Abstract
We assessed the ex vivo reactivity of peptidic constructs of Tet1 (analog of tetanus toxin non-virulent C fragment) with sequence homology to the cysteine-active site of thioredoxin (Tet1THO) or tetralysine (Tet1PLYS) with oxidative species or axonopathic sodium cyanate (NaOCN), respectively. We then assessed their neuronal uptake in vivo in laboratory animals. The reactivity of Tet1PLYS with NaOCN (1:2.5 to 1:37.5 molar ratios) or Tet1THO with hydrogen peroxide (1:0.4 to 1:6.2 molar ratios) was assessed by mass spectrometry. Green fluorescence protein (GFP)-tagged Tet1-derivatives (3-mg/ml in artificial cerebrospinal fluid) were administered daily to rats by intramuscular injection in latissimus dorsi at lumborum at the dose of 1 µl/g of body weight, for 3 days. Motor neuron uptake was assessed after double immunolabeling for GFP and choline acetyltransferase. Mass spectrometry analysis successfully demonstrated the ex vivo reactivity of Tet1-derivatives in a concentration-dependent manner. Confocal microscopy revealed the localization of Tet1-derivatives in axons and motor neuron cell bodies. Intramuscular delivery of Tet1-derivatives appears to be a practical approach to circumvent the blood nerve barrier and selectively deliver small molecules to the nervous system, for diagnostic and/or treatment purposes.
Keywords: Axonal transport, Blood nerve barrier, Drug delivery, Tetanus toxin, Neurotoxicity, Neurodegeneration
Introduction
Treatment of neurological diseases has been ineffective for several reasons, including a poor understanding of the pathogenic mechanisms underlying neurodegenerative processes and limited options for effective delivery of drugs to the nervous system. A novel approach for the delivery of therapeutics to the central nervous system via retrograde axonal transport has been proposed but still needs to be tested. This approach exploits the physicochemical properties of the Tet1-peptide (sequence HLNILSTLWKYR), a tetanus toxin-derived peptide with no neurotoxic properties that is retrogradely transported and selectively translocated into neuronal networks (Federici et al. 2007). Our laboratory focuses on the role of protein post-translational modifications (PTM) by chemical adduction or oxidation in neurodegeneration. We first established a proof-of-principle for structure–activity relationships by showing that a protein-reactant γ-diketo group on the side chain of hydrocarbons was sufficient for the compounds to acquire neurotoxic properties and induce proximal giant axonopathy, a pathological hallmark of amyotrophic lateral sclerosis (Tshala-Katumbay et al. 2006). We also confirmed that cyanate, a protein carbamoylating agent, induces neuropathy through mechanisms possibly linked to protein loss of function due to carbamoylation and/or oxidative damage of proteins (Kassa et al. 2011). In this study, we sought to assess the diagnostic and therapeutic potential of peptidic derivatives of Tet1 in neurological diseases. Tet1 constructs were made by conjugating Tet1 to tetrapeptides with a sequence homology to the cysteine-active site of thioredoxin (Tet1THO) or tetralysine (Tet1PLYS; New England Peptide, Gardner, MA, USA). The constructs were then assessed for their ex vivo reactivity with either hydrogen peroxide or sodium cyanate (NaOCN) for Tet1THO or Tet1PLYS, respectively, in addition to their ability to translocate into axons and motor neurons.
Materials and Methods
Unmodified peptides (Tet1: H2N-HLNILSTLWKYR-amide; Tet1PLYS: Tet1KKKK-amide; and Tet1THO: Tet1CGHC-amide) were synthesized, conjugated to green fluorescence protein (GFP), and purified by New England Peptide (Gardner, MA). For ex vivo proteomics, 0.4 mM Tet1PLYS was incubated in 20 mM K2PO4, pH 7.0, 150 mM KCl, at 37 °C for 24 h in the presence of 0, 1, 5, 10, and 15 mM NaOCN to generate carbamoylated peptides. The reaction was stopped with addition of formic acid to a final concentration of 0.1 %, peptides desalted using C18 spin columns (Product # 89870, Thermo Scientific), and Tet1PLYS peptide diluted to 10 µM concentration of aqueous 20%acetonitrile, 0.1%formic acid. To oxidize Tet1THO, 0.8 mM fluorescein-labeled Tet1THO was incubated for 2 h at 37 °C in 50 mM ammonium bicarbonate (pH 8) with 0, 0.156, 0.312, 0.625, and 1.25 mM H2O2. Samples were then diluted to 10 µM final concentration of Tet1THO peptide by dilution in aqueous 20 % acetonitrile, 0.1 % formic acid. Masses of carbamoylated Tet1PLYS and oxidized Tet1THO were then determined by direct infusion into an LTQ linear ion trap mass spectrometer (Thermo Scientific). Electrospray ionization data were collected in profile mode and averaged spectra deconvoluted using ProMass software (Thermo Scientific).
For in vivo studies, young adult male heterozygous rats (Crl: NIH-Fox1 rnu/Fox 1+, 6–8 weeks old) were injected IM in paravertebral lumbar muscles (latissimus dorsi at lumborum) with Tet1-derivatives (2 mM solution of Tet1 in artificial cerebrospinal fluid containing 2 % acetone) at the dose of 1 µl/g of body weight (n=3/each Tet1-derivative), once per day, on alternate sides, for 3 days. On day 4, animals were deeply anesthetized with 4 % isofluorane and systemically perfused with 0.1 M phosphate buffer, pH 7.4 (PB) followed by 4 % paraformaldehyde in PB. The spinal cord was dissected out and put in 20 % sucrose in PB overnight for cryoprotection. The lumbar segment of the spinal cord was cut under a dissecting stereomicroscope, embedded in optimal cutting temperature compound, and frozen on dry ice. Forty-micrometer-thick transverse sections were cut at the cryostat and every 11th section collected free-floating in PB.
To determine if Tet1-derivatives target lower motor neurons, one series of sections from all rats was processed for double immunofluorescence with anti-GFP and anti-choline acetyltransferase (ChAT) antibodies. Sections were preincubated in 3 % bovine serum albumin (BSA) and 0.2 % Triton X-100 in PB for 2 h and then incubated in rabbit polyclonal anti-GFP antibodies (Invitrogen, Carlsbad, CA, USA; diluted 1:500) and goat polyclonal anti-ChAT antibodies (Millipore, Billerica, MA, USA; 1:80) in 3 % BSA and 0.2 % Triton X-100 in PB overnight. After repeated washes in PB, the sections were incubated with CyTM2-conjugated donkey anti-rabbit or CyTM3-conjugated mouse anti-goat (Jackson ImmunoResearch Laboratories, West Grove, PA, USA; diluted 1:100), respectively, in 1 % BSA and 0.2 % Triton X-100 in PB for 2 h. Control sections were processed as above, omitting the primary antibodies as well as incubating at the same dilution of the primary antibodies with respective normal host sera. No immunoreactivity was seen in these materials. After repeated washes, sections were mounted on slides, air dried, and coverslipped with an anti-fading mounting medium (Dako, Glostrup, Denmark). Immunofluorescence was examined by fluorescence microscopy and images acquired with a Zeiss LSM 710 laser scanning confocal microscope, using the 488-nm and 533-nm excitation beams. Experimental protocols were approved by the OHSU Institutional Animal Care and Use Committee.
Results and Discussion
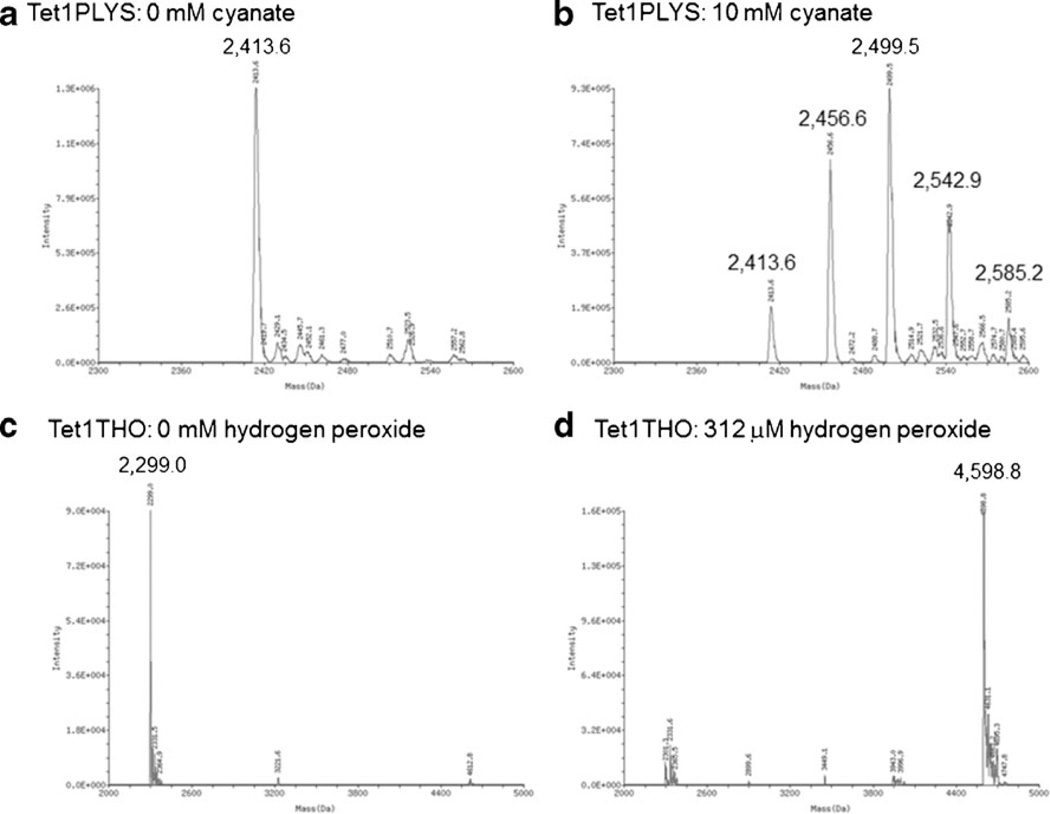
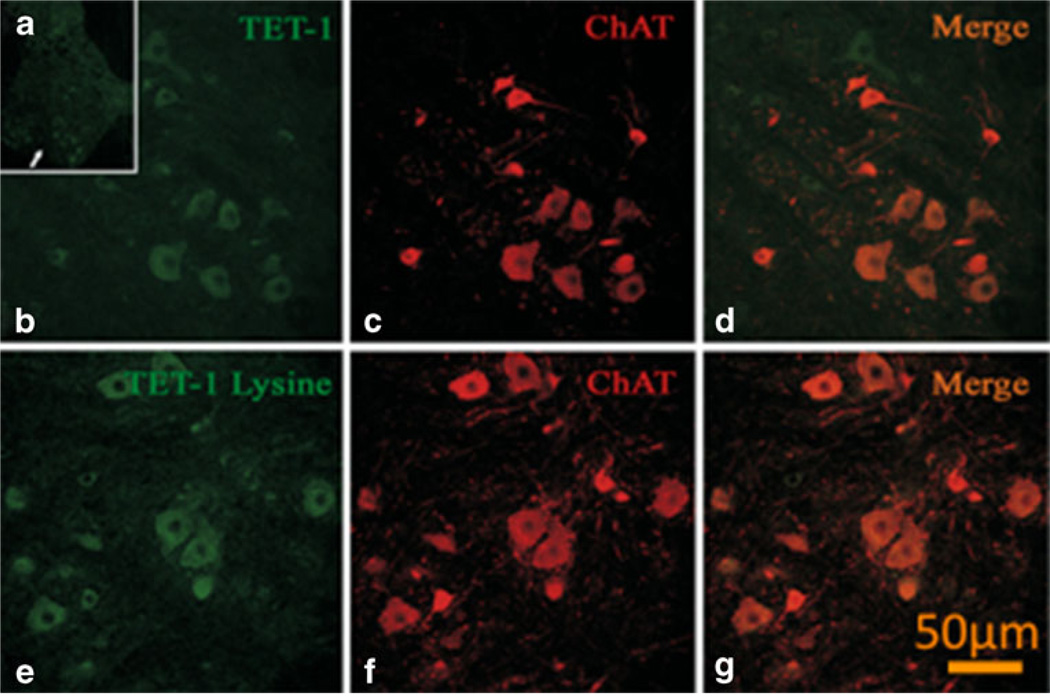
Mass spectrometry was used to characterize the ex vivo reactivity of Tet1PLYS or Tet1THO with NaOCN or hydrogen peroxide, respectively. Unmodified TET1PLYS had a mass of 2,413.6 (Fig. 1A) and increased in mass by increments of 43 following 24-h incubation in 10 mM cyanate (Fig. 1B), consistent with carbamoylation at its four additional lysine residues. Unmodified Tet1THO had a mass of 2,299.0 (Fig. 1C) that increased in mass to 4,598.8 following incubation in 312 µM H2O2, consistent with disulfide formation between two Tet1THO molecules (Fig. 1D). Animals showed no signs of toxicity. Microscopic examination of lumbar spinal cord sections from all Tet1-injected rats revealed immunoreactivity for this peptide in the gray and white matter. In particular, immunoreactive neuronal cell bodies were detected in the ventral horn (Fig. 2), while an occasional and sparse immunosignal was observed in the dorsal horn. Confocal microscopy confirmed that most Tet1-positive neurons were motor neurons (Fig. 3).
Fig. 1.
Masses of Tet1-derivatives following carbamoylation and cysteine oxidation. A Unmodified Tet1PLYS. B Tet1PLYS carbamoylated by 24-h incubation in 10 mM cyanate showing 43 mass unit increase due to successive carbamoylation at lysine residues. C Unmodified Tet1THO. D MS spectrum of Tet1THO oxidized by 2-h incubation in 312 µM hydrogen peroxide, suggesting disulfide bonding between two Tet1THO molecules
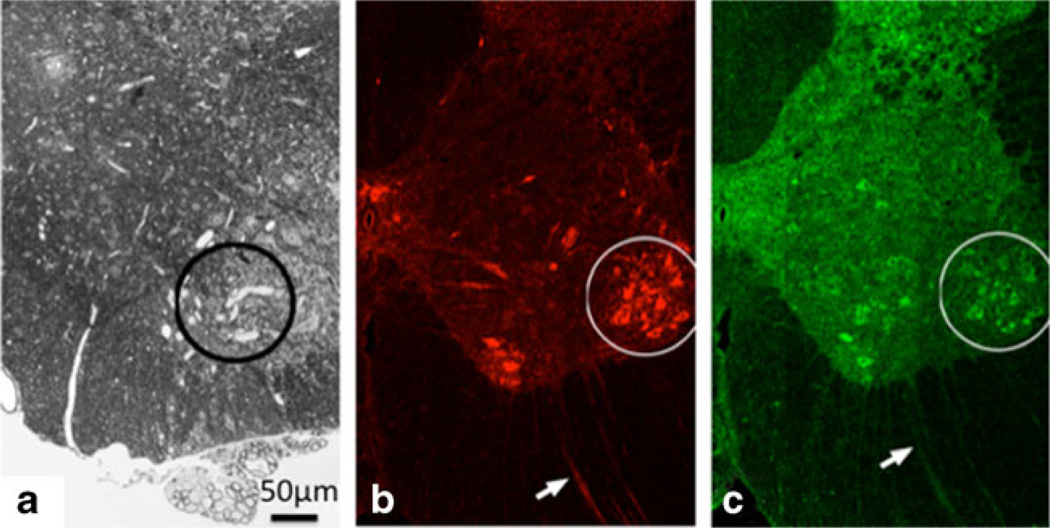
Fig. 2.
A Illustrative axonal swellings in spinal cord anterior horn (circle) in animals treated with neuroprotein reactant and axonopathic 1,2-diacetylbenze (prior studies). B ChAT immunostaining of motor neurons (MN) in the same region. C Uptake of Tet1PLYS in the same region. A comparable level of uptake was seen for Tet1THO. Uptake of Tet1-derivatives was also seen in axons (arrows)
Fig. 3.
Representative confocal microscopy images of in vivo immunofluorescent labeling of lumbar rat motor neurons with GFP-conjugated Tet1 and derivatives (Tet1-Lysine=Tet1PLYS). A GFPimmunolabeling of neurons in the anterior horn of the lumbar spinal cord (arrow). Higher magnification images of GFP-immunopositive neurons in the anterior horn of the lumbar spinal cord (B, E), choline acetyltransferase (ChAT) immunolabeling (C, F), and overlap images (D, G). Note the localization of Tet1 and Tet1PLYS within motor neurons
Our findings indicate that intramuscular delivery of Tet1-derivatives appears to be a practical approach in target delivery of small molecules to the nervous system. We also suggest that short-sequence peptides with chemical affinity for neurotoxic speciesmay be tested for neuroprotective properties or diagnostic value in animal models of neurodegeneration. While Tet1PLYSmay act as a scavenger for cyanate, Tet1THO or derivatives may possibly be tested to modulate cellular thiol redox states. Further studies, however, will need to be conducted in vivo to characterize Tet1 modifications and rule out immunogenic adverse effects. If successfully developed, the proposed approach will help circumvent the blood nerve barrier and deliver drug candidates to the nervous system for the diagnosis and/or inhibition of PTMs associated with neurological diseases. This would be achieved by means of bioactive molecules conjugated to synthetic analogs of the tetanus toxin non-virulent C fragment, which would serve as a vector (Federici et al. 2007). Other forms of administration of drugs to enhance their bioavailability may include Tet1 nanoscaled carriers and/or nasal spray preparations for emergency needs (Bhaskar et al. 2010; Zhang et al. 2012).
Acknowledgments
This work was supported by NIEHS/FIC grant R21ES017225 and NINDS P30-NS061800 from the National Institutes of Health, Bethesda, MD, USA. The technical expertise of Deborah McMillen (OHSU Proteomic Shared Resource) is greatly appreciated.
Contributor Information
R. Kassa, Center for Research on Occupational & Environmental Toxicology, Oregon Health & Science University (OHSU), 3181 Sam Jackson Park Road, mail code L606, Portland, OR 97239, USA
V. Monterroso, Department of Neurology, Oregon Health & Science University (OHSU), 3181 Sam Jackson Park Road, mail code L606, Portland, OR 97239, USA
L. L. David, Department of Comparative Medicine, Oregon Health & Science University (OHSU), 3181 Sam Jackson Park Road, mail code L606, Portland, OR 97239, USA
D. Tshala-Katumbay, Email: tshalad@ohsu.edu, Center for Research on Occupational & Environmental Toxicology, Oregon Health & Science University (OHSU), 3181 Sam Jackson Park Road, mail code L606, Portland, OR 97239, USA; Department of Biochemistry and Molecular Biology & Proteomics Shared Resource, Oregon Health & Science University (OHSU), 3181 Sam Jackson Park Road, mail code L606, Portland, OR 97239, USA.
References
- Bhaskar S, Tian F, Stoeger T, et al. Multifunctional nanocarriers for diagnostics, drug delivery and targeted treatment across blood–brain barrier: perspectives on tracking and neuroimaging. Particle and fibre toxicology. 2010;7:3. doi: 10.1186/1743-8977-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federici T, Liu JK, Teng Q, Yang J, Boulis NM. A means for targeting therapeutics to peripheral nervous system neurons with axonal damage. Neurosurgery. 2007;60:911–918. doi: 10.1227/01.NEU.0000255444.44365.B9. [DOI] [PubMed] [Google Scholar]
- Kassa RM, Kasensa NL, Monterroso VH, et al. On the biomarkers and mechanisms of konzo, a distinct upper motor neuron disease associated with food (cassava) cyanogenic exposure. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 2011;49:571–578. doi: 10.1016/j.fct.2010.05.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tshala-Katumbay DD, Palmer VS, Lasarev MR, Kayton RJ, Sabri MI, Spencer PS. Monocyclic and dicyclic hydrocarbons: structural requirements for proximal giant axonopathy. Acta neuropathologica. 2006;112:317–324. doi: 10.1007/s00401-006-0106-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhang W, Johnston AH, Newman TA, Pyykko I, Zou J. Targeted delivery of Tet1 peptide functionalized polymersomes to the rat cochlear nerve. International journal of nanomedicine. 2012;7:1015–1022. doi: 10.2147/IJN.S28185. [DOI] [PMC free article] [PubMed] [Google Scholar]