Abstract
Aberrant angiogenesis can cause or contribute to a number of diseases such as neovascular age-related macular degeneration (NVAMD). While current NVAMD treatments target angiogenesis, these treatments are not effective for all patients and also require frequent intravitreal injections. New agents and delivery systems to treat NVAMD could be beneficial to many patients. We have recently developed a serpin-derived peptide as an anti-angiogenic agent. Here, this peptide is investigated for activity in human retinal endothelial cells in vitro and for reducing angiogenesis in a laser-induced choroidal neovascularization mouse model of NVAMD in vivo. While frequent intravitreal injections can be tolerated clinically, reducing the number of injections can improve patient compliance, safety, and outcomes. To achieve this goal, and to maximize the in vivo activity of injected peptide, we have developed biodegradable polymers and controlled release particle formulations to extend anti-angiogenic therapy. To create these devices, the anionic peptides are first self-assembled into nanoparticles using a biodegradable cationic polymer and then as a second step, these nanoparticles are encapsulated into biodegradable poly(lactic-co-glycolic acid) (PLGA) microparticles. In situ, these particles show approximately zero-order, linear release of the anionic peptide over 200 days. These particles are made of safe, hydrolytically degradable polymers and have low endotoxin. Long-term in vivo experiments in the laser-induced neovascularization model for NVAMD show that these peptide-releasing particles decrease angiogenesis for at least fourteen weeks in vivo following a single particle dose and therefore are a promising treatment strategy for NVAMD.
Keywords: Angiogenesis, controlled drug release, drug delivery, microsphere, ophthalmology, peptide
INTRODUCTION
Pathologic angiogenesis plays an important role in several classes of diseases. In cancer, angiogenesis supports the growth of tumors [1]. In patients with neovascular age-related macular degeneration (NVAMD), angiogenesis leads to the loss of central vision [2]. There are several angiogenic factors that contribute to pathologic angiogenesis, such as vascular endothelial growth factor (VEGF-A), platelet-derived growth factor (PDGF-BB), and stromal derived factor (SDF-1) and neutralization of one or more of these can provide therapeutic benefits [3]. Patients with NVAMD have experienced improved visual outcomes from intraocular injections of various types of VEGF antagonists including ranibizumab (Lucentis®), an Fab; bevacizumab (Avastin®), a full-length antibody; and aflibercept (EYLEA®), a fusion protein consisting of the binding domains of VEGF receptors 1 and 2 and Fc fragment [4, 5], but frequent injections over a prolonged period are needed to maintain visual benefits. Failure to return for follow up which can occur for a variety of reasons such as illness, travel, or transportation difficulties can result in permanent loss of vision. More durable treatments are needed to mitigate these risks. Biomaterials for controlled drug delivery can potentially facilitate both protection of sensitive biological molecules from quick clearance and degradation as well as provide a mechanism for sustained and long-term release.
We have discovered classes of peptides with very strong anti-angiogenic properties, including collagen IV-derived, thrombospondins, CXC chemokines, somatotropins and serpins [6]. These peptides have been developed by combining experimental and computational approaches and several have been validated by inhibiting tumor growth in cancer models [7]. One class of these peptides, the serpin-derived peptides, are able to inhibit angiogenesis by both inducing endothelial cell apoptosis as well as decreasing their migration by increasing adhesion [8]. One of these serpin-derived peptides, which we refer to as SP6001, more specifically derived from DEAH box polypeptide 8 protein, was selected and evaluated unencapsulated, in nanoparticles, and in microparticles in the mouse model of laser-induced choroidal neovascularization.
Generally, small peptides possess many advantageous characteristics as therapeutic agents, such as high specificity and low toxicity [9]; the main disadvantage is their short half-life. Biomaterials, nanoparticles, and microparticles have the potential to significantly impact medicine as delivery systems for diverse biological molecules, including peptides. A long-term controlled release system can help overcome problems associated with current AMD treatments. A number of different polyester polymers, such as poly(lactic-co-glycolic acid) (PLGA), have been commonly used in long-term release systems. PLGA has been used in several FDA approved devices such as sutures and drug delivery devices. It is a material that is biodegradable in water and is generally recognized as safe. PLGA nanoparticles have been used to increase the half-life of therapeutics, such as in the encapsulation of a peptide integrin antagonist in PLA/PLA-PEO nanoparticles [10], as well as encapsulation of the antibody bevacizumab [11]. In contrast to nanoparticles, which generally act short-term, larger implantable devices are a drug delivery strategy that has been investigated to enable controlled long-term delivery [12, 13]. By using polymers such as PLGA, implantable devices can be designed to be biodegradable so that they do not need to be surgically removed at a future time [14].
In order to protect the SP6001 peptide from degradation and to extend its delivery, the peptide can be complexed and/or encapsulated by biodegradable polymers. The SP6001 peptide is negatively charged due to a number of glutamic acid residues. Therefore, a cationic polymer, such as a poly(beta-amino ester), PBAE, can be used to self-assemble with the peptide. PBAEs are also hydrolytically degradable due to the ester bonds in the polymer backbone. As such, these polymers have been previously used to self-assemble with DNA and RNA to form effective gene delivery nanoparticles [15–17]. To further extend release, these polymer-peptide nanoparticles can be encapsulated into PLGA microparticles. These microparticles degrade over time to release the nanoparticles and peptide into the eye to treat NVAMD.
METHODS
Chemicals
PLGA [Poly(D,L-lactide-co-glycolide); lactide:glycolide (65:35); Mw 40,000–75,000] and DCM [Dichloromethane] were purchased from Sigma (St. Louis, MO). We synthesized PBAE [Poly(beta-amino ester)], as previously described [18], from the following monomers: 3-amino-1-propanol (S3) purchased from Alfa Aesar (Ward Hill, MA), 1,3-propanediol diacrylate (B3) purchased from Dajac laboratories (Trevose, PA), and 2-(3-aminopropylamino)ethanol (E6) purchased from Fluka/Sigma. The PBAE polymer, 2-(3-aminopropylamino)ethanol end-capped 1,3-propanediol diacrylate-co-3-amino-1-propanol (abbreviated based on its constituent monomers as B3-S3-E6), was synthesized at a B3 to S3 molar ratio of 1.05:1. Polymer B3-S3-E6 was kept stored in anhydrous DMSO at 100 mg/mL with desiccant at −20°C. Peptides (SP6001 and FITC-SP6001) were purchased from American Peptide (Sunnyvale, CA). Sodium Acetate buffer (NaAc) (pH=5) was purchased from Invitrogen (Grand Island, NY). PVA [Poly(vinyl alcohol); Mw 25,000] was purchased from Polysciences (Warrington, PA).
Nanoparticle formation
For sizing with a Nanosight NS500: In an eppendorf tube, SP6001 peptide (20 µg/µL in DMSO) was diluted to 1.2 µg/µL in milli-Q water. In a second tube, 25 mM NaAc was added to the PBAE to obtain the desired PBAE concentration. For example, for 5:1 weight/weight (w/w) of PBAE to peptide, 125.3 µL NaAc was added to 8 µL (100 µg/µL) of B3-S3-E6. 100 µL of PBAE solution was added to 100 µL of peptide solution, vortexed, and incubated at room temperature for 10 min to allow for nanoparticle formation. To characterize nanoparticle size by nanoparticle tracking analysis, 100 µL of nanoparticle solution was diluted into 400 µL milli-Q water and run on a Nanosight NS500.
For in vivo injections: In separate eppendorf tubes 1.25 µL (100 µg/µL) B3-S3-E6 + 8.75 µL NaAc was prepared as was 1.25 µL (20 µg/µL) SP6001 + 8.75 µL PBS. The solutions were mixed together and then an additional 5 µL PBS was added to bring the total peptide concentration to 1.0 µg/µL. For corresponding controls: Buffer only contained 2.5 µL DMSO + 13.75 µL PBS + 8.75 µL NaAc; Peptide only contained 1.25 µL (20 µg/µL) peptide + 13.75 µL PBS mixed with 1.25 µL DMSO + 8.75 µL NaAc; Polymer only contained 1.25 µL (100 µg/µL) PBAE + 8.75 µL NaAc mixed with 1.25 µL DMSO + 13.75 µL PBS. For all samples of nanoparticles containing peptide and corresponding peptide controls, 1 µL of 1 µg/µL peptide solutions were intravitreally injected into each mouse eye.
Microparticle formulation
One hundred mg of PLGA was first dissolved into 2.5 mL of DCM in a test tube and vortexed to fully dissolve. The aqueous phase was prepared by mixing peptide (SP6001 or FITC-SP6001), PBAE (B3-S3-E6), and milli-Q water in an eppendorf tube. First 12.5 µL (20 µg/µL) SP-6001 + 8.33 µL water were mixed, then 2.5 µL (100 µg/µL) B3-S3-E6 1.05:1 + 18.33 µL water was added, and then this was diluted with an additional 26.67 µL water. For blank microparticles, the aqueous phase was 41.67 µL water. The aqueous phase was micropipetted to the PLGA/DCM solution and vortexed on high. The mixture was sonicated with the test tube on ice to create the first w/o emulsion. Sonication was performed with an amplitude setting of ‘30’, which equals approximately 10 W for 20 seconds. The primary emulsion was poured into 50 mL of 1% PVA solution and homogenized at 3.6–3.8 krpm for 1 minute to create the w/o/w secondary emulsion. The full volume was transferred into 100 mL of 0.5% PVA solution and stirred in a chemical hood for 3 hours. Three wash steps were then performed. For each wash step, the microparticle solution was centrifuged at 4°C, 4 krpm, for 5 minutes, and then the supernatant was removed. Subsequently, 40 mL of refrigerated water was added, the microparticle pellet was resuspended and the washing steps were repeated. After the last centrifugation step, 5 mL of water was added. Samples were snap frozen in liquid nitrogen and immediately placed in a lyophilizer. Following lyophilization, all microparticles were stored at −20°C. For release and in vivo studies, an appropriate amount of microparticles were weighed out and suspended in an appropriate amount of PBS to reach the desired concentration.
SEM imaging of microparticles and ImageJ quantification
Lyophilized particles were placed on carbon tape (Electron Microscopy Sciences, Hatfield, PA) placed on aluminum mounts. Samples were sputtered with gold-palladium, and SEM imaging was performed with a LEO/Zeiss FESEM at the JHU School of Medicine MicFac.
Microparticle loading and release profiles
Microparticles were prepared as described with 10% or 100% of the peptide labeled with FITC. Loading efficiency was quantified by dissolving the microparticles in DMSO and adding to PBS. The solution was centrifuged to separate out the PLGA precipitate and the supernatant was collected for fluorescence measurement. For release studies, microparticles were diluted in PBS at 40 mg/mL in a 1.5 mL tube and incubated at 37°C with light shaking. At the specified time points, samples were vortexed, spun down, supernatant was collected, and new PBS added to the microparticle pellet. DMSO was added to the supernatant so that the final solution for fluorescence measurements was constant 5% v/v DMSO/PBS. Fluorescence measurements were obtained using a BioTek Synergy 2 plate reader with an excitation filter of 485 +/− 20 nm and an emission filter of 528 +/− 20 nm. Peptide concentration was obtained by comparison to a standard curve for 6001-FITC in 5% v/v DMSO/PBS.
In vitro assays for determination of peptide effects
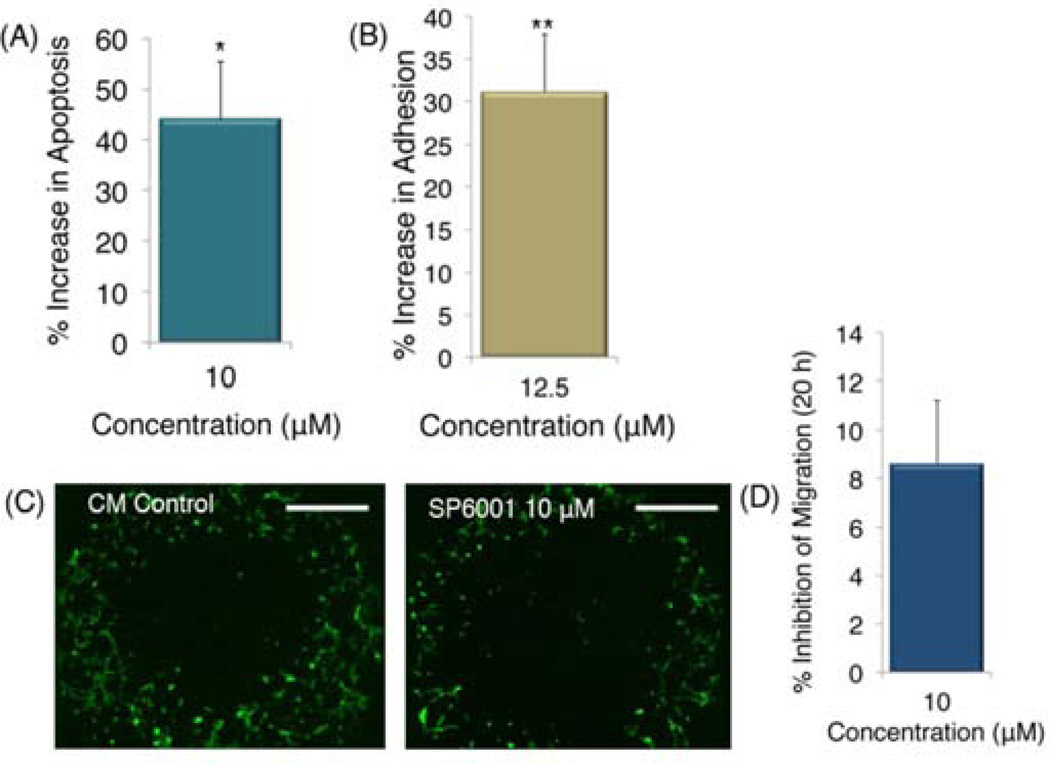
Human retinal endothelial cells (HRECs) (all cells used were P8-P12) were tested in three separate assays. SP6001’s effect on HREC apoptosis was tested by the caspase-glo 3/7 assay purchased from Promega (Madison, WI). Cells were plated at 5,000 cells/well in opaque 96-well plates to minimize well-to-well cross-talk. After 24 h, complete endothelial cell media was replaced with serum free media. Next, media with 30/10 ng/mL (bFGF/VEGF) was added with or without peptide at 10 µM. After 48 h, caspase-glo luminescent reagent was added at 100 µL/well, and luminescence measured with a Victor V plate reader (Perkin Elmer). The experiment was repeated twice.
We used the ACEA cell migration assay to assess SP6001 effect on cell adhesion, SP6001 was added to complete endothelial cell medium at 12.5 µM, and cells allowed to adhere in special E-plate (Roche, IN), suitable for cell culture with sensing electrodes. Impedance, correlated to cell adhesion, was measured using a RT-CIM system (ACEA Biosciences, Inc., San Diego, CA). HRECs were trypsinized and plated at 25,000 cells/well. Cells settled for 30 minutes before being loaded into the ACEA machine. Values are scaled to percent increase above the negative control (complete endothelial cell media), at 10 h time point.
HREC migration was tested using the Platypus migration assay. Specialized plates with stoppers were purchased from Platypus Technologies (Madison, WI). HRECs were plated at 20,000 cells/well in the presence or absence of SP6001 at 10 µM in complete endothelial cell media for 2 h, then stoppers were removed and cells allowed to migrate. After 20 h cells were stained with calcein AM (Invitrogen, Carlsbad, CA) and read with a Victor V plate reader (Perkin Elmer, Waltham, MA). Digital micrographs were taken using a Nikon Inverted Scope Eclipse T-100 scope (Nikon Instruments, Inc., Melville, NY), and are representative of each image.
Mouse model of choroidal neovascularization
Choroidal NV was induced by laser photocoagulation-induced rupture of Bruch’s membrane, as previously described [19]. Briefly, 5- to 6-wk-old female C57BL/6 mice were anesthetized with ketamine hydrochloride (100 mg/kg body weight) and pupils were dilated. Laser photocoagulation was performed in the 9, 12, and 3 o’clock positions of the posterior pole of each eye with the slit lamp delivery system of an OcuLight GL diode laser (Iridex, Mountain View, CA, USA) and a coverslip as a contact lens to view the retina. Production of a tissue bubble by the laser, which indicates rupture of Bruch’s membrane, is an important factor in obtaining choroidal NV; therefore, only burns in which a bubble was produced were included in the study. After 14 days, the mice were perfused with 1 ml of PBS containing 50 mg/ml of fluorescein-labeled dextran (2×106 Da average molecular mass; Sigma-Aldrich, St. Louis, MO, USA) and choroidal flat mounts were examined by fluorescence microscopy. Image analysis software (Image-Pro Plus; Media Cybernetics, Silver Spring, MD, USA) was used to measure the area of choroidal NV at each rupture site. To measure the long-term efficacy, Bruch’s membrane was ruptured at various time points after intravitreous injection (of 1.0 µL of peptide, buffer without peptide, nanoparticles containing peptide, polymer without peptide, microparticles containing peptide, or empty microparticles). Intravitreous injections were done under a dissecting microscope with a Harvard Pump Microinjection System (Harvard Apparatus, Holliston, MA, USA) and pulled glass micropipettes, as previously described [20].
Mouse model statistical comparisons
Data are presented graphically as mean+s.e.m. Experiments were designed so that there were fellow-eye controls and comparisons were done using a two-way analysis of variance or paired t test. P-values are two-tailed, * indicates p < 0.05 and ** indicates p < 0.01.
RESULTS
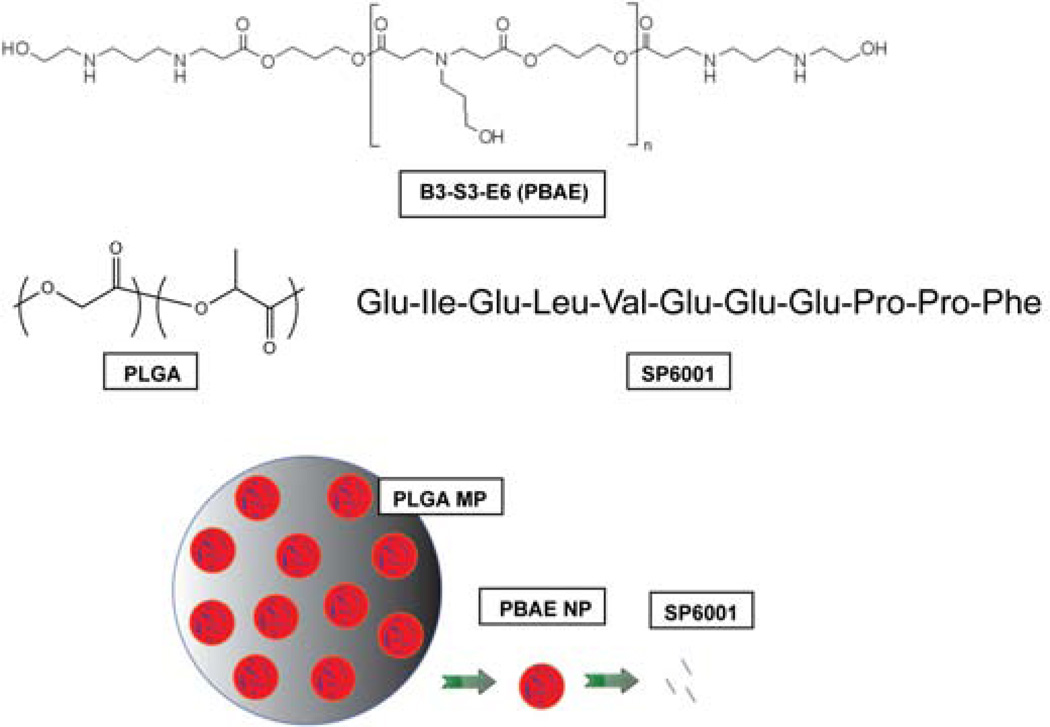
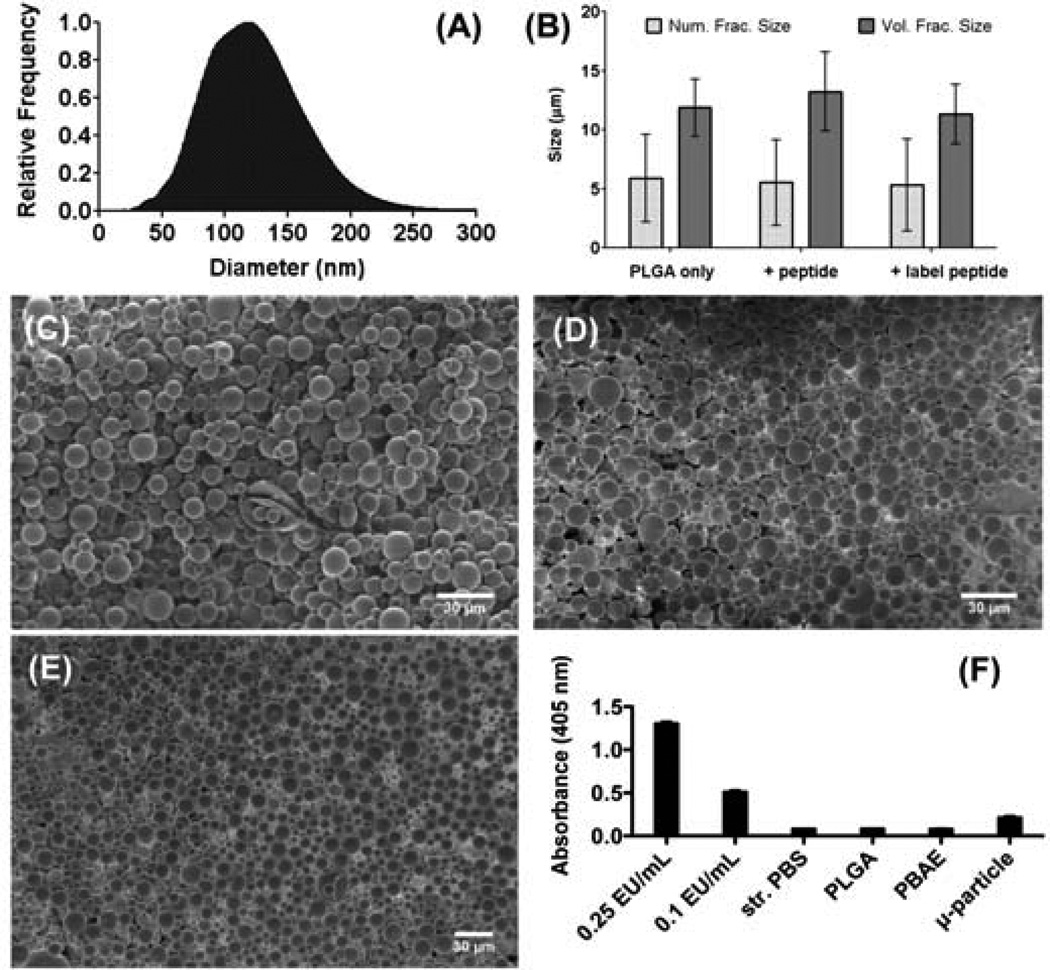
The serpin-derived peptide, SP6001 (sequence shown in Figure 1), has been previously shown to have anti-angiogenic properties in macrovascular endothelial cells and in a cancer model [8]. However, its potential inhibitory effect on retinal microvascular endothelial cells, its effects on ocular NV, and whether or not a sustained delivery formulation could be achieved were unknown. SP6001 statistically significantly increases both apoptosis and adhesion in HRECs, as well as inhibits the migration of these cells (Figure 2). Biodegradable materials were used to construct a long-term peptide delivery system. In the first step, a peptide-polymer nanoparticle was formed with a PBAE, a biodegradable and cationic polymer. In the second step, these nanoparticles were encapsulated into larger PLGA microparticles that serve as a reservoir for long-term release. The polymer structures, peptide structure, and particle diagram are shown in Figure 1. The negatively charged peptide forms nanoparticles with the positively charged, biodegradable polymer through electrostatic self-assembly. Polymer B3-S3-E6 was chosen due to its biodegradability, positive charge, biocompatibility with cells, and for its ability to form self-assembled particles with SP6001. The size of the self-assembled peptide-polymer nanoparticles formed was determined by use of the Nanosight Nanoparticle Tracking Analysis instrument and software. The B3-S3-E6/SP6001 nanoparticles had a mode size of 119 nm as shown in Figure 3A.
Figure 1. Polymer, peptide, and particle structures.
Structure of PBAE (B3-S3-E6), structure of PLGA, SP6001 peptide sequence, and the peptide particle delivery system.
Figure 2. SP6001 effects on HRECs.
(A) SP6001 evaluated in the caspase-glo 3/7 assay to measure apoptosis, normalized to the negative control. (B) ACEA cell migration assay. Values are scaled to percent increase above the negative control (complete endothelial cell media), at the 10 hour time point, (C) Platypus cell migration assay. Digital micrographs are shown representative of each condition. Scale bars represent 500 µm. (D) Quantification from the cell migration assay. Values are significant for *p<0.05.
Figure 3. Peptide delivery system characterization.
(A) Nanoparticle formation between the SP6001 peptide and PBAE B3-S3-E6, as measured by NanoSight NTA. The mode of the particle distribution is 119 nm. SEM of microparticles, (B) ImageJ size quantification. Error bars represent standard deviations of the mean of each sample, (C) Empty microparticles with aqueous phase only, (D) Microparticles encapsulating peptide SP6001/PBAE B3-S3-E6 nanoparticles, (E) Microparticles encapsulating peptide FITC-SP6001/PBAE B3-S3-E6 nanoparticles, (E) Limulus amebocyte lysate endotoxin assay test performed on LPS controls, as well as polymer and microparticle samples. All polymeric samples contained less than the 0.1 EU/mL control and are comparable to sterile PBS. Error bars represent standard deviation of the mean of each sample.
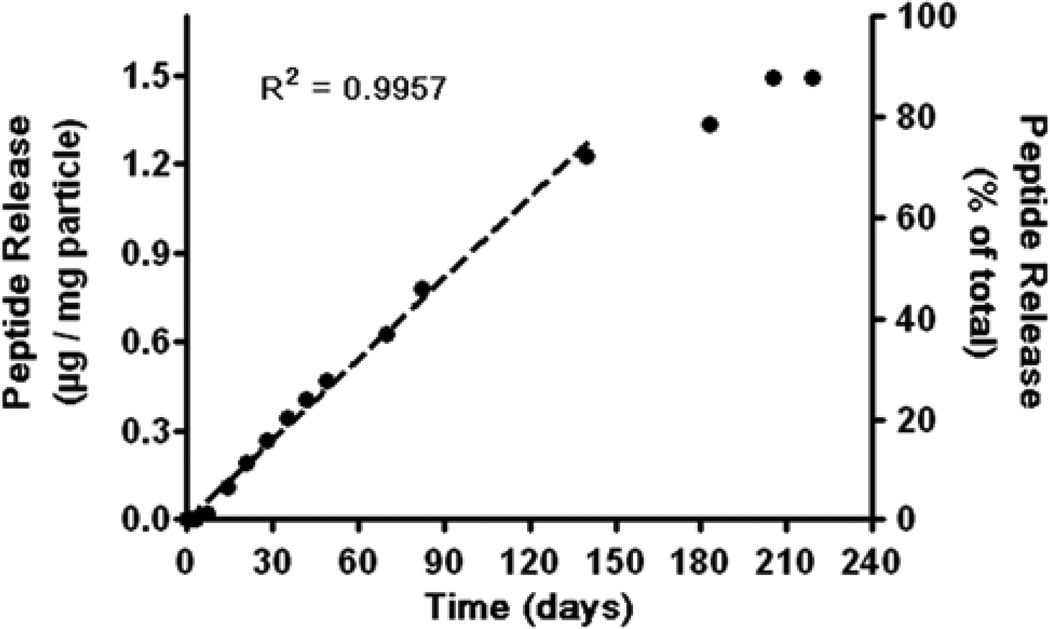
In the next step, microparticles were formed using PLGA via a standard double emulsion method. The resulting microparticles were observed using SEM and sizes were quantified using imageJ (Figure 3B). The number fraction average size was approximately 6 µm and the volume fraction weighted size was approximately 12 µm. Addition of peptide-polymer nanoparticles did not affect microparticle size or morphology of the microparticles. The presence or absence of labeled peptide as compared to unlabeled peptide also did not affect particle size or morphology. The encapsulation efficiency of the labeled peptide was determined to be approximately 70% of the initially loaded peptide amount. The microparticle fabrication process was also evaluated for endotoxin level to ensure that the particles were appropriate to use for subsequent in vivo experiments. According to the LAL endotoxin assay, all polymer and particle samples contained less than the 0.1 EU/mL of the lowest control sample (Figure 3F). The release of labeled peptide from the microparticles was quantified in situ under physiological conditions and observed to last for over 200 days, as seen in Figure 4. The release curve demonstrates that there is near linear release for approximately 140 days at ~0.008 µg peptide / mg particle released per day. This is followed by slightly slower release phase at additional 60 days. The full release extends over 7 months under physiological conditions in situ.
Figure 4. Microparticle release profile.
Microparticles are loaded with FITC-SP6001/B3-S3-E6 nanoparticles and release experiments are performed in phosphate buffered saline at 37°C on a shaker.
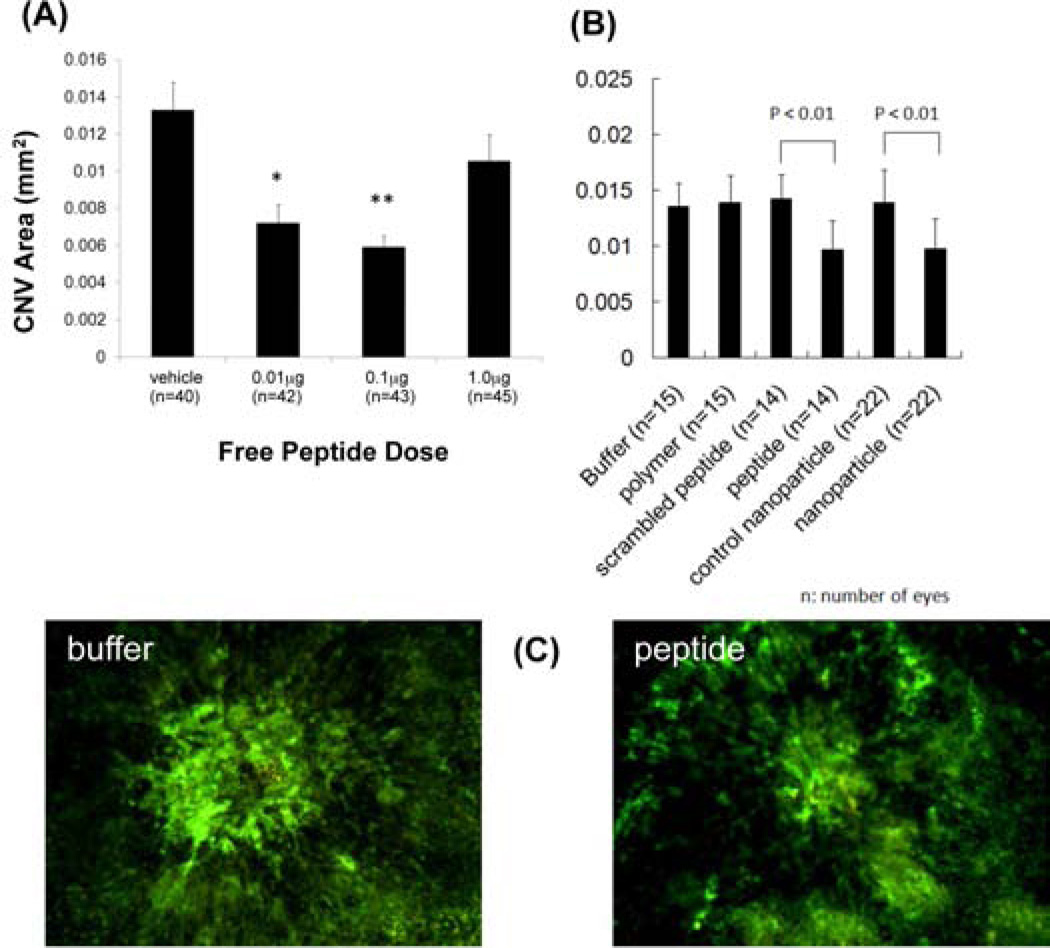
After developing the peptide release system, we sought to compare its effects with the naked peptide in vivo. Free SP6001 was injected at different concentrations on the same day as rupture of Bruch’s membrane and after 2 weeks, there was significant suppression of choroidal NV in eyes that had been injected with 0.01 µg or 0.1 µg (Figure 5A). The 0.1 µg dose was chosen as the total peptide dose to use in all subsequent experiments. Next, the SP6001/B3-S3-E6 nanoparticles were tested for activity as compared to a scrambled control peptide. While none of the controls (buffer, scrambled peptide, PBAE polymer) had any anti-angiogenic effect, both the free peptide and nanoparticle-complexed peptides caused significant suppression (Figure 5B).
Figure 5. In vivo efficacy of peptide and nanoparticles.
In vivo fluorescein isothiocyanate-dextran stained mouse eyes were analyzed in the laser-induced CNV model. (A) Free peptide injections, * = p < 0.05, ** = p < 0.01. (B) Polymer, peptide, and nanoparticle injections including polymer = B3-S3-E6, nanoparticle = B3-S3-E6 + SP6001, control nanoparticle = B3-S3-E6 + scrambled SP6001. (C) Representative fluorescence micrographs from buffer injected and peptide injected conditions.
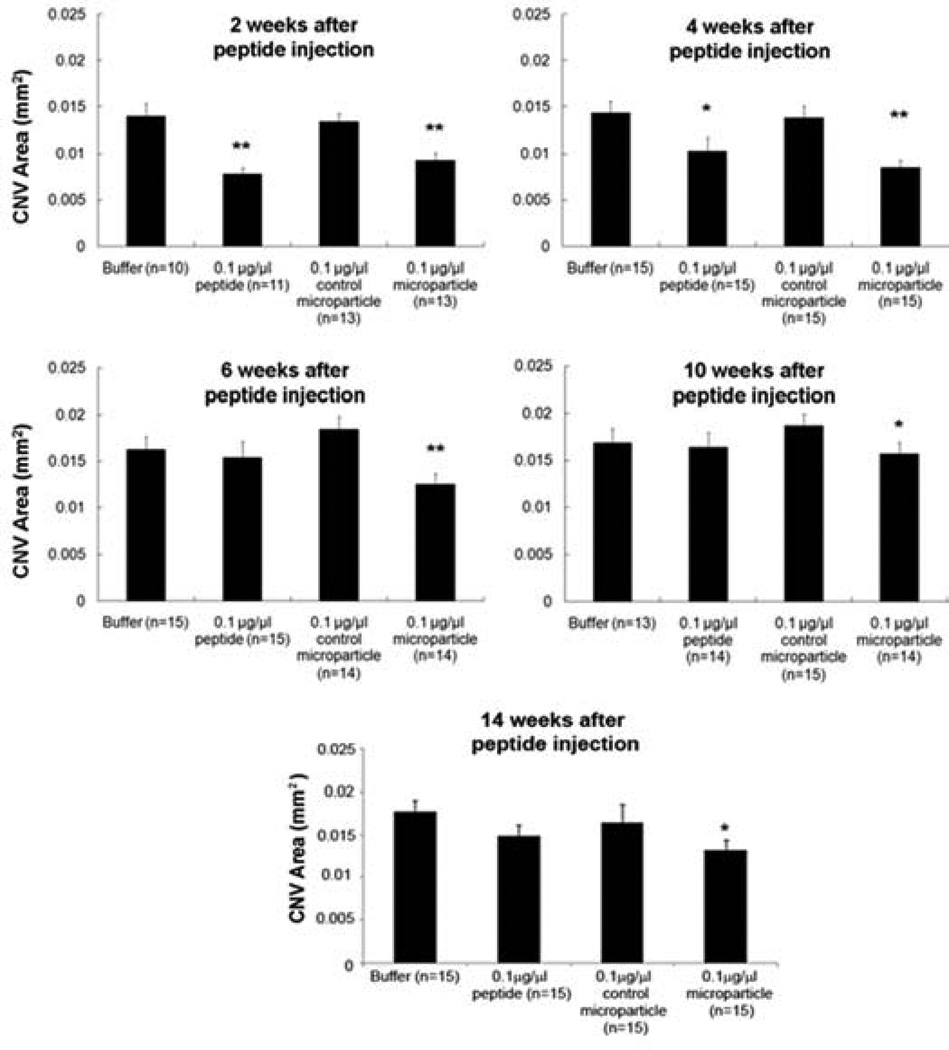
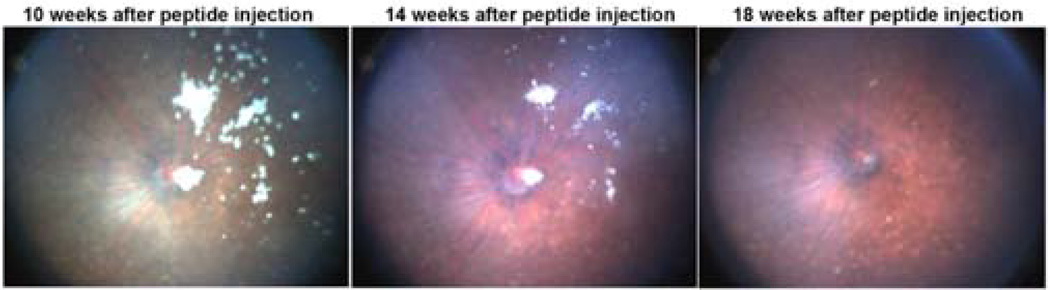
Next, we tested the effect of encapsulating the peptide-containing nanoparticles into microparticles. At short time points (2–4 weeks), both the free peptide and the peptide in nanoparticles and microparticles significantly suppresses choroidal NV; however, at time points longer than 1 month, there was good suppression by the encapsulated peptide but not the free peptide (Figure 6). A single injection of the encapsulated peptide inhibited choroidal NV for at least 14 weeks. It is important to note that even though the microparticle groups contain the same total peptide dose as the free peptide dose, and only release a small fraction of peptide at a given time point, the microparticle group performed similarly to free peptide at the early time points (<1 month). This demonstrates both that the peptide is potent at low doses and that controlled constant release, rather than injection of a bolus, may be especially advantageous for treating NVAMD. Fundus photographs showed slow disappearance of the microparticles from mouse eyes that correlated well with the duration of bioactivity (Figure 7).
Figure 6. In vivo efficacy of microparticles.
In vivo fluorescein isothiocyanate-dextran stained mouse eyes were analyzed in the laser-induced CNV model. “Control microparticles” do not contain peptide whereas “0.1 µg/µl microparticles” contain 0.1 µg of peptide per injection. * = p < 0.05, ** = p < 0.01.
Figure 7. Persistence of microparticles in vivo.
Fundus photographs showed slow degradation of the microparticles in the mouse eyes over 18 weeks.
DISCUSSION
The eye is a relatively isolated tissue compartment and local delivery can facilitate high drug levels within the eye and low systemic levels in other tissues. Systemic administration of VEGF antagonists in patients with cancer provides some benefits, but also has potential complications including hypertension, thromboembolic events, and renal damage [21, 22]. These problems have been largely circumvented in patients with NVAMD by intravitreous injections of VEGF antagonists, which neutralize VEGF in the eye for 1–2 months in most patients with little effect on systemic VEGF levels. However, a month after injection of ranibizumab and possibly as long as 2 months after an injection of aflibercept, VEGF is no longer neutralized causing recurrent leakage and collection of fluid in the macula that reduces vision. Timely reinjection of a VEGF antagonist can stop leakage allowing vision to be regained, but failure to re-inject allows growth of the NV, recruitment of retinal pigmented epithelial cells and glia, and scarring that damages photoreceptors resulting in permanent reduction in vision. Attempts to reduce follow up and frequency of anti-VEGF injections have resulted in poorer visual outcomes than those achieved with monthly injections. Therefore, sustained suppression of choroidal NV is needed to achieve the best long-term outcomes in patients with NVAMD, and this is difficult to sustain with current treatments that require very frequent follow up and injections.
In this study, we have demonstrated sustained suppression of choroidal NV for at least 14 weeks after a single injection of an anti-angiogenic peptide encapsulated in nanoparticles and microparticles. Specifically, we report on the efficacy of an anti-angiogenic serpin-derived peptide, SP6001, to treat AMD and its improved long-term efficacy in vivo when released from a biodegradable drug delivery system composed of PBAE nanoparticles in PLGA microparticles. The peptide SP6001 shows anti-angiogenic efficacy comparable to a recently approved AMD therapeutic, aflibercept, using the same mouse model [23]. Statistically significant suppression of choroidal NV was caused by the microparticles encapsulating peptide compared to empty control microparticles for at least 14 weeks after a single intravitreal injection. The degradation rate of the particles in vivo was observed to be faster (approximately twice as fast) as what was observed in situ. This is not unexpected as the in vivo microenvironment in the eye contains additional degradative enzymes and clearance mechanisms that are not captured in an in situ degradation experiment. Biomaterial modification (i.e. PLGA copolymer composition) can be used to further slow degradation rate if needed.
PLGA, a biodegradable polymer that has been used in FDA approved devices, has been used to deliver a number of different drugs in the eye and has been shown to be generally well tolerated [11, 24, 25]. For example, Shelke et al. have observed safe and sustained release of an encapsulated hydrophilic drug in vivo [24]. Mordenti et al. delivered a humanized antibody encapsulated in PLGA to rabbit eyes and observed some initial immune response, but no resulting safety issues [26]. Pan et al. have shown long-term release of PLGA-encapsulated bevacizumab in a similar laser photocoagulation model in rats over the course of a few weeks [27]. In this study they observed a statistically significant decrease in CNV area at four weeks and at eight weeks post-injection, but not at six weeks post-injection. In another example, Xu et al. delivered dexamethasone acetone loaded PLGA nanoparticles using a rat laser photocoagulation model and observed inhibition of CNV [28]. In contrast, here we show a peptide controlled release system that maintains anti-angiogenic activity in this laser-induced choroidal neovascularization model that lasts for at least 14 weeks following a single injection. In this manuscript, we report a potent anti-angiogenic peptide for NVAMD, SP6001, and a biodegradable polymeric particle delivery system able to maintain long-term peptide efficacy in the eye.
CONCLUSION
We have demonstrated that the combination of a serpin-derived peptide and its polymeric delivery system is promising as a potential therapeutic for NVAMD. The peptide is able to inhibit angiogenesis through multiple mechanisms including interfering with proliferation, adhesion, and migration. The peptide has anti-angiogenic efficacy in mice with choroidal NV that peaks at ~50% inhibition at 2 weeks and persists for an additional two weeks. By complexing the serpin-derived peptide with a poly(beta-amino ester) to form nanoparticles and then encapsulating these nanoparticles within PLGA microparticles, inhibition of angiogenesis using the same peptide dose can be extended to at least 14 weeks following a single intravitreal injection. The particles are made of safe, hydrolytically degradable polymers and have low endotoxin. By delivering the peptide in a long-term release system, this treatment may be able to improve patient outcomes, both by sustaining suppression of choroidal NV for long periods and through the action of a multimodal anti-angiogenic therapeutic.
ACKNOWLEDGEMENTS
The authors thank the Edward N. & Della L. Thome Memorial Foundation (Bank of America, Trustee) Awards Program in AMD Research, the NIH (1R21EY022986-01 and R01EY012609), and the Wallace H. Coulter Foundation for support of this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE
JJG and ASP are co-founders and officers of AsclepiX Therapeutics, LLC; Potential conflicts of interest are managed by the Johns Hopkins Medical Institutions Committee on Outside Interests.
REFERENCES
- 1.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285(21):1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 2.Bressler SB. Introduction: Understanding the role of angiogenesis and antiangiogenic agents in age-related macular degeneration. Ophthalmology. 2009;116(10 Suppl):S1–S7. doi: 10.1016/j.ophtha.2009.06.045. [DOI] [PubMed] [Google Scholar]
- 3.Distler JH, Hirth A, Kurowska-Stolarska M, Gay RE, Gay S, Distler O. Angiogenic and angiostatic factors in the molecular control of angiogenesis. Q J Nucl Med. 2003;47(3):149–161. [PubMed] [Google Scholar]
- 4.Holash J, Davis S, Papadopoulos N, Croll SD, Ho L, Russell M, et al. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci U S A. 2002;99(17):11393–11398. doi: 10.1073/pnas.172398299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD, et al. Intravitreal aflibercept (VEGF Trap-Eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–2548. doi: 10.1016/j.ophtha.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Karagiannis ED, Popel AS. A systematic methodology for proteome-wide identification of peptides inhibiting the proliferation and migration of endothelial cells. Proc Natl Acad Sci U S A. 2008;105(37):13775–13780. doi: 10.1073/pnas.0803241105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koskimaki JE, Karagiannis ED, Rosca EV, Vesuna F, Winnard PT, Jr, Raman V, et al. Peptides derived from type IV collagen, CXC chemokines, and thrombospondin-1 domain-containing proteins inhibit neovascularization and suppress tumor growth in MDA-MB-231 breast cancer xenografts. Neoplasia. 2009;11(12):1285–1291. doi: 10.1593/neo.09620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koskimaki JE, Rosca EV, Rivera CG, Lee E, Chen W, Pandey NB, et al. Serpin-derived peptides are antiangiogenic and suppress breast tumor xenograft growth. Transl Oncol. 2012;5(2):92–97. doi: 10.1593/tlo.11244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saladin PM, Zhang BD, Reichert JM. Current trends in the clinical development of peptide therapeutics. IDrugs : the investigational drugs journal. 2009;12:779–784. [PubMed] [Google Scholar]
- 10.Kim H, Csaky KG. Nanoparticle-integrin antagonist C16Y peptide treatment of choroidal neovascularization in rats. J Control Release. 2010;142(2):286–293. doi: 10.1016/j.jconrel.2009.10.031. [DOI] [PubMed] [Google Scholar]
- 11.Li F, Hurley B, Liu Y, Leonard B, Griffith M. Controlled release of bevacizumab through nanospheres for extended treatment of age-related macular degeneration. Open Ophthalmol J. 2012;6:54–58. doi: 10.2174/1874364101206010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campochiaro PA, Hafiz G, Shah SM, Bloom S, Brown DM, Busquets M, et al. Sustained ocular delivery of fluocinolone acetonide by an intravitreal insert. Ophthalmology. 2010;117(7):1393–1399. e1393. doi: 10.1016/j.ophtha.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 13.Lee JH, Pidaparti RM, Atkinson GM, Moorthy RS. Design of an implantable device for ocular drug delivery. J Drug Deliv. 2012;2012:527516. doi: 10.1155/2012/527516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yasukawa T, Kimura H, Kunou N, Miyamoto H, Honda Y, Ogura Y, et al. Biodegradable scleral implant for intravitreal controlled release of ganciclovir. Graefes Arch Clin Exp Ophthalmol. 2000;238(2):186–190. doi: 10.1007/s004170050031. [DOI] [PubMed] [Google Scholar]
- 15.Green JJ, Zugates GT, Tedford NC, Huang YH, Griffith LG, Lauffenburger DA, et al. Combinatorial modification of degradable polymers enables transfection of human cells comparable to adenovirus. Advanced Materials. 2007;19(19):2836–2842. [Google Scholar]
- 16.Shmueli RB, Sunshine JC, Xu Z, Duh EJ, Green JJ. Gene delivery nanoparticles specific for human microvasculature and macrovasculature. Nanomedicine. 2012;8(7):1200–1207. doi: 10.1016/j.nano.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tzeng SY, Hung BP, Grayson WL, Green JJ. Cystamine-terminated poly(beta-amino ester)s for siRNA delivery to human mesenchymal stem cells and enhancement of osteogenic differentiation. Biomaterials. 2012;33(32):8142–8151. doi: 10.1016/j.biomaterials.2012.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green JJ, Langer R, Anderson DG. A combinatorial polymer library approach yields insight into nonviral gene delivery. Acc Chem Res. 2008;41(6):749–759. doi: 10.1021/ar7002336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tobe T, Ortega S, Luna JD, Ozaki H, Okamoto N, Derevjanik NL, et al. Targeted disruption of the FGF2 gene does not prevent choroidal neovascularization in a murine model. Am J Pathol. 1998;153(5):1641–1646. doi: 10.1016/S0002-9440(10)65753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mori K, Duh E, Gehlbach P, Ando A, Takahashi K, Pearlman J, et al. Pigment epithelium-derived factor inhibits retinal and choroidal neovascularization. J Cell Physiol. 2001;188(2):253–263. doi: 10.1002/jcp.1114. [DOI] [PubMed] [Google Scholar]
- 21.Miki K, Miki A, Matsuoka M, Muramatsu D, Hackett SF, Campochiaro PA. Effects of intraocular ranibizumab and bevacizumab in transgenic mice expressing human vascular endothelial growth factor. Ophthalmology. 2009;116(9):1748–1754. doi: 10.1016/j.ophtha.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Escalante CP, Zalpour A. Vascular endothelial growth factor inhibitor-induced hypertension: basics for primary care providers. Cardiol Res Pract. 2011;2011:816897. doi: 10.4061/2011/816897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saishin Y, Takahashi K, Lima e Silva R, Hylton D, Rudge JS, Wiegand SJ, et al. VEGFTRAP( R1R2) suppresses choroidal neovascularization and VEGF-induced breakdown of the blood-retinal barrier. J Cell Physiol. 2003;195(2):241–248. doi: 10.1002/jcp.10246. [DOI] [PubMed] [Google Scholar]
- 24.Shelke NB, Kadam R, Tyagi P, Rao VR, Kompella UB. Intravitreal poly(L-lactide) microparticles sustain retinal and choroidal delivery of TG-0054, a hydrophilic drug intended for neovascular diseases. Drug Deliv Transl Res. 2011;1(1):76–90. doi: 10.1007/s13346-010-0009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Short BG. Safety evaluation of ocular drug delivery formulations: techniques and practical considerations. Toxicol Pathol. 2008;36(1):49–62. doi: 10.1177/0192623307310955. [DOI] [PubMed] [Google Scholar]
- 26.Mordenti J, Thomsen K, Licko V, Berleau L, Kahn JW, Cuthbertson RA, et al. Intraocular pharmacokinetics and safety of a humanized monoclonal antibody in rabbits after intravitreal administration of a solution or a PLGA microsphere formulation. Toxicol Sci. 1999;52(1):101–106. doi: 10.1093/toxsci/52.1.101. [DOI] [PubMed] [Google Scholar]
- 27.Pan CK, Durairaj C, Kompella UB, Agwu O, Oliver SC, Quiroz-Mercado H, et al. Comparison of long-acting bevacizumab formulations in the treatment of choroidal neovascularization in a rat model. J Ocul Pharmacol Ther. 2011;27(3):219–224. doi: 10.1089/jop.2010.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu J, Wang Y, Li Y, Yang X, Zhang P, Hou H, et al. Inhibitory efficacy of intravitreal dexamethasone acetate-loaded PLGA nanoparticles on choroidal neovascularization in a laser-induced rat model. J Ocul Pharmacol Ther. 2007;23(6):527–540. doi: 10.1089/jop.2007.0002. [DOI] [PubMed] [Google Scholar]