Abstract
Inflammation is a central part of innate immunity, but its role in anti-pathogen defenses has been overshadowed by recent interest in the contribution of inflammation to a wide range of chronic degenerative diseases. Current research on chronic inflammation is conducted primarily in affluent populations with low levels of infectious disease; comparative research in different ecological settings is needed to advance understandings of the causes and consequences of variation in the regulation of inflammation. This paper investigates the levels and predictors of interleukin-6 (IL-6) and interleukin-10 (IL-10)–two cytokines important to the regulation of inflammation—in a large, population-based study in the Philippines. Concentrations of IL-6 and IL-10 were determined in N=1569 healthy young adults (20-22 yrs) in Metro Cebu, Philippines. IL-6 and IL-10 concentrations were positively correlated, and body mass index and symptoms of infectious disease were both associated with higher concentrations of IL-6 and IL-10. Median concentrations of IL-6 (1.0 pg/mL) and IL-10 (7.56 pg/mL) were substantially lower and higher, respectively, than levels reported for other populations based on a systematic review of prior research. This study contributes to a growing body of research in human ecological immunology, and suggests that there may be substantial population differences in the regulation of inflammation that has implications for the association between inflammation and disease.
Keywords: ecological immunology, innate immunity, cytokines, cardiovascular disease
Research in human ecological immunology has demonstrated the value of applying an adaptationist approach to understanding the development and function of the human immune system (Blackwell et al. 2010; McDade 2003; McDade and Worthman 1999; Muehlenbein and Bribiescas 2005). The field-based, comparative perspective of human ecological immunology is important for documenting the range of variation in key immune processes, and for examining the contextual factors that shape this variation. We contribute to research in this area by investigating the levels and predictors of interleukin-6 (IL-6) and interleukin-10 (IL-10)–two cytokines critical to the regulation of inflammation—in healthy young adults in the Philippines.
Inflammation is a central part of innate immunity, and acute inflammation initiates a rapid, coordinated mobilization of non-specific cellular and biochemical defenses that promote pathogen clearance and healing (Kumar et al. 2004). Recently, inflammation's role in anti-pathogen defenses has been overshadowed by intense clinical and epidemiological interest in the contribution of inflammation to the pathophysiology of a wide range of chronic diseases (Festa et al. 2000; Pearson et al. 2003; Pickup 2004). Elevated concentrations of C-reactive protein (CRP)—a prototypical acute phase protein—have been consistently associated with increased risk for cardiovascular disease (Ballou and Kushner 1992; Libby et al. 2002), type II diabetes (Pradhan et al. 2001a; Pradhan et al. 2001b), late-life disability (Kuo et al. 2006), and mortality (Harris 1999). While acute inflammation is typically viewed as an adaptive response to infection, this new line of inquiry suggests that chronic, low-grade activation of inflammatory pathways may have long-term, maladaptive consequences.
There are two important limitations to prior research in this area. First, most population-based studies have focused primarily on CRP as a biomarker of inflammation without attention to the upstream pathways that up- and downregulate inflammatory processes. Interleukin-6 is a pro-inflammatory cytokine that is produced by endothelial cells, monocytes, macrophages, mast cells, and adipocytes, and is a primary determinant of CRP production and release (Bermudez et al. 2002; Du Clos 2000; Yudkin et al. 1999a). While other cytokines are also involved in the activation of inflammation (e.g., TNFα, IL-1β), IL-6 has received the most attention as a contributor to chronic degenerative diseases. Previous work has shown that individual correlations between concentrations of IL-6 and CRP are typically high (Esposito et al. 2003a) and elevated concentrations of IL-6 are associated with increased risk for cardiovascular disease and rheumatoid arthritis (Ridker et al. 2000b; Tziakas et al. 2003; Robak 1998).
Interleukin-10 is a cytokine secreted primarily by T and B lymphocytes, monocytes, and macrophages (Tedgui and Mallat 2001), and it is a potent inhibitor of pro-inflammatory activity, including suppression of IL-6 production (Moore et al. 2001). Although relatively few studies have measured IL-10 in relation to health outcomes, lower concentrations of IL-10 have been associated with increased risk for metabolic syndrome (Choi et al. 2007; Esposito et al. 2003b; van Exel et al. 2002b), type 2 diabetes (van Exel et al. 2002b), stroke (van Exel et al. 2002a), and heart disease (Pradhan et al. 2001a; Tziakas et al. 2003). In sum, IL-10 and IL-6 appear to play counter-regulatory roles with respect to inflammation, and insufficient anti-inflammatory signaling may be an important, but relatively overlooked, mechanism through which inflammation contributes to chronic degenerative diseases.
A second limitation derives from the fact that current understandings of chronic inflammation and disease are based on research conducted primarily in relatively affluent western populations. These populations are typically characterized by low levels of infectious disease and high levels of caloric excess. Since the human immune system evolved in environments with marginal nutrition and substantially higher levels of microbial exposure, it is reasonable to suggest that over-nourished, “under-infected” western populations may not represent the most enlightening contexts in which to study inflammation (Gurven et al. 2008; McDade 2003). Therefore research in different ecological settings, grounded by the adaptationist perspective of human ecological immunology, is needed to complement current biomedical research on the determinants of inflammation.
The Philippines represents such a setting. It is a lower-middle income nation undergoing significant economic, dietary and lifestyle changes. Although rates of overweight/obesity, CVD, and metabolic syndrome are relatively low, they are on the rise (Adair et al 2011; Pedro et al. 2007; Tanchoco et al. 2003). At the same time, infectious disease accounts for more than 30% of all mortality in Southeast Asia, with pneumonia, diarrhea, and tuberculosis serving as major contributors (WHO 2004). In the Philippines, respiratory infections rank beside ischemic heart disease as the top causes of mortality (WHO 2006). This level of infectious disease exposure, in combination with recent trends toward increased body weight, is characteristic of many transitional populations globally and thus provides an interesting and important setting in which to investigate the dynamics of inflammation.
In prior research we have reported exceptionally low concentrations of CRP among young adults in the Philippines compared with young adults in the US (McDade et al. 2009). In this paper we seek to build on these findings by investigating two cytokines centrally involved in pro- and anti-inflammatory processes. Specifically, the objectives of this analysis are threefold: 1) To report concentrations of IL-6 and IL-10 among healthy young adults in the Philippines, and to evaluate adiposity, infectious, and socioeconomic predictors of individual variation in IL-6 and IL-10; 2) To investigate patterns of association among IL-6, IL-10, and CRP; and 3) To compare IL-6 and IL-10 concentrations to prior studies in other populations. Based on our prior analysis of CRP, we hypothesized that concentrations of IL-6 would be lower and concentrations of IL-10 would be higher in the Philippines than in more affluent, industrialized populations. We also expected measures of adiposity and pathogen exposure to be significant predictors of inflammatory cytokine production. These analyses provide important comparative information for research on the regulation of inflammation, and contribute to a growing body of research in human ecological immunology.
Methods
Participants and data collection
The Cebu Longitudinal Health and Nutrition Survey (CLHNS) began in 1983 with the recruitment of 3327 pregnant women in Metro Cebu, which is among the largest and fastest growing metropolitan areas in the Philippines. For these women, 3080 singleton infants were born between 1983-84, representative of births in Metro Cebu for this period (Adair et al. 2010). The women and their children have been followed through multiple rounds of data collection since 1983. The data for the present analyses come from the 2005 survey, when all of the offspring of the original cohort were 20-22 years of age. Complete data were available for 1598 participants. However, 29 women who were pregnant at the time of survey were excluded from the analyses resulting in a final sample of N=1569. Participants provided information on household demographics and income levels, environmental quality, and health behaviors in face-to-face interviews conducted in their homes. All data were collected under conditions of informed consent with institutional review board approval from the University of North Carolina, Chapel Hill.
We evaluated the degree to which the analytic sample differed from the original cohort as assessed when the study started in 1983. Compared to those lost to follow up, participants remaining in the study were born to fathers with less formal education (mean (SE) difference = (0.54 (0.15) years; p<0.001), to mothers with marginally less formal education (0.24 (0.13) years; p<0.10), and into homes in slightly more rural communities (2.05 (0.45); p<0.001) points on 70 point urbanicity scale (Dahly and Adair 2007). Participants did not differ with respect to household income or assets at baseline. Attrition in the CLHNS is due primarily to factors related to out-migration rather than refusal to participate (Adair et al. 2010), and as a result, participants in the 2005 survey represent households that are less mobile compared with all participants in the original cohort.
Standard anthropometric techniques were used to measure body weight, height, waist circumference, and triceps, subscapular, and suprailiac skinfold thicknesses (Lohman et al. 1988). The body mass index (BMI) was calculated as the ratio of weight (kg)/height (m2).
Following prior research in the Philippines and elsewhere (Nurgalieva et al. 2002; Prado et al. 2003; VanDerslice et al. 1994), we collected multiple proxy measures of the likelihood of exposure to infectious microbes, including household crowding (number of persons/number of rooms), type of toilet (no toilet, pit, flush/water sealed), and source of drinking water (bottled, piped municipal supply, closed well with pump, open sources: uncovered well, spring, river, rain). We also constructed a pathogen exposure scale based on five variables, each scored on a three point scale (0=low exposure, 1=moderate, 2=high): cleanliness of the food preparation area, means of garbage disposal, presence of excrement near the house, level of garbage and excrement present in the neighborhood surrounding the household (McDade et al. 2009). In addition, at the time of blood collection we asked participants if they were currently experiencing any symptoms of infection, including runny nose, cough, fever, diarrhea, sore throat, as well as the more general categories of “flu,” “cold,” and “sinusitis”. Responses were used to construct a summary variable indicating the presence or absence of any infectious symptoms at the time of blood collection.
Information on health behaviors of relevance to inflammation was collected during face-to-face interviews. Variables were constructed for smoking (none vs. ≥1 cigarette/day), alcohol consumption (never, occasionally, weekly, daily), and oral contraceptive use (yes/no). Measures of socioeconomic status included highest grade completed, household income, household assets, and home ownership. We also used a previously validated measure of the degree of urban development in the community in which participants lived (Dahly and Adair 2007). This scale is based on population size and density, availability of communications (e.g., telephone, internet), transportation infrastructure, and presence of educational facilities, health services, and markets for food and other consumer goods. Higher scores (range 0 to 70) on the scale indicate a higher degree of urban development.
Analysis of IL-6 and IL-10
Blood samples were collected using EDTA-coated vacutainer tubes in the participants’ homes in the morning after an overnight fast. Blood samples were kept in coolers on ice packs for no more than 2 hours and were then centrifuged to separate plasma prior to freezing at -70° C. Samples were express shipped to Northwestern University on dry ice and stored frozen at -80° C until analysis. Samples were analyzed for CRP using a high sensitivity immunoturbidimetric method as previously described (McDade et al. 2009).
Concentrations of IL-6 and IL-10 were determined in the Laboratory for Human Biology Research at Northwestern University using a high sensitivity multiplex immunoassay protocol (HSCYTO-60SK, Millipore, Billerica, MA) on the Luminex platform (Luminex Corporation, Austin, TX). Briefly, samples were incubated with sets of polystyrene microspheres covalently coupled with capture antibodies specific for IL-6 and IL-10. Samples were then incubated with detection antibody labeled with a fluorescent reporter molecule. Data were acquired by running the samples through a modified flow cytometer that identifies and separates each set of microspheres, and quantifies the amount of bound analyte. The manufacturer-reported lower detection limits for IL-6 and IL-10 were 0.10 and 0.15 pg/mL, respectively, but for many samples we were able to estimate values for samples below these limits. Samples with undetectable concentrations were assigned a value of 0.0001 pg/mL. For IL-6, the between-assay percent coefficient of variation (%CV; SD/mean) for low and high control samples included with each assay was 14.7 and 12.4, respectively. For IL-10, the %CV for low and high controls was 15.4 and 11.6, respectively. All samples were analyzed in duplicate, with average within-assay %CVs of 13.3 for IL-6, and 14.1 for IL-10. These levels of analytic variation are comparable to, or lower than, previously reported applications of this method to multiplexed cytokine analysis (Prabhakar et al. 2002).
Data analysis
Analyses proceeded in three stages. First, we tested the hypothesis that adiposity, pathogen exposure, and health behaviors are significant predictors of cytokine levels by implementing a series of regression models with log-transformed IL-6 and IL-10 as dependent variables. We applied Tobit regression models for censored data to account for non-normality in the distribution of IL-6 and IL-10 values. Even after log transformation the distribution was left-censored due to the large number of observations with values below the lower detection limit of the cytokine assays. The application of ordinary least squares regression procedures would likely result in biased and unstable parameter estimates, whereas Tobit regression takes into account the censored nature of the distribution to provide more reliable parameter estimates (Greene 2000).
Measures of adiposity (waist circumference, BMI, skinfold thickness), pathogen exposure (crowding, water source, toilet type, pathogen exposure scale, presence of infectious symptoms), and health behaviors (smoking, alcohol consumption, oral contraception) were considered in separate regression models as predictors of log-IL-6 and log-IL-10. Measures of socioeconomic status and urbanicity were also evaluated in an attempt to capture other unmeasured aspects of environmental quality and lifestyle that may be related to inflammation. Variables with p<0.10 were retained and considered in a final model to evaluate their independent and combined contributions to explaining cytokine levels. All statistical analyses were conducted with Stata for Windows, version 10 (StataCorp, College Station, TX). Since episodes of acute inflammation may obscure assessment of chronic, low grade inflammatory activity in studies using samples collected at a single time point (Pearson et al. 2003), we conducted analyses for the entire sample, as well as analyses that excluded individuals reporting symptoms of infectious disease at the time of blood collection.
Second, bivariate associations between IL-6 and CRP, and between IL-10 and CRP, were evaluated to consider the degree to which each cytokine may be involved in the regulation of a key product of inflammation. We also investigated the bivariate association between IL-6 and IL-10 to evaluate the degree to which they may be playing counter-regulatory roles.
Third, descriptive analyses were used to investigate median levels of IL-6 and IL-10 in relation to previously reported values in other populations. We used electronic databases (Medline, Google Scholar) as well as bibliographies of past publications to conduct a comprehensive search for prior peer-reviewed publications containing mean or median IL-6 or IL-10 concentrations from healthy individuals. In many cases an overall median or mean concentration was not provided; rather, values were presented for subgroups in a comparative study design. We report overall concentrations when available, and concentrations for subgroups otherwise. For comparative purposes, we calculated weighted averages for IL-6 and IL-10 across these studies.
Results
Predictors of IL-6 and IL-10
The young adults in this sample were lean, with low waist circumference compared to young adults in the U.S., and mean BMI values are at the low end of “normal” as defined by recent CDC guidelines (Okosun et al. 2004) (Table 1). Median IL-6 concentration for the entire sample was 1.00 pg/mL (interquartile range 0.28, 3.21). At the time of blood collection N=224 individuals reported a symptom of infectious disease. With those individuals removed from the sample, median IL-6 was 0.95 pg/mL (0.24, 2.93). There was no evidence for any gender difference in IL-6 concentration.
Table 1.
Descriptive statistics for female and male participants. Mean (SD) values are presented for continuous variables; % values are presented for categorical variables.
| Female (N=703) |
Male (N=866) |
Total (N=1569) |
|
|---|---|---|---|
| Age (yrs) | 20.9 (0.4) | 20.9 (0.3) | 20.9 (0.3) |
| Household income, weekly (pesos) | 666.9 (1721.9) | 571.9 (896.3) | 614.5 (1331.5) |
| Highest grade of school completed | 11.6 (3.3) | 10.5 (3.9) | 11.0 (3.6) |
| Currently in school | 18.9 | 18.6 | 18.7 |
| Urbanicity (0-70) | 41.3 (13.2) | 41.0 (13.7) | 41.1 (13.5) |
| BMI (kg/m2) | 20.3 (3.2) | 21.1 (3.1) | 20.7 (3.2) |
| Waist circumference (cm) | 68.0 (7.7) | 72.2 (7.6) | 70.3 (7.9) |
| Sum of skinfold thickness (mm) | 62.1 (19.8) | 37.8 (18.1) | 48.7 (22.4) |
| Symptoms of infection (%) | 15.1 | 13.6 | 14.3 |
| Current smoker (%) | 3.0 | 42.5 | 24.8 |
| Oral contraceptive use (%) | 3.7 | ||
| CRP (median, 25th, 75th percentile) | 0.2 (0.1, 0.9) | 0.3 (0.1, 0.9) | 0.2 (0.1, 0.9) |
| IL-6 (median, 25th, 75th percentile) | 1.00 (.28, 3.21) | ||
| IL-10 (median, 25th, 75th percentile) | 7.56 (3.09, 14.67) |
Body mass index (BMI), waist circumference, and skinfold thickness were highly correlated and were therefore considered separately in regression models predicting IL-6 to avoid problems associated with collinearity. Body mass index was the strongest predictor of the three variables, and was positively associated with IL-6 concentration (Table 2, model 1). Among the pathogen exposure variables, the presence of infectious disease symptoms at the time of blood collection was the only significant predictor, and was positively associated with IL-6 (Table 2, model 2). Measures of socioeconomic status and urbanicity were not significantly associated with IL-6. Among the health behaviors, oral contraceptive use among women was rare (N=26), but was associated with significantly lower concentrations of IL-6 (Table 2, model 3).
Table 2.
Results of Tobit regression models predicting log-transformed IL-6 (N=1,569).
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
|---|---|---|---|---|---|---|---|---|
| B | SE | B | SE | B | SE | B | SE | |
| Female | 0.022 | 0.099 | -0.013 | 0.098 | 0.032 | 0.099 | 0.060 | 0.100 |
| BMI (kg/m2) | 0.039* | 0.016 | 0.043** | 0.015 | ||||
| Symptoms of infection (0, 1) | 0.424** | 0.139 | 0.431** | 0.139 | ||||
| Oral contraception (0, 1) | -1.102** | 0.396 | -1.148** | 0.394 | ||||
| Constant | -1.414*** | 0.334 | -0.651*** | 0.069 | -0.593*** | 0.066 | -1.557*** | 0.334 |
| Model p value | <0.05 | <0.01 | <0.05 | <0.001 | ||||
p<0.10;
p<0.05;
p<0.01;
p<0.001
Note: Models 1-3 consider gender as well as the separate associations of adiposity (model 1), pathogen exposure (model 2), and health behavior variables (model 3) shown in bivariate analyses to predict IL-6 at p<0.10. Model 4 considers all variables simultaneously to evaluate their independence in predicting IL-6.
Patterns of association were similar when BMI, infectious symptoms, and oral contraceptive use were considered simultaneously (Table 2, model 4), indicating that their associations with IL-6 were relatively independent. When individuals with current symptoms of infection were eliminated from the final model, the association between BMI and IL-6 strengthened (B=0.057, SE=0.017, p<0.001), while the association with oral contraceptive use was essentially the same (B=-1.143, SE=0.429, p<0.01).
Median IL-10 concentration in the sample was 7.56 pg/mL (3.09, 14.67). When individuals with symptoms of infectious disease were removed, median IL-10 was lower at 7.38 pg/mL (2.88, 14.51). Concentrations of IL-10 were slightly, but not significantly, lower in females (7.09 pg/mL, 2.71, 13.57) than in males (7.88 pg/mL, 3.51, 15.15).
As with IL-6, BMI was positively associated with IL-10, and was the strongest predictor among the adiposity variables (Table 3, model 1). Infectious symptoms were also strongly and positively associated with IL10 (Table 3, model 2). Oral contraceptive use was associated with lower IL-10, while urbanicity was negatively associated with IL-10. Formal schooling was marginally associated with higher IL-10. Coefficients were similar when these variables were considered simultaneously, suggesting independent associations with IL-10. Years of schooling was an exception, which was attenuated in the full model. When individuals with current symptoms of infection were eliminated from the final model, the association between BMI and IL-10 strengthened (B=0.037, SE=0.013, p<0.01), while associations with oral contraceptive use (B=-0.310, SE=0.327, p=0.3) and education were attenuated (B=0.136, SE=0.109, p=0.2). The association with urbanicity remained essentially unchanged (B=-0.007, SE=0.003, p<0.05).
Table 3.
Results of Tobit regression models predicting log-transformed IL-10 (N=1,569).
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
|---|---|---|---|---|---|---|---|---|
| B | SE | B | SE | B | SE | B | SE | |
| Female | -0.209** | 0.076 | -0.232** | 0.075 | -0.202** | 0.076 | -0.183* | 0.076 |
| BMI (kg/m2) | 0.024* | 0.012 | 0.029* | 0.012 | ||||
| Symptoms of infection (0, 1) | 0.302** | 0.107 | 0.300** | 0.106 | ||||
| Oral contraception (0, 1) | -0.637* | 0.298 | -0.666* | 0.297 | ||||
| Urbanicity (0-70) | -0.007** | 0.003 | -0.008** | 0.003 | ||||
| Education (yrs) | 0.161+ | 0.096 | 0.153 | 0.096 | ||||
| Constant | 0.102 | 0.468 | 0.573*** | 0.052 | 0.885*** | 0.124 | 0.251 | 0.273 |
| Model p value | <0.01 | <0.001 | <0.001 | <0.001 | ||||
p<0.10;
p<0.05;
p<0.01;
p<0.001
Note: Models 1-3 consider gender as well as the separate associations of adiposity (model 1), pathogen exposure (model 2), and health behavior variables (model 3) shown in bivariate analyses to predict IL-10 at p<0.10. Model 4 considers all variables simultaneously to evaluate their independence in predicting IL-10.
Associations among IL-6, IL-10, and CRP
Concentrations of IL-6 and IL-10 were each positively associated with CRP. Spearman rank correlation indicates a moderately strong and significant association between IL-6 and CRP for the entire sample (rho=0.26, p<0.001). The correlation was reduced when individuals with symptoms of infectious disease were removed (rho=0.23, p<0.001), but was substantially higher in the subset of individuals with infectious symptoms at the time of blood collection (rho=0.36, p<0.001).
The correlation between IL-10 and CRP was significant but relatively weak (rho=0.10, p<0.001), and was similar in magnitude when individuals with symptoms of infectious disease were excluded (rho=0.10, p<0.001), or considered separately (rho=0.09, p=0.17). Patterns of association between IL-6/IL-10 and CRP were very similar for males and females.
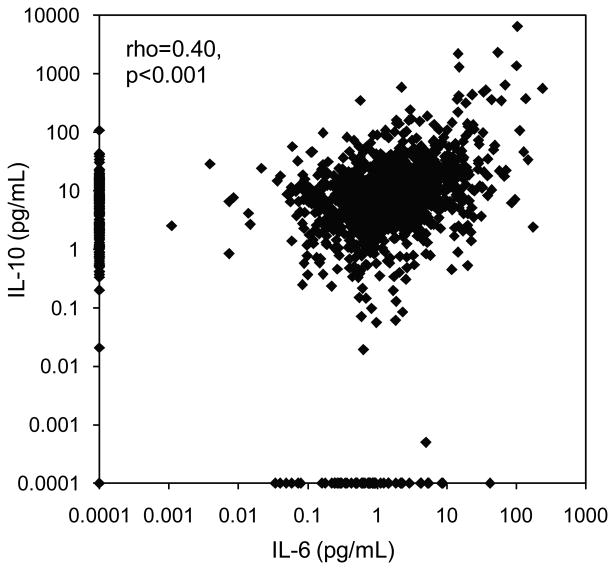
Concentrations of IL-6 and IL-10 were positively correlated across the entire range of measurement (Figure 1). Spearman rank correlation indicates a strong association between levels of IL-6 and IL-10 within individuals (rho=0.40, p<0.001). The correlation was attenuated slightly when individuals with symptoms of infectious disease were removed (rho=0.38, p<0.001), but was stronger in the subset of individuals with infectious symptoms at the time of blood collection (rho=0.46, p<0.001).
Figure 1.
Bivariate association between concentration of IL-6 and IL-10 in young adults in the Philippines (N=1,569).
Concentrations of IL-6 and IL-10 in comparison to other populations
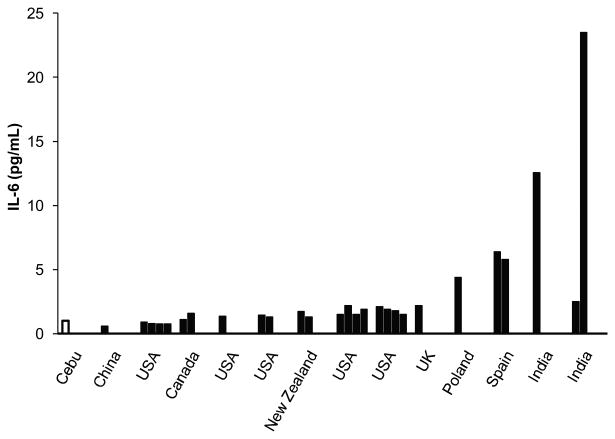
Concentrations of IL-6 in the Philippines were low relative to previously reported values in other populations (Table 4). Of studies reporting medians, the weighted average IL-6 concentration was 1.91 pg/mL, with a range of 0.61 to 23.5. For studies reporting means, the weighted average IL-6 concentration was 2.83 pg/mL, and values ranged from a low of 0.39 to a high of 10.9. It should be emphasized that differences in study design complicate attempts to make valid comparisons of central tendency across populations. Figure 2 presents IL-6 results from Cebu in relation to the subset of prior studies in Table 4 likely to yield the most meaningful comparisons. Studies were included in Figure 2 if they reported median IL-6 concentrations, included healthy participants, did not focus exclusively on older adults (>60 yrs), and had a sample size of at least 25.
Table 4.
Prior research reporting concentrations of IL-6 (pg/mL) in healthy adults.
| Location | N | IL-6 median (IQR) |
IL-6 mean (SD) |
Age (yrs) mean (SD) |
BMI (kg/m2) mean (SD) |
Sample Characteristics | Reference |
|---|---|---|---|---|---|---|---|
| Bolivia | 394 | 5.2 (9.0) | 23.2 (2.9) | Healthy adults | (Vasunilashorn et al. 2010) | ||
| Canada | 102 | 1.12 (.77-1.6) | 28.1 (5.5) | 25.8 (4.5) | Healthy young adult males | (Cartier et al. 2009) | |
| Canada | 106 | 1.6 (1.09-2.28) | 55.8 (6.7) | 27.4 (3.9) | Healthy middle-aged males | (Cartier et al. 2009) | |
| China | 30 | .61 (0-4.42) | 34 (8) | Healthy adults | (Wong et al. 2001) | ||
| France | 8 | .39 (.06) | 42 (5) | 20.6 (.6) | Healthy adult females | (Bastard et al. 2000) | |
| France | 14 | 2.78 (.30) | 45 (4) | 39.5 (1.1) | Healthy adult females (android obesity) | (Bastard et al. 2000) | |
| India | 50 | 12.56 (1.8-77.6) | 45 (11) | 22.5 (4.4) | Healthy adults | (Deepa et al. 2006) | |
| India | 40 | 7.15 (3.92–22.4) | 38 | 23.3 | Healthy adults (Urban middle-class) | (Yudkin et al. 1999b) | |
| India | 28 | 23.5 (6.60–26.9) | 35 | 22.3 | Healthy adults (Urban slum) | (Yudkin et al. 1999b) | |
| India | 43 | 2.50 (1.62–14.5) | 28 | 18.7 | Healthy adults (Rural Village) | (Yudkin et al. 1999b) | |
| Ireland | 29 | .56 (.26) | 31.0 (10.2) | Healthy white adults | (O'Donovan et al. 2010) | ||
| Italy | 20 | 1.36 (.6) | 31 (1.7) | 23.5 (.8) | Healthy young adults | (Targher et al. 2001) | |
| Italy | 34 | 2.6 (1.3) | 32 (7) | Healthy pre-menopausal women | (Cioffi et al. 2002) | ||
| Italy | 48 | 10.9 (4.1) | 54.8 (6) | Healthy post-menopausal women | (Cioffi et al. 2002) | ||
| Italy | 20 | 2 (.7) | 35 (4) | 24.2 (1.1) | Healthy young adults | (Esposito et al. 2002) | |
| Italy | 60 | 4.1 (2.0-9.0) | 35 (5.1) | 34.7 (2.4) | Healthy obese females (Control Diet) | (Esposito et al. 2003a) | |
| Italy | 60 | 4.3 (1.9-9.0) | 34.2 (4.8) | 35 (2.3) | Healthy obese females (Intervention Diet) | (Esposito et al. 2003a) | |
| Japan | 382 | 1.7 (1.99) | 55.5 | 22.58 (2.96) | Healthy adults | (Coe et al. 2010) | |
| Korea | 312 | 1.1 (.5-1.9) | 70 (5) | 24.6 (3.1) | Healthy older adults | (Choi et al. 2007) | |
| New Zealand | 38 | 1.73 (.93-2.67) | 39 (5) | Healthy adult females (Asian Indian immigrants) | (Rush et al. 2007) | ||
| New Zealand | 41 | 1.31 (.8- 2.24) | 39 (5) | Healthy adult males (Asian Indian immigrants) | (Rush et al. 2007) | ||
| Poland | 24 | 5.3 (.5-16.6) | 5.1 (3) | Healthy adults | (Robak 1998) | ||
| Poland | 51 | 1.7 (.6) | 51 (11) | Healthy middle-aged adults | (Wykretowicz et al. 2004) | ||
| Poland | 28 | 4.4 (.5-14.6) | 4.49 (2.91) | 63 | Healthy adults | (Urbańska-Ryś 2000) | |
| Spain | 132 | 6.4 (1-13.1) | 40.5 (11) | 25.4 (4) | Healthy white adult males | (Fernandez-Real et al. 2001) | |
| Spain | 96 | 5.8 (1.8-14) | 37.5 (8.9) | 24.6 (4.4) | Healthy white adult females | (Fernandez-Real et al. 2001) | |
| USA | 202 | 1.46 (1.04-2.28) | 59.1 (8.8) | 24.9 (3) | Healthy middle-aged males | (Ridker et al. 2000b) | |
| USA | 244 | 1.3 (1-2.03) | 59.3 | 26.0 | Healthy middle-aged females | (Ridker et al. 2000a) | |
| USA | 362 | 1.38 (0.91-2.05) | 54.7 | 25.6 | Healthy middle-aged females | (Pradhan et al. 2001a) | |
| USA | 850 | 1.84 (1.31-2.83) | 73.9 (2.9) | 26.9 (3.7) | Healthy older white males | (Visser et al. 2002) | |
| USA | 494 | 2.06 | 73.5 (2.8) | 27.1 (4.2) | Healthy older black males | (Visser et al. 2002) | |
| USA | 764 | 1.63 | 73.5 (2.8) | 25.9 (4.5) | Healthy older white females | (Visser et al. 2002) | |
| USA | 638 | 1.92 | 73.3 (3) | 29.4 (5.8) | Healthy older black females | (Visser et al. 2002) | |
| USA | 304 | 1.47 (1.05-2.15) | 69 (6.6) | 26.6 (4.48) | Healthy older females | (Pradhan et al. 2002) | |
| USA | 50 | 1.9 (.22) | 30.2 (10.1) | Healthy young adults | (Miller et al. 2003) | ||
| USA | 176 | 0.913 (.64-1.49) | 44.7 (3.91) | 25.5 (3.04) | Healthy white middle-aged males | (Haddy et al. 2003) | |
| USA | 179 | 0.807 (.57-1.31) | 43.1 (4.00) | 24.1 (4.14) | Healthy white middle-aged females | (Haddy et al. 2003) | |
| USA | 224 | 0.78 (.47-1.32) | 16 (3.86) | 20.1 (3.15) | Healthy white teenage males | (Haddy et al. 2003) | |
| USA | 212 | 0.78 (.47-1.48) | 15.9 (3.85) | 20.4 (3.08) | Healthy white teenage females | (Haddy et al. 2003) | |
| USA | 11 | 0.7 | 0.74 (.12) | 38.0 (13.27) | 24.4 (3.61) | Healthy adults | (Dhabhar et al. 2009) |
| USA | 343 | 3.10 | 63.7 | 29.0 | Healthy adults (low cumulative socioeconomic position) | (Loucks et al. 2010) | |
| USA | 553 | 2.88 | 58.8 | 28.5 | Healthy adults (medium cumulative socioeconomic position) | (Loucks et al. 2010) | |
| USA | 617 | 2.69 | 57.9 | 27.7 | Healthy adults (high cumulative socioeconomic position) | (Loucks et al. 2010) | |
| USA | 976 | 2.79 (2.3) | 58.4 | 29.1 | Healthy white adults | (Coe et al. 2010) | |
| USA | 233 | 4.16 (3.7) | 53.6 | 32.9 | Healthy black adults | (Coe et al. 2010) | |
| USA | 9 | 3.4 (.8) | 34.3 (8.9) | 25.8 | Healthy adults | (Alesci et al. 2005) | |
| USA | 944 | 1.5 (.9-2.5) | 45.7 (3.4) | 27 (6.4) | Healthy middle-aged white females | (Gruenewald et al. 2009) | |
| USA | 857 | 2.2 (1.3-3.4) | 44.7 (3.9) | 31.4 (7.3) | Healthy middle-aged black females | (Gruenewald et al. 2009) | |
| USA | 868 | 1.5 (0.9-2.4) | 45.7 (3.4) | 28.5 (6) | Healthy middle-aged white males | (Gruenewald et al. 2009) | |
| USA | 597 | 1.9 (1.1-3.0) | 44.6 (3.7) | Healthy middle-aged black males | (Gruenewald et al. 2009) | ||
| USA | 1744 | 3.6 (2.85) | 62 (10) | 27.6 (5.8) | Healthy older adult females | (Loucks et al. 2006) | |
| USA | 1487 | 3.95(3.26) | 62 (10) | 28.8 (4.5) | Healthy older adult males | (Loucks et al. 2006) | |
| USA | 1028 | 2.8 (2.8) | 58 (11.6) | 29.2 (6) | Healthy older adults | (Morozink et al. 2010) | |
| USA | 3044 | 1.83 (1.27-2.79) | 74.2 (2.8) | 27.4 (2.9) | Healthy older black and white adults | (Koster et al. 2006) | |
| USA | 822 | 2.73(2.81) | 58.7 (11.7) | 29.1 (6.0) | Healthy white adults | (Slopen et al. 2010) | |
| USA | 177 | 4.11(3.45) | 54.2 (10.7) | 33.4 (8.9) | Healthy black adults | (Slopen et al. 2010) | |
| USA | 104 | 2.1 (1.8-2.3) | 53.8 | 29.6 (.5) | Healthy middle-aged males (Mediterranean Diet Score: 0-3) | (Dai et al. 2008) | |
| USA | 70 | 1.9 (1.6-2.2) | 54.3 | 29.5 (.6) | Healthy middle-aged males (Mediterranean Diet Score:4) | (Dai et al. 2008) | |
| USA | 81 | 1.8 (1.6-2.1) | 54.5 | 29.5 (.5) | Healthy middle-aged males (Mediterranean Diet Score:5) | (Dai et al. 2008) | |
| USA | 90 | 1.5 (1.3-1.7) | 54.8 | 28.5 (.5) | Healthy middle-aged males (Mediterranean Diet Score: 6-9) | (Dai et al. 2008) | |
| USA | 58 | 2.6 (1.5) | 30 (7) | 32.5 (6.5) | Healthy Pima Indian adults | (Vozarova et al. 2001) | |
| USA | 400 | .93 | 54.5 (6.6) | 24.4 (3.9) | Healthy middle-aged females | (Sesso et al. 2007) | |
| UK | 1520 | 2 (1.48) | 59.9 (5.7) | 26.2 (4.2) | Healthy adults (low positive affect) | (Steptoe et al. 2008) | |
| UK | 675 | 1.84 (1.22) | 59.8 (5.6) | 26.1 (4.2) | Healthy adults (moderate positive affect) | (Steptoe et al. 2008) | |
| UK | 678 | 1.71 (1.03) | 61.8 (5.7) | 26.3 (3.9) | Healthy adults (high positive affect) | (Steptoe et al. 2008) | |
| UK | 48 | 1.15 (.62) | 52.5 (2.6) | 25.7 (2.9) | Healthy middle-aged males (high SES) | (Steptoe et al. 2002) | |
| UK | 40 | 1.40 (.67) | 51.1 (2.3) | 25.7 (4.3) | Healthy middle-aged females (high SES) | (Steptoe et al. 2002) | |
| UK | 42 | 1.26 (.82) | 51.8 (2.4) | 25.9 (4.1) | Healthy middle-aged males (intermediate SES) | (Steptoe et al. 2002) | |
| UK | 35 | 1.04 (.57) | 52.5 (2.9) | 25.0 (3.9) | Healthy middle-aged females (intermediate SES) | (Steptoe et al. 2002) | |
| UK | 35 | 1.17 (.72) | 53.7 (2.8) | 25.7 (3.2) | Healthy middle-aged males (low SES) | (Steptoe et al. 2002) | |
| UK | 30 | 1.66 (1.2) | 52.1 (2.8) | 25.4 (4.1) | Healthy middle-aged females (low SES) | (Steptoe et al. 2002) | |
| UK | 107 | 2.19 (1.18-4.40) | 59 (10.9) | 25.9 (4.5) | Healthy middle-aged white adults | (Yudkin et al. 1999a) | |
| UK | 96 | .6 (.1) | 23.7 (.5) | 24 (.3) | Healthy young adult males | (Miles et al. 2008) | |
| UK | 31 | .8 (.1) | 53.6 (1) | 26.8 (.5) | Healthy middle-aged males | (Miles et al. 2008) | |
| UK | 35 | 1.4 (.2) | 64.7 (.8) | 28.2 (.8) | Healthy older males | (Miles et al. 2008) | |
| UK | 17 | 1.54 (.5) | 46.1 (6.2) | 26.1 (2.5) | Healthy middle-aged males (high SES) | (Brydon et al. 2004) | |
| UK | 21 | 1.24 (.8) | 39.7 (6.7) | 25.2 (2.7) | Healthy middle-aged males (low SES) | (Brydon et al. 2004) |
Figure 2.
Median IL-6 concentration in Cebu in comparison with prior studies reporting median values for healthy adults, middle-age and younger, with a sample size of N>25. References for the included studies can be found in Table 2.
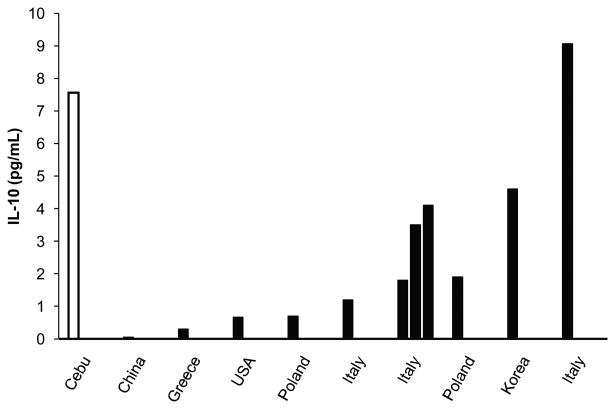
Only a handful of studies have investigated IL-10 concentrations in healthy adults. Of the nine studies reporting median values, the weighted average IL-10 concentration was 3.17 pg/mL. Only one study in Italy (Galizia et al. 2002) reported median IL-10 concentrations higher than Cebu, with the other studies reporting substantially lower concentrations. Figure 3 compares results from Cebu with all prior studies reporting median IL-10 concentrations. Of the seven studies reporting IL-10 means, the weighted average IL-10 concentration was 8.64 pg/mL. For purposes of comparison, the mean IL-10 concentration in our Cebu sample was 25.48 pg/mL. Due to the high degree of skew in the distribution of IL-10, median values better represent the central tendency in our dataset.
Figure 3.
Median IL-10 concentration in Cebu in comparison with prior studies reporting median values for healthy adults. References for the included studies can be found in Table 3.
It is important to note that prior research has demonstrated substantial variation in cytokine concentrations across assay methods (Eishal and McCoy 2006). The vast majority of the studies listed in tables 4 and 5 applied enzyme-linked immunosorbent assay (ELISA) methods using reagents supplied by R&D Systems. In a recent, direct comparison of results produced by R&D Systems ELISA and the high sensitivity multiplex immunoassay protocol applied in our study, concentrations of IL-6 were found to be significantly lower when samples were analyzed with the R&D ELISA protocol (Dossus et al. 2009). Differences in assay methods are therefore not likely to account for the low concentrations of IL-6 in our sample. Similar comparisons have not been reported for IL-10, and we therefore cannot eliminate assay method as a contributor to the high IL-10 in our sample.
Table 5. Prior research reporting concentrations of IL-10 (pg/mL) in healthy adults.
| Location | N | IL-10 | IL-10 | Age (yrs) | BMI | Sample Characterstics | Reference |
|---|---|---|---|---|---|---|---|
| median | mean (SD) | mean | (kg/m2) | ||||
| (IQR) | (SD) | mean | |||||
| (SD) | |||||||
| China | 30 | .05 (0-4.26) | 34 (8) | Healthy adults | (Wong et al. 2001) | ||
| Greece | 16 | .3 (.01-1.1) | 54 (5) | Healthy middle-aged adults | (Tziakas et al. 2003) | ||
| Italy | 50 | 1.2 (.7-2.9) | 35.9 (4.9) | 23.8(1.2) | Healthy young females | (Esposito et al. 2003b) | |
| Italy | 34 | 13.5 (8) | 32 (7) | Healthy pre-menopausal females | (Cioffi et al. 2002) | ||
| Italy | 48 | 16 (6.6) | 54.8 (6) | Healthy post-menopausal females | (Cioffi et al. 2002) | ||
| Italy | 25 | 9.07 (7.4-12) | 9.26 (1.5) | Healthy adults | (Galizia et al. 2002) | ||
| Italy | 20 | 4.1 (3.5-4.8) | 46 (11) | 25.2 (2.2) | Healthy adult females | (Manigrasso et al. 2005) | |
| Italy | 20 | 3.5 (2.9-4.3) | 49 (11) | 33.4 (2.6) | Healthy adult females (gynoid obesity) | (Manigrasso et al. 2005) | |
| Italy | 64 | 1.8 (1.2-3.3) | 49 (14) | 37.1(5.3) | Healthy adult females (android obesity) | (Manigrasso et al. 2005) | |
| Japan | 11 | 1.1 (.6) | 45 | Healthy adults | (Kakumu et al. 1997) | ||
| Korea | 312 | 4.6 (3.9-5.5) | 70 (5) | 24.6 (3.1) | Healthy older adults | (Choi et al. 2007) | |
| Poland | 51 | 9 (3.0) | 51 (11) | Healthy middle-aged adults | (Wykretowicz et al. 2004) | ||
| Poland | 30 | .92 (.17) | 42.5 (8.2) | 24.7 (2.9) | Healthy middle-aged adults | (Myśliwska 2005) | |
| Poland | 93 | .7 (.6-1.0) | 28.1 (8.4) | 26.1 (4.8) | Healthy young adults | (Straczkowski et al. 2005) | |
| Poland | 28 | 1.9 (.9-21.5) | 3.23(3.97) | 63 | Healthy adults | (Urbańska-Ryś 2000) | |
| USA | 11 | .67 | .83 (.19) | 38 (13.3) | 24.4 (3.6) | Healthy adults | (Dhabhar et al. 2009) |
Discussion
In this study we investigated concentrations of IL-6 and IL-10 in healthy young adults in the Philippines to advance comparative research on the regulation of inflammation. Our study is among the largest analyses to date of these important inflammatory cytokines, and we find lower concentrations of IL-6, and substantially elevated concentrations of IL-10, in comparison to prior research. These results suggest the possibility of substantial population differences in the regulation of the inflammation, and point toward productive directions for future research.
Although concentrations of IL-6 in Cebu are among the lowest on record, patterns of association between IL-6 and other variables are similar to prior research in other settings. IL-6 is positively correlated with CRP, as expected given the well-established, potent effect of IL-6 on hepatic production of CRP (Castell et al. 1989; Friedman and Herd 2010; Harris 1999). Similarly, symptoms of infectious disease predict elevated IL-6, likely reflecting acute pro-inflammatory cytokine production by monocytes and other activated immune cells in response to pathogenic challenge. Finally, BMI is positively associated with IL-6, despite the relatively low levels of overweight/obesity and the restricted range of BMI variation in our sample. Adipose tissue is an important source of circulating IL-6, and prior research in post- nutrition transition populations with higher rates of overweight/obesity reports similar associations between adiposity and inflammation (Bermudez et al. 2002; Esposito et al. 2003a; Howren et al. 2009).
Relatively few studies have taken a population-based approach to investigating the correlates of IL-10, and our study is the largest reported analysis of this key anti-inflammatory cytokine. Concentrations in the Philippines stand out as high in comparison to prior studies in other populations. As with IL-6, IL-10 is positively—although weakly—correlated with CRP. It is also positively associated with BMI and symptoms of infectious disease.
While this pattern of results may appear inconsistent with IL-10's putative role as an anti-inflammatory cytokine, it is important to note that the counter-regulatory activity of IL-10 may only be necessary when pro-inflammatory pathways are activated. The strong and positive correlation between IL-10 and IL-6—particularly among individuals with active infections—supports this interpretation, and is consistent with prior research reporting positive associations between IL-10 and IL-6 (Dhabhar et al. 2009; Urbańska-Ryś 2000). The low concentrations of IL-6 and/or high IL-10 in Cebu may reflect a balance of pro- to anti-inflammatory signaling that differs significantly from other populations, perhaps explaining the exceptionally low concentrations of CRP in the Philippines (McDade et al. 2010; McDade et al. 2009).
The origin of this inflammatory phenotype is not clear, and likely reflects ecological, developmental, and/or genetic factors that influence inflammation. For example, micronutrients common in the Filipino diet may reduce pro-inflammatory cytokine production (Barber et al. 1999; Walston et al. 2006), and similar dietary differences have been proposed as a partial explain for low concentrations of IL-6 in Japanese adults compared to adults in the US (Coe et al. 2010). In addition, we have suggested that low CRP concentrations among Filipino adults may trace back to relatively high levels of microbial exposure in infancy (McDade et al. 2010), consistent with a wider body of research on the “hygiene” or “old friends” hypothesis (Radon et al. 2004; Rook 2010; Rook and Stanford 1998; Yazdanbakhsh et al. 2002). Similar exposures early in development may also shape the balance of pro- and anti-inflammatory signaling in Cebu. Lastly, several polymorphisms are known to influence production of IL-6 and IL-10 (Fishman et al. 1998; Gibson et al. 2001), although we have recently shown that genetic differences cannot explain population differences in CRP concentration (Curocichin et al. under review). Each of these factors represents promising areas for future research into population variation in the regulation of inflammation.
The long-term health implications of our findings are not known, but to the extent that inflammation contributes to the pathophysiology of cardiovascular, metabolic, or atopic/autoimmune diseases, low IL-6 and high IL-10 may represent a favorable balance of pro- vs. anti-inflammatory signaling. In a recent study of obesity and the metabolic syndrome, concentrations of IL-6 and IL-10 were elevated in obese women compared to non-obese controls (Esposito et al. 2003b). However, obese women with symptoms of the metabolic syndrome had lower levels of IL-10—levels that were indistinguishable from non-obese women. This pattern suggests that the metabolic syndrome may be characterized by dysregulated inflammation, in which an important anti-inflammatory signal is absent. Similarly, in a prospective study of patients with acute coronary syndromes, baseline IL-10 concentration was a significant predictor of death or myocardial infarction during 6 months of follow up, but only in conjunction with baseline CRP: 6.8% of patients with high CRP and high IL-10 experienced an adverse outcome, compared to 21.8% of patients with high CRP but low IL-10 (Heeschen et al. 2003). These findings implicate uncontrolled inflammation, rather than inflammation in general, in adverse health outcomes, and suggest that additional research on IL-10 may be a particularly promising direction for future investigation.
Limitations of our study include the use of a single IL-6 and IL-10 measure, which makes it more difficult to differentiate acute episodes of inflammation from chronic, low-grade inflammatory activity. We also do not evaluate other cytokines (e.g., IL-1β, TNFα) which play important roles in the regulation of inflammation and should be considered in subsequent comparative research. In addition, differences in sampling strategy, study design, and assay methodology make comparisons with prior studies difficult, and conclusions drawn from such comparisons should be considered tentative. We hope the findings reported here will motivate future research into population differences in the regulation of inflammation and their genetic, ecological, and developmental antecedents.
Acknowledgments
Funding for this study was provided by the National Institutes of Health (RO1 HL085144; 5 RO1 TW05596); biomarker data collection was supported by pilot funds from the Interdisciplinary Obesity Center (RR20649) and the Center for Environmental Health and Susceptibility (ES10126; project 7-2004-E).
Literature Cited
- Adair LS, Gultiano S, Suchindran C. Twenty-year trends in Filipino women's weight reflect substantial secular and age effects. Journal of Nutrition. 2011 doi: 10.3945/jn.110.134387. (early access) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adair LS, Kuzawa CW, Borja J. Maternal energy stores and diet composition during pregnancy program adolescent blood pressure. Circulation. 2001:1034–1039. doi: 10.1161/hc3401.095037. [DOI] [PubMed] [Google Scholar]
- Adair LS, Popkin BM, John AS, Guilkey DK, Gultiano S, Borja JB, Perez L, Kuzawa CW, McDade T, Hindin M. Cohorts Profile: The Cebu Longitudinal Health and Nutrition Survey. Int J Epid. 2010 doi: 10.1093/ije/dyq085. (early access) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alesci S, Martinez PE, Kelkar S, Ilias I, Ronsaville DS, Listwak SJ, Ayala AR, Licinio J, Gold HK, Kling MA, et al. Major Depression is associated with significant diurnal elevations in plasma interleukin-6 levels, a shift of its circadian rhythm, and loss of physiological complexity in its secretion: clinical implications. J Clin Endocrinol Metab. 2005;90:2522–2530. doi: 10.1210/jc.2004-1667. [DOI] [PubMed] [Google Scholar]
- Ballou SP, Kushner I. C-reactive protein and the acute phase response. Adv Intern Med. 1992;37:313–336. [PubMed] [Google Scholar]
- Barber MD, Ross JA, Preston T, Shenkin A, Fearon KCH. Fish oil–enriched nutritional supplement attenuates progression of the acute-phase response in weight-losing patients with advanced pancreatic cancer. J Nutr. 1999;129:1120–1125. doi: 10.1093/jn/129.6.1120. [DOI] [PubMed] [Google Scholar]
- Bastard JP, Jardel C, Bruckert E, Blondy P, Capeau J, Laville M, Vidal H, Hainque B. Elevated levels of interleukin 6 are reduced in serum and subcutaneous adipose tissue of obese women after weight loss. J Clin Endocrinol Metab. 2000;85:3338–3342. doi: 10.1210/jcem.85.9.6839. [DOI] [PubMed] [Google Scholar]
- Bermudez EA, Rifai N, Buring J, Manson JE, Ridker PM. Interrelationships among circulating interleukin-6, C-Reactive Protein, and traditional cardiovascular risk Factors in Women. Arterioscler Thromb Vasc Biol. 2002;22:1668–1673. doi: 10.1161/01.atv.0000029781.31325.66. [DOI] [PubMed] [Google Scholar]
- Blackwell AD, Snodgrass JJ, Madimenos FC, Sugiyama LS. Life history, immune function, and intestinal helminths: Trade-offs among immunoglobulin E, C-reactive protein, and growth in an Amazonian population. Am J Hum Biol. 2010;22:836–848. doi: 10.1002/ajhb.21092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brydon L, Edwards S, Mohamed-Ali V, Steptoe A. Socioeconomic status and stress-induced increases in interleukin-6. Brain Behav Immun. 2004;18:281–290. doi: 10.1016/j.bbi.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Cartier A, Côté M, Lemieux I, Pérusse L, Tremblay A, Bouchard C, Després JP. Age-related differences in inflammatory markers in men: contribution of visceral adiposity. Metabolism. 2009;58:1452–1458. doi: 10.1016/j.metabol.2009.04.025. [DOI] [PubMed] [Google Scholar]
- Castell JV, Gómez-Lechón MJ, David M, Andus T, Geiger T, Trullenque R, Fabra R, Heinrich PC. Interleukin-6 is the major regulator of acute phase protein synthesis in adult human hepatocytes. FEBS Lett. 1989;242:237–239. doi: 10.1016/0014-5793(89)80476-4. [DOI] [PubMed] [Google Scholar]
- Choi KM, Ryu OH, Lee KW, Kim HY, Seo JA, Kim SG, Kim NH, Choi DS, Baik SH. Serum adiponectin, interleukin-10 levels and inflammatory markers in the metabolic syndrome. Diabetes Res Clin Pract. 2007;75:235–240. doi: 10.1016/j.diabres.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Cioffi M, Esposito K, Vietri MT, Gazzerro P, D'Auria A, Ardovino I, Puca GA, Molinari AM. Cytokine pattern in postmenopause. Maturitas. 2002;41:187–192. doi: 10.1016/s0378-5122(01)00286-9. [DOI] [PubMed] [Google Scholar]
- Coe CL, Love GD, Karasawa M, Kawakami N, Kitayama S, Markus HR, Tracy RP, Ryff CD. Population differences in proinflammatory biology: Japanese have healthier profiles than Americans. Brain Behav Immun. 2010 doi: 10.1016/j.bbi.2010.11.013. (early access) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curocichin G, Wu Y, McDade TW, Kuzawa CK, Borja J, Qin L, Lange EM, Adair LS, Lange LA, Mohlke KL. Single nucleotide polymorphisms at five loci are associated with C-reactive protein levels in a cohort of young adult Filipinos. doi: 10.1038/jhg.2011.106. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahly DL, Adair LS. Quantifying the urban environment: A scale measure of urbanicity outperforms the urban-rural dichotomy. Soc Sci Med. 2007;64:1407–1419. doi: 10.1016/j.socscimed.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J, Miller AH, Bremner JD, Goldberg J, Jones L, Shallenberger L, Buckham R, Murrah NV, Veledar E, Wilson PW, et al. Adherence to the mediterranean diet is inversely associated with circulating interleukin-6 among middle-aged men: a twin study. Circulation. 2008;117:169–175. doi: 10.1161/CIRCULATIONAHA.107.710699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deepa R, Velmurugan K, Arvind K, Sivaram P, Sientay C, Uday S, Mohan V. Serum levels of interleukin 6, C-reactive protein, vascular cell adhesion molecule 1, and monocyte chemotactic protein 1 in relation to insulin resistance and glucose intolerance--the Chennai Urban Rural Epidemiology Study (CURES) Metabolism. 2006;55:1232–1238. doi: 10.1016/j.metabol.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, Burke HM, Epel ES, Mellon SH, Rosser R, Reus VI, Wolkowitz OM. Low serum IL-10 concentrations and loss of regulatory association between IL-6 and IL-10 in adults with major depression. J Psychiatr Res. 2009;43:962–969. doi: 10.1016/j.jpsychires.2009.05.010. [DOI] [PubMed] [Google Scholar]
- Dossus L, Becker S, Achaintre D, Kaaks R, Rinaldi S. Validity of multiplex-based assays for cytokine measurements in serum and plasma from “non-diseased” subjects: Comparisons with ELISA. J Immunol Methods. 2009;350:125–132. doi: 10.1016/j.jim.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Du Clos TW. Function of C-reactive protein. Ann Med. 2000;32:274–278. doi: 10.3109/07853890009011772. [DOI] [PubMed] [Google Scholar]
- Eishal MF, McCoy JP. Multiplex bead array assays: Performance evaluation and comparison of sensitivity to ELISA. Methods. 2006;38:317–323. doi: 10.1016/j.ymeth.2005.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, Quagliaro L, Ceriello A, Giugliano D. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002;106:2067–2072. doi: 10.1161/01.cir.0000034509.14906.ae. [DOI] [PubMed] [Google Scholar]
- Esposito K, Pontillo A, Di Palo C, Giugliano G, Masella M, Marfella R, Giugliano D. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women. JAMA. 2003a;289:1799–1804. doi: 10.1001/jama.289.14.1799. [DOI] [PubMed] [Google Scholar]
- Esposito K, Pontillo A, Giugliano F, Giugliano G, Marfella R, Nicoletti G, Giugliano D. Association of low interleukin-10 levels with the metabolic syndrome in obese women. J Clin Endocrinol Metab. 2003b;88:1055–1058. doi: 10.1210/jc.2002-021437. [DOI] [PubMed] [Google Scholar]
- Fernandez-Real JM, Vayreda M, Richart C, Gutierrez C, Broch M, Vendrell J, Ricart W. Circulating interleukin 6 levels, blood pressure, and insulin sensitivity in apparently healthy men and women. J Clin Endocrinol Metab. 2001;86:1154–1159. doi: 10.1210/jcem.86.3.7305. [DOI] [PubMed] [Google Scholar]
- Festa A, D'Agostino R, Jr, Howard G, Mykkanen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome : the insulin resistance atherosclerosis study (IRAS) Circulation. 2000;102:42–47. doi: 10.1161/01.cir.102.1.42. [DOI] [PubMed] [Google Scholar]
- Fishman D, Faulds G, Jeffery R, Mohamed-Ali V, Yudkin JS, Humphries S, Woo P. The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. J Clin Invest. 1998;102:1369–1376. doi: 10.1172/JCI2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman EM, Herd P. Income, education, and inflammation: differential associations in a national probability sample (the MIDUS study) Psychosom Med. 2010;72:290–30. doi: 10.1097/PSY.0b013e3181cfe4c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galizia G, Orditura M, Romano C, Lieto E, Castellano P, Pelosio L, Imperatore V, Catalano G, Pignatelli C, De Vita F. Prognostic significance of circulating IL-10 and IL-6 serum levels in colon cancer patients undergoing surgery. Clin Immunol. 2002;102:169–178. doi: 10.1006/clim.2001.5163. [DOI] [PubMed] [Google Scholar]
- Gibson A, Edberg J, Wu J, Westendorp R, Huizinga T, Kimberly R. Novel single nucleotide polymorphisms in the distal IL-10 promoter affect IL-10 production and enhance the risk of systemic lupus erythematosus. J Immunol. 2001;166:3915–3922. doi: 10.4049/jimmunol.166.6.3915. [DOI] [PubMed] [Google Scholar]
- Greene WH. Econometric analysis. New Jersey, NJ: Prentice Hal; 2000. [Google Scholar]
- Gruenewald TL, Cohen S, Matthews KA, Tracy R, Seeman TE. Association of socioeconomic status with inflammation markers in black and white men and women in the Coronary Artery Risk Development in Young Adults (CARDIA) study. Soc Sci Med. 2009;69:451–459. doi: 10.1016/j.socscimed.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurven M, Kaplan H, Winking J, Finch C, Crimmins EM. Aging and inflammation in two epidemiological worlds. J Gerontol A Biol Sci Med Sci. 2008;63:196–199. doi: 10.1093/gerona/63.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddy N, Sass C, Droesch S, Zaiou M, Siest G, Ponthieux A, Lambert D, Visvikis S. IL-6, TNF-[alpha] and atherosclerosis risk indicators in a healthy family population: the STANISLAS cohort. Atherosclerosis. 2003;170:277–283. doi: 10.1016/s0021-9150(03)00287-9. [DOI] [PubMed] [Google Scholar]
- Harris TBF L, Tracy RP, Corti MC, Wacholder S, Ettinger WH, Heimovitz H, Cohen HJ, Wallace R. Associations of elevated interleukin-6 and C-Reactive protein levels with mortality in the elderly. Am J Med. 1999;106:506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- Heeschen C, Dimmeler S, Hamm CW, Fichtlscherer S, Boersma E, Simoons ML, Zeiher AM, for the CAPTURE Study Investigators Serum level of the anti-inflammatory cytokine interleukin-10 is an important prognostic determinant in patients with acute coronary syndromes. Circulation. 2003;107:2109–2114. doi: 10.1161/01.CIR.0000065232.57371.25. [DOI] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-Reactive Protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71:171–86. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Kakumu S, Okumura A, Ishikawa T, Yano M, Enomoto A, Nishimura H, Yoshioka K, Yoshikai Y. Serum levels of IL-10, IL-15 and soluble tumour necrosis factor-alpha (TNF-α) receptors in type C chronic liver disease. Clin Exp Immunol. 1997;109:458–463. doi: 10.1046/j.1365-2249.1997.4861382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster A, Bosma H, Penninx BWJH, Newman AB, Harris TB, van Eijk JTM, Kempen GIJM, Simonsick EM, Johnson KC, Rooks RN, et al. Association of inflammatory markers with socioeconomic status. J Gerontol A Biol Sci Med Sci. 2006;61:284–290. doi: 10.1093/gerona/61.3.284. [DOI] [PubMed] [Google Scholar]
- Kumar R, Clermont G, Vodovotz Y, Chow CC. The dynamics of acute inflammation. J Theor Biol. 2004;230:145–155. doi: 10.1016/j.jtbi.2004.04.044. [DOI] [PubMed] [Google Scholar]
- Kuo HK, Bean JF, Yen CJ, Leveille SG. Linking C-reactive protein to late-life disability in the National Health and Nutrition Examination Survey (NHANES) 1999-2002. J Gerontol A Biol Sci Med Sci. 2006;61:380–387. doi: 10.1093/gerona/61.4.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002a;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- Lohman TG, Roche AF, Martorell R. Anthropometric Standardization Reference Manual. Champaign, IL: Human Kinetics Books; 1988. [Google Scholar]
- Loucks EB, Pilote L, Lynch JW, Richard H, Almeida ND, Benjamin EJ, Murabito JM. Life course socioeconomic position is associated with inflammatory markers: The Framingham Offspring Study. Soc Sci Med. 2010;71:187–195. doi: 10.1016/j.socscimed.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loucks EB, Sullivan LM, Agostino RB, Sr, Larson MG, Berkman LF, Benjamin EJ. Social networks and inflammatory markers in the Framigham heart study. J Biosoc Sci. 2006;38:835–842. doi: 10.1017/S0021932005001203. [DOI] [PubMed] [Google Scholar]
- Manigrasso MR, Ferroni P, Santilli F, Taraborelli T, Guagnano MT, Michetti N, Davi G. Association between circulating adiponectin and interleukin-10 levels in android obesity: effects of weight loss. J Clin Endocrinol Metab. 2005;90:5876–5879. doi: 10.1210/jc.2005-0281. [DOI] [PubMed] [Google Scholar]
- McDade T. Life history theory and the immune system: Steps toward a human ecological immunology. Yearb Phys Anthropol. 2003;4:100–125. doi: 10.1002/ajpa.10398. [DOI] [PubMed] [Google Scholar]
- McDade T, Rutherford JN, Adair L, Kuzawa C. Population differences in C-reactive protein concentration and associations with adiposity: Comparing young adults in the Philippines and the U.S. Am J Clin Nutr. 2009;89:1237–1245. doi: 10.3945/ajcn.2008.27080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade TW, Rutherford J, Adair L, Kuzawa CW. Early origins of inflammation: microbial exposures in infancy predict lower levels of C-reactive protein in adulthood. Proc R Soc Lond [Biol] 2010;277:1129–1137. doi: 10.1098/rspb.2009.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade TW, Rutherford JN, Adair L, Kuzawa C. Adiposity and pathogen exposure predict C-Reactive protein in Filipino women. J Nutr. 2008;138:2442–2447. doi: 10.3945/jn.108.092700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade TW, Worthman CM. Evolutionary process and the ecology of human immune function. Am J Hum Biol. 1999;11:705–717. doi: 10.1002/(SICI)1520-6300(199911/12)11:6<705::AID-AJHB1>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Miles EA, Rees D, Banerjee T, Cazzola R, Lewis S, Wood R, Oates R, Tallant A, Cestaro B, Yaqoob P, et al. Age-related increases in circulating inflammatory markers in men are independent of BMI, blood pressure and blood lipid concentrations. Atherosclerosis. 2008;196:298–305. doi: 10.1016/j.atherosclerosis.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Miller GE, Freedland KE, Carney RM, Stetler CA, Banks WA. Pathways linking depression, adiposity, and inflammatory markers in healthy young adults. Brain Behav Immun. 2003;17:276–285. doi: 10.1016/s0889-1591(03)00057-6. [DOI] [PubMed] [Google Scholar]
- Moore KW, Malefyt RD, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- Morozink JA, Friedman EM, Coe CL, Ryff CD. Socioeconomic and Psychosocial Predictors of Interleukin-6 in the MIDUS National Sample. Health Psychol. 2010;29:626–635. doi: 10.1037/a0021360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muehlenbein MP, Bribiescas RG. Testosterone-mediated immune functions and male life histories. Am J Hum Biol. 2005;17:527–558. doi: 10.1002/ajhb.20419. [DOI] [PubMed] [Google Scholar]
- Myśliwska JZ K, Semetkowska-Jurkiewicz E, Rachoń D, Suchanek H, Myśliwsk A. High levels of circulating interleukin-10 in diabetic nephropathy patients. Eur Cytokine Netw. 2005;16:117–122. [PubMed] [Google Scholar]
- Nurgalieva ZZ, Malaty HM, Graham DY, Almuchambetova R, Machmudova A, Kapsultanova D, Osato MS, Hollinger FB, Zhangabylov A. Helicobacter pylori infection in Kazakhstan: effect of water source and household hygiene. Am J Trop Med Hyg. 2002;67:201–206. doi: 10.4269/ajtmh.2002.67.201. [DOI] [PubMed] [Google Scholar]
- O'Donovan A, Hughes BM, Slavich GM, Lynch L, Cronin MT, O'Farrelly C, Malone KM. Clinical anxiety, cortisol and interleukin-6: Evidence for specificity in emotion-biology relationships. Brain Behav Immun. 2010;24:1074–1077. doi: 10.1016/j.bbi.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okosun IS, Chandra KMD, Boev A, Boltri JM, Choi ST, Parish DC, Dever GEA. Abdominal adiposity in U.S. adults: prevalence and trends. Prev Med. 2004;39:197–206. doi: 10.1016/j.ypmed.2004.01.023. [DOI] [PubMed] [Google Scholar]
- Papanicolaou DA, Wilder RL, Manolagas SC, Chrousos GP. The pathophysiologic roles of interleukin-6 in human disease. Ann Intern Med. 1998;128:127–137. doi: 10.7326/0003-4819-128-2-199801150-00009. [DOI] [PubMed] [Google Scholar]
- Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, et al. Markers of inflammation and cardiovascular disease: Application to clinical and public health practice. Circulation. 2003a;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- Pedro MR, Barba CVC, Benavides-de Leon R. Nutrition Transition in the Philippines, Philippine Population Review. 2007;6:1–19. [Google Scholar]
- Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care. 2004;27:813–823. doi: 10.2337/diacare.27.3.813. [DOI] [PubMed] [Google Scholar]
- Prabhakar U, Eirikis E, Davis HM. Simultaneous quantification of proinflammatory cytokines in human plasma using the LabMAP assay. J Immunol Methods. 2002;260:207–218. doi: 10.1016/s0022-1759(01)00543-9. [DOI] [PubMed] [Google Scholar]
- Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001a;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- Pradhan AD, Manson JE, Rossouw JE, Siscovick DS, Mouton CP, Rifai N, Wallace RB, Jackson RD, Pettinger MB, Ridker PM. Inflammatory biomarkers, hormone replacement therapy, and incident coronary heart disease. JAMA. 2002;288:980–987. doi: 10.1001/jama.288.8.980. [DOI] [PubMed] [Google Scholar]
- Prado MS, Strina A, Barreto ML, Oliveira-Assis AM, Paz LM, Cairncross S. Risk factors for infection with Giardia duodenalis in pre-school children in the city of Salvador, Brazil. Epidemiol Infect. 2003;131:899–906. doi: 10.1017/s0950268803001018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radon K, Ehrenstein V, Praml G, Nowak D. Childhood visits to animal buildings and atopic diseases in adulthood: an age-dependent relationship. Am J Ind Med. 2004;46:349–356. doi: 10.1002/ajim.20000. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Hennekens CH, Buring JE, Rifai N. C-Reactive Protein and other markers of inflammation in the prediction of cardiovascular disease in women. New Engl J Med. 2000a;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000b;101:1767–1772. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- Robak T, G A, Stepien H, Robak E. Serum levels of interleukin-6 type cytokines and soluble interleukin-6 receptor in patients with rheumatoid arthritis. Mediat Inflamm. 1998;7:347–353. doi: 10.1080/09629359890875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook GAW. 99th Dahlem conference on infection, inflammation and chronic inflammatory disorders: darwinian medicine and the ‘hygiene’ or ‘old friends’ hypothesis. Clin Exp Immunol. 2010;160:70–79. doi: 10.1111/j.1365-2249.2010.04133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook GAW, Stanford JL. Give us this day our daily germs. Immunol Today. 1998;19:113–116. doi: 10.1016/s0167-5699(97)01204-8. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis: an inflammatory disease. New Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Rush EC, Plank LD, Yajnik CS. Interleukin-6, tumour necrosis factor-alpha and insulin relationships to body composition, metabolism and resting energy expenditure in a migrant Asian Indian population. Clin Endocrinol (Oxf) 2007;66:684–90. doi: 10.1111/j.1365-2265.2007.02801.x. [DOI] [PubMed] [Google Scholar]
- Slopen N, Lewis TT, Gruenewald TL, Mujahid MS, Ryff CD, Albert MA, Williams DR. Early life adversity and inflammation in African Americans and Whites in the Midlife in the United States Survey. Psychosom Med. 2010;72:694–701. doi: 10.1097/PSY.0b013e3181e9c16f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, O'Donnell K, Badrick E, Kumari M, Marmot M. Neuroendocrine and inflammatory factors associated with positive affect in healthy men and women. Am J Epidemiol. 2008;167:96–102. doi: 10.1093/aje/kwm252. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Owen N, Kunz-Ebrecht S, Mohamed-Ali V. Inflammatory cytokines, socioeconomic status, and acute stress responsivity. Brain Behav Immun. 2002;16:774–784. doi: 10.1016/s0889-1591(02)00030-2. [DOI] [PubMed] [Google Scholar]
- Straczkowski M, Kowalska I, Nikolajuk A, Krukowska A, Gorska M. Plasma interleukin-10 concentration is positively related to insulin sensitivity in young healthy individuals. Diabetes Care. 2005;28:2036–37. doi: 10.2337/diacare.28.8.2036. [DOI] [PubMed] [Google Scholar]
- Tanchoco CC, Cruz AJ, Duante CA, Litonjua AD. Prevalence of metabolic syndrome among Filipino adults aged 20 years and over. Asia Pac J Clin Nutr. 2003;12:271–276. [PubMed] [Google Scholar]
- Targher G, Zenari L, Bertolini L, Muggeo M, Zoppini G. Elevated levels of interleukin-6 in young Adults with type 1 diabetes without clinical evidence of microvascular and macrovascular complications. Diabetes Care. 2001;24:956–957. doi: 10.2337/diacare.24.5.956. [DOI] [PubMed] [Google Scholar]
- Tedgui A, Mallat Z. Anti-inflammatory mechanisms in the vascular wall. Circ Res. 2001;88:877–887. doi: 10.1161/hh0901.090440. [DOI] [PubMed] [Google Scholar]
- Tziakas DN, Chalikias GK, Hatzinikolaou HI, Parissis JT, Papadopoulos ED, Trypsianis GA, Papadopoulou E, Tentes IK, Karas SM, Hatseras DI. Anti-inflammatory cytokine profile in acute coronary syndromes: behavior of interleukin-10 in association with serum metalloproteinases and proinflammatory cytokines. Int J Cardiol. 2003;92:169–175. doi: 10.1016/s0167-5273(03)00084-6. [DOI] [PubMed] [Google Scholar]
- Urbańska-Ryś H, W A, Stepień H, Robak T. Relationship between circulating interleukin-10 (IL-10) with interleukin-6 (IL-6) type cytokines (IL-6, interleukin-11 (IL-11), oncostatin M (OSM)) and soluble interleukin-6 (IL-6) receptor (sIL-6R) in patients with multiple myeloma. Eur Cytokine Netw. 2000;11:443–451. [PubMed] [Google Scholar]
- van Exel E, Gussekloo J, de Craen AJM, Bootsma-van der Wiel A, Frolich M, Westendorp RGJ. Inflammation and stroke: the Leiden 85-plus study. Stroke. 2002a;33:1135–1138. doi: 10.1161/01.str.0000014206.05597.9e. [DOI] [PubMed] [Google Scholar]
- van Exel E, Gussekloo J, de Craen AJM, Frölich M, Bootsma-van der Wiel A, Westendorp RGJ. Low production capacity of interleukin-10 associates with the metabolic syndrome and type 2 diabetes. Diabetes. 2002b;51:1088–1092. doi: 10.2337/diabetes.51.4.1088. [DOI] [PubMed] [Google Scholar]
- VanDerslice J, Popkin B, Briscoe J. Drinking-water quality, sanitation, and breastfeeding: their interactive effects on infant health. Bull World Health Organ. 1994;72:589–601. [PMC free article] [PubMed] [Google Scholar]
- Vasunilashorn S, Crimmins E, Kim J, Winking J, Gurven M, Kaplan H, Finch C. Blood lipids, infection, and inflammatory markers in the Tsimane of Bolivia. Am J Hum Biol. 2010;22:731–40. doi: 10.1002/ajhb.21074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser M, Pahor M, Taaffe DR, Goodpaster BH, Simonsick EM, Newman AB, Nevitt M, Harris TB. Relationship of interleukin-6 and tumor necrosis factor-α with muscle mass and muscle strength in elderly men and women. J Gerontol A Biol Sci Med Sci. 2002;57:M326–M332. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]
- Vozarova B, Weyer C, Hanson K, Tataranni PA, Bogardus C, Pratley RE. Circulating interleukin-6 in relation to adiposity, insulin action, and insulin secretion. Obesity. 2001;9:414–417. doi: 10.1038/oby.2001.54. [DOI] [PubMed] [Google Scholar]
- Walston J, Xue Q, Semba RD, Ferrucci L, Cappola AR, Ricks M, Guralnik J, Fried LP. Serum antioxidants, inflammation, and total mortality in older women. Am J Epidemio. 2006;163:18–26. doi: 10.1093/aje/kwj007. [DOI] [PubMed] [Google Scholar]
- WHO. The World Health Report 2004 - Changing History. World Health Organization; 2004. [Google Scholar]
- WHO. Mortality Country Fact Sheet 2006. World Health Organization; 2006. [Google Scholar]
- Wong CK, Ho CY, Ko FWS, Chan CHS, Ho ASS, Hui DSC, Lam CWK. Proinflammatory cytokines (IL-17, IL-6, IL-18 and IL-12) and Th cytokines (IFN-γ, IL-4, IL-10 and IL-13) in patients with allergic asthma. Clin Exp Immunol. 2001;125:177–183. doi: 10.1046/j.1365-2249.2001.01602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wykretowicz A, Furmaniuk J, Smielecki J, Deskur-Smielecka E, Szczepanik A, Banaszak A, Wysocki H. The oxygen stress index and levels of circulating interleukin-10 and interleukin-6 in patients with chronic heart failure. Int J Cardiol. 2004;94:283–287. doi: 10.1016/j.ijcard.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Yazdanbakhsh M, Kremsner PG, van Ree R. Allergy, parasites, and the hygiene hypothesis. Science. 2002;296:490–494. doi: 10.1126/science.296.5567.490. [DOI] [PubMed] [Google Scholar]
- Yudkin JS, Stehouwer CDA, Emeis JJ, Coppack SW. C-Reactive Protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction : a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999a;19:972–978. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]
- Yudkin JS, Yajnik CS, Mohamed-Ali V, Bulmer K. High levels of circulating proinflammatory cytokines and leptin in urban, but not rural, Indians. A potential explanation for increased risk of diabetes and coronary heart disease. Diabetes Care. 1999b;22:363–364. doi: 10.2337/diacare.22.2.363. [DOI] [PubMed] [Google Scholar]