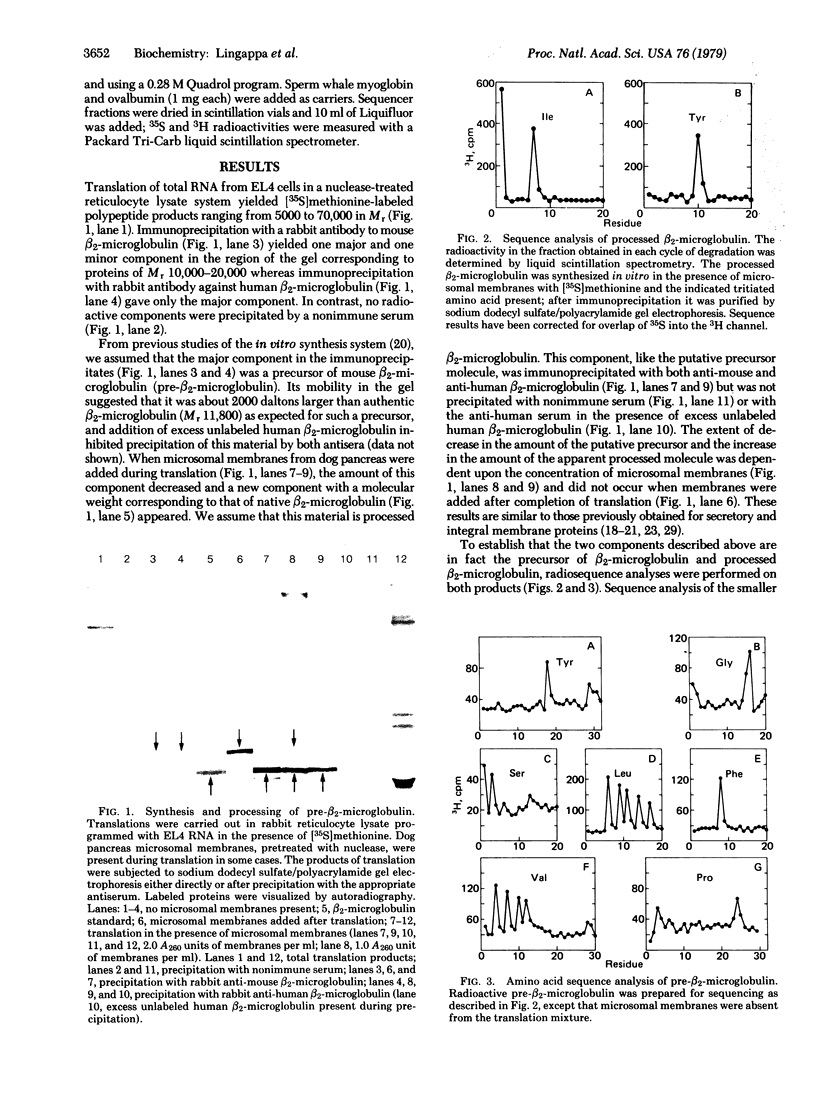
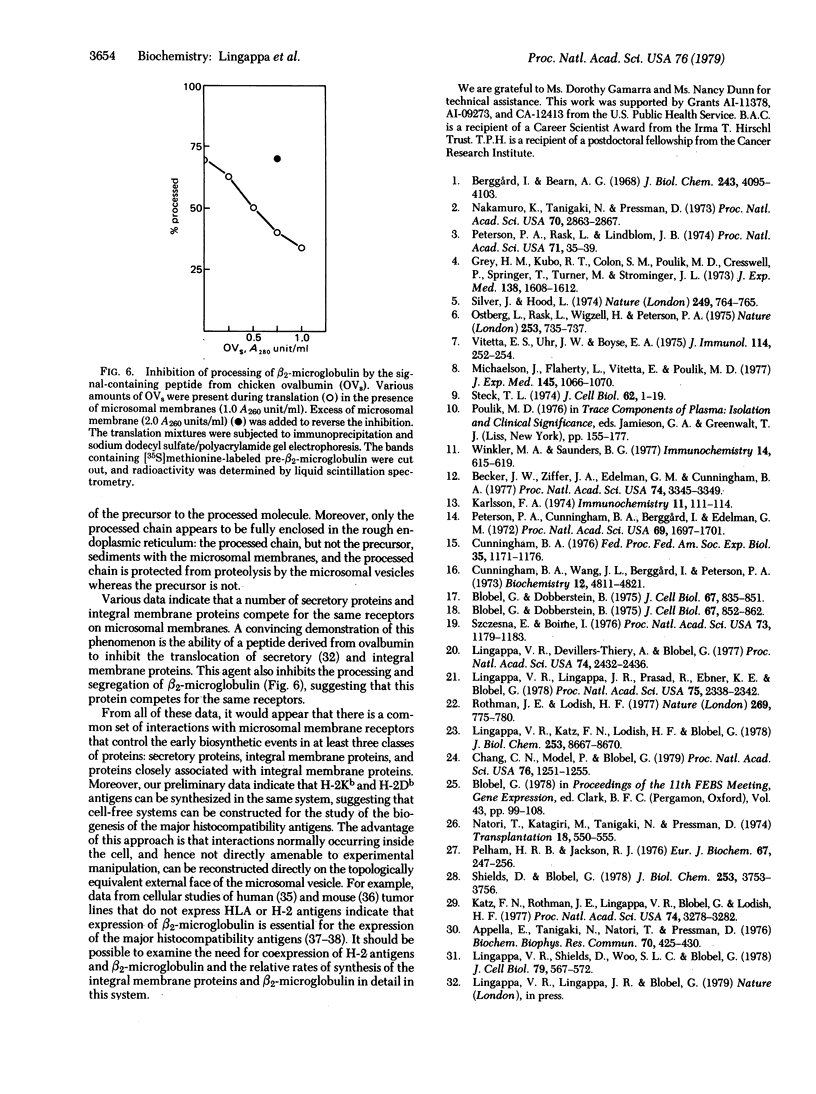
beta2-Microglobulin has been synthesized in vitro by using a rabbit reticulocyte lysate system and mRNA from the mouse tumor cell line EL4. The molecule is synthesized as a precursor with an NH2-terminal extension of 19 amino acids: Ser-X-Ser-Val-X-Leu-Val-Phe-Leu-Val-Leu-Val-Ser-Leu-X-Gly-Leu-Tyr-X. The processing and segregation of this peripheral membrane protein are directly comparable to those of secretory proteins and integral membrane proteins: addition of dog pancreas microsomal membranes during translation caused conversion to the processed chain, but addition of membranes after synthesis did not; only the processed chain sedimented with the membrane vesicles and was protected from proteolysis by the vesicles; and processing of nascent beta 2-microglobulin was blocked by competitive inhibitors that prevent processing and segregation of secretory and integral membrane proteins. These results suggest that the signal sequences of secretory proteins, integral membrane proteins, and peripheral membrane proteins have a common function and a common receptor on the cytoplasmic face of dog pancreas microsomal membranes. This system also provides a means for studying in vitro the expression and function of the major histocompatibility antigens that are associated with beta 2-microglobulin on cell surfaces.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appella E., Tanigaki N., Natori T., Pressman D. Partial amino acid sequence of mouse beta2-microglobulin. Biochem Biophys Res Commun. 1976 May 17;70(2):425–430. doi: 10.1016/0006-291x(76)91063-9. [DOI] [PubMed] [Google Scholar]
- Becker J. W., Ziffer J. A., Edelman G. M., Cunningham B. A. Crystallographic studies of bovine beta2-microglobulin. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3345–3349. doi: 10.1073/pnas.74.8.3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berggård I., Bearn A. G. Isolation and properties of a low molecular weight beta-2-globulin occurring in human biological fluids. J Biol Chem. 1968 Aug 10;243(15):4095–4103. [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J Cell Biol. 1975 Dec;67(3):852–862. doi: 10.1083/jcb.67.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. N., Model P., Blobel G. Membrane biogenesis: cotranslational integration of the bacteriophage f1 coat protein into an Escherichia coli membrane fraction. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1251–1255. doi: 10.1073/pnas.76.3.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham B. A. Structure and significance of beta2-microglobulin. Fed Proc. 1976 Apr;35(5):1171–1176. [PubMed] [Google Scholar]
- Cunningham B. A., Wang J. L., Berggård I., Peterson P. A. The complete amino acid sequence of beta 2-microglobulin. Biochemistry. 1973 Nov 20;12(24):4811–4822. doi: 10.1021/bi00748a001. [DOI] [PubMed] [Google Scholar]
- Fellous M., Hors M. C., Rebourcet R., Finaz C., Weil D., Heuertz S., Wiels J., Mahouy G., Fridman W. H. The expression and relation of HLA, beta2-microglobulin and receptor for marmoset red blood cells on man/mouse and man/Chinese hamster hybrid cells. Eur J Immunol. 1977 Jan;7(1):22–26. doi: 10.1002/eji.1830070106. [DOI] [PubMed] [Google Scholar]
- Goodfellow P. N., Jones E. A., Van Heyningen V., Solomon E., Bobrow M., Miggiano V., Bodmer W. F. The beta2-microglobulin gene is on chromosome 15 and not in the HL-A region. Nature. 1975 Mar 20;254(5497):267–269. doi: 10.1038/254267a0. [DOI] [PubMed] [Google Scholar]
- Grey H. M., Kubo R. T., Colon S. M., Poulik M. D., Cresswell P., Springer T., Turner M., Strominger J. L. The small subunit of HL-A antigens is beta 2-microglobulin. J Exp Med. 1973 Dec 1;138(6):1608–1612. doi: 10.1084/jem.138.6.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning R., Milner R. J., Reske K., Cunningham B. A., Edelman G. M. Subunit structure, cell surface orientation, and partial amino-acid sequences of murine histocompatibility antigens. Proc Natl Acad Sci U S A. 1976 Jan;73(1):118–122. doi: 10.1073/pnas.73.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman R., Trowbridge I. Analysis of lymphocyte surface antigen expression by the use of variant cell lines. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 1):407–415. doi: 10.1101/sqb.1977.041.01.049. [DOI] [PubMed] [Google Scholar]
- Karlsson F. A. Physical-chemical properties of beta 2-microglobulin. Immunochemistry. 1974 Mar;11(3):111–114. doi: 10.1016/0019-2791(74)90207-9. [DOI] [PubMed] [Google Scholar]
- Katz F. N., Rothman J. E., Lingappa V. R., Blobel G., Lodish H. F. Membrane assembly in vitro: synthesis, glycosylation, and asymmetric insertion of a transmembrane protein. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3278–3282. doi: 10.1073/pnas.74.8.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingappa V. R., Devillers-Thiery A., Blobel G. Nascent prehormones are intermediates in the biosynthesis of authentic bovine pituitary growth hormone and prolactin. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2432–2436. doi: 10.1073/pnas.74.6.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingappa V. R., Katz F. N., Lodish H. F., Blobel G. A signal sequence for the insertion of a transmembrane glycoprotein. Similarities to the signals of secretory proteins in primary structure and function. J Biol Chem. 1978 Dec 25;253(24):8667–8670. [PubMed] [Google Scholar]
- Lingappa V. R., Lingappa J. R., Prasad R., Ebner K. E., Blobel G. Coupled cell-free synthesis, segregation, and core glycosylation of a secretory protein. Proc Natl Acad Sci U S A. 1978 May;75(5):2338–2342. doi: 10.1073/pnas.75.5.2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingappa V. R., Shields D., Woo S. L., Blobel G. Nascent chicken ovalbumin contains the functional equivalent of a signal sequence. J Cell Biol. 1978 Nov;79(2 Pt 1):567–572. doi: 10.1083/jcb.79.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelson J., Flaherty L., Vitetta E., Poulik M. D. Molecular similarities between the Qa-2 alloantigen and other gene products of the 17th chromosome of the mouse. J Exp Med. 1977 Apr 1;145(4):1066–1070. doi: 10.1084/jem.145.4.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamuro K., Tanigaki N., Pressman D. Multiple common properties of human beta2-microglobulin and the common portion fragment derived from HL-A antigen molecules. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2863–2865. doi: 10.1073/pnas.70.10.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natori T., Katagiri M., Tanigaki N., Pressman D. The 11,000-dalton component of mouse H-2. Isolation and identification. Transplantation. 1974 Dec;18(6):550–555. doi: 10.1097/00007890-197412000-00015. [DOI] [PubMed] [Google Scholar]
- Ostberg L., Rask L., Wigzell H., Peterson P. A. Thymus leukaemia antigen contains beta2-microglobulin. Nature. 1975 Feb 27;253(5494):735–737. doi: 10.1038/253735a0. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Gagnon J., Walsh K. A. Ovalbumin: a secreted protein without a transient hydrophobic leader sequence. Proc Natl Acad Sci U S A. 1978 Jan;75(1):94–98. doi: 10.1073/pnas.75.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Peterson P. A., Cunningham B. A., Berggård I., Edelman G. M. 2 -Microglobulin--a free immunoglobulin domain. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1697–1701. doi: 10.1073/pnas.69.7.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson P. A., Rask L., Lindblom J. B. Highly purified papain-solubilized HL-A antigens contain beta2-microglobulin. Proc Natl Acad Sci U S A. 1974 Jan;71(1):35–39. doi: 10.1073/pnas.71.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. E., Lodish H. F. Synchronised transmembrane insertion and glycosylation of a nascent membrane protein. Nature. 1977 Oct 27;269(5631):775–780. doi: 10.1038/269775a0. [DOI] [PubMed] [Google Scholar]
- Shields D., Blobel G. Efficient cleavage and segregation of nascent presecretory proteins in a reticulocyte lysate supplemented with microsomal membranes. J Biol Chem. 1978 Jun 10;253(11):3753–3756. [PubMed] [Google Scholar]
- Silver J., Hood L. Detergent-solubilised H-2 alloantigen is associated with a small molecular weight polypeptide. Nature. 1974 Jun 21;249(459):764–765. doi: 10.1038/249764a0. [DOI] [PubMed] [Google Scholar]
- Steck T. L. The organization of proteins in the human red blood cell membrane. A review. J Cell Biol. 1974 Jul;62(1):1–19. doi: 10.1083/jcb.62.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczesna E., Boime I. mRNA-dependent synthesis of authentic precursor to human placental lactogen: conversion to its mature hormone form in ascites cell-free extracts. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1179–1183. doi: 10.1073/pnas.73.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitetta E. S., Uhr J. W., Boyse E. A. Association of a beta2-microglobulin-like subunit with H-2 and TL alloantigens on murine thymocytes. J Immunol. 1975 Jan;114(1 Pt 1):252–254. [PubMed] [Google Scholar]
- Winkler M. A., Sanders B. G. Chemical and immunologic characterization of a beta2-microglobulin-like protein isolated from chicken sera. Immunochemistry. 1977 Aug;14(8):615–619. doi: 10.1016/0019-2791(77)90158-6. [DOI] [PubMed] [Google Scholar]