Abstract
The symptoms of major psychotic illness are diverse and vary widely across individuals. Furthermore, the prepsychotic phase is indistinct, providing little indication of the precise pattern of symptoms that may subsequently emerge. Likewise, although in some individuals who have affected family members the occurrence of disease may be predicted, the specific symptom profile may not. An important question, therefore, is whether predictive physiological markers of symptom expression can be identified. We conducted a placebo-controlled, within-subjects study in healthy individuals to investigate whether individual variability in baseline physiology, as assessed using functional magnetic resonance imaging, predicted psychosis elicited by the psychotomimetic drug ketamine and whether physiological change under drug reproduced those reported in patients. Here we show that brain responses to cognitive task demands under placebo predict the expression of psychotic phenomena after drug administration. Frontothalamic responses to a working memory task were associated with the tendency of subjects to experience negative symptoms under ketamine. Bilateral frontal responses to an attention task were also predictive of negative symptoms. Frontotemporal activations during language processing tasks were predictive of thought disorder and auditory illusory experiences. A subpsychotic dose of ketamine administered during a second scanning session resulted in increased basal ganglia and thalamic activation during the working memory task, paralleling previous reports in patients with schizophrenia. These results demonstrate precise and predictive brain markers for individual profiles of vulnerability to drug-induced psychosis.
Keywords: fMRI, schizophrenia, NMDA, endophenotype, psychotomimetic, cognition
Introduction
Symptoms of schizophrenia may be principally understandable in terms of a dysfunction of primary cognitive processes. Auditory hallucinations, for example, may occur as a failure of self-monitoring of internal verbal processing and consequent misattribution to an external source (Frith, 1992). Similarly, delusional beliefs may represent attempts to rationalize inappropriate associations, constructed on the basis of aberrant salience in the environment (Kapur, 2003). Expression of these symptoms varies considerably within the clinical population, suggesting that patients may differ in an inherent vulnerability to specific symptoms. Individual variability in cognitive functioning and associated physiological processes may therefore provide early markers of susceptibility to symptoms that occur when such processes become compromised. Here, we used functional magnetic resonance imaging (fMRI) to examine whether patterns of physiological response to specific cognitive challenges confer a vulnerability to associated symptoms.
We used a drug model of psychosis to relate presymptomatic physiology to symptom outcome. Ketamine induces transient psychotic symptoms in healthy volunteers (Krystal et al., 1994) and exacerbates existing symptoms in patients (Lahti et al., 1995). Cognitive dysfunction similar to that observed in patients is also reported (Morgan and Curran, 2006). Psychotic symptoms under ketamine are therefore associated with disruption of cognitive processes and brain mechanisms putatively involved in schizophrenia.
We assessed brain responses, separately under placebo and ketamine treatments, in healthy volunteers across four cognitive challenges, each theoretically related to a symptom of psychosis. Two of the tasks (verbal working memory and attention) are associated with negative symptoms, which may result from social and cognitive disengagement attributable to reduced processing capacity of prefrontal cortex (Silver and Feldman, 2005), leading to difficulties in concentration and maintaining task set (Nuechterlein et al., 1986). We predicted that prefrontal activity during the attention and working memory tasks would be associated with vulnerability to negative symptoms under ketamine.
A failure to monitor “inner speech” may provide a mechanism leading to auditory hallucinations, whereby self-generated speech is misattributed externally (Frith, 1992). Comparing verbal self-monitoring (imagining speech spoken by another person) with inner speech (minimal self-monitoring) increases prefrontal and temporal cortex activation in patients with auditory hallucinations (McGuire et al., 1995). Ketamine produces auditory illusory experiences similar to the heightened auditory and visual awareness described by patients during the prodromal phase (McGhie and Chapman, 1961), and it has been suggested that these contribute to the development of hallucinations (Chapman, 1966). We predicted that prefrontal and temporal cortex activation during a self-monitoring task would be associated with vulnerability to the auditory illusory experiences under ketamine.
Finally, a sentence completion task was used to engage brain regions associated with semantic processing. Thought disorder involves difficulty in constraining semantic threads of language, making speech disjointed and chaotic (Kerns and Berenbaum, 2002), as also observed under ketamine (Adler et al., 1999). In patients, the requirement to generate an appropriate semantic response to complete a sentence is associated with increased activation of left frontal and temporal cortex (Kircher et al., 2001). We predicted that frontotemporal responses to a sentence completion task would predict vulnerability to thought disorder induced by ketamine.
Materials and Methods
Subjects
Fifteen healthy, right-handed volunteers (eight male) with a mean ± SD age of 29 ± 7 years and a mean ± SD predicted full scale intelligence quotient of 113 ± 4 [as indexed by the National Adult Reading Test (Nelson, 1982)] were recruited from the local community by advertisement. Exclusion criteria included a history of psychiatric or physical illness (particularly cardiovascular or neurological disorders), head injury, any history of drug or alcohol dependence, as well as contraindications for fMRI scanning. The study was approved by the Cambridge Local Research and Ethics Committee. Written informed consent was given by all subjects.
Experimental design
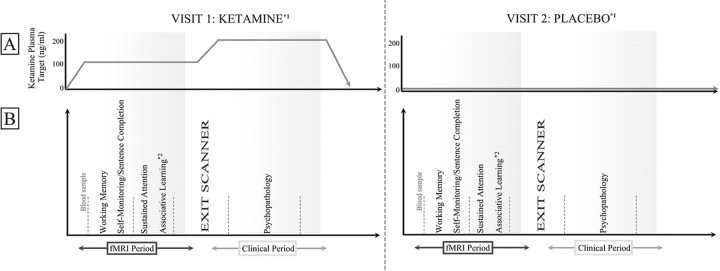
For the experimental design, see Figure 1.
Figure 1.
Experimental design across two visits. Subjects received either a low-dose infusion of ketamine (100 ng/ml plasma) or placebo while they performed a series of cognitive tasks in the scanner. 2Data from the associative learning task has been described previously (Corlett et al., 2006). Racemic ketamine (1 mg/ml) was administered by bolus and continuous target controlled infusion using a computerized pump using the pharmacokinetic parameters of a three-compartment model described by Domino et al. (1982). On completion of fMRI scanning, subjects were removed from the scanner into an adjacent clinical observation room, and the ketamine dose was either increased to 200 ng/ml or subjects continued to receive placebo. Subjects then underwent a series of clinical interviews to evaluate and quantify experience of psychotic phenomenology. 1The order of visits was counterbalanced across subjects. Drug and placebo visits for each subject were spaced by at least 1 month.
Cognitive tasks
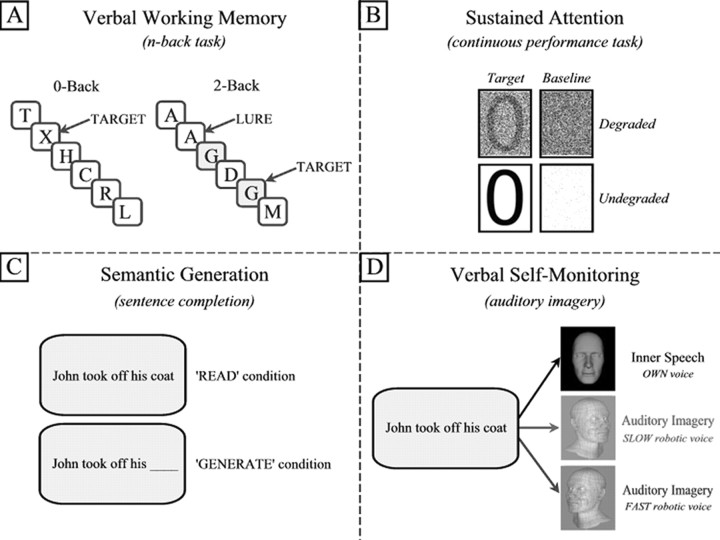
Working memory (n-back).
Letters were individually presented for 0.5 s, with an interstimulus interval (ISI) of 2.5 s; targets were repetitions of letters presented n trials previously; load ranged from zero to three trials and were presented as 43 s blocks, consisting of 14 trials and a 1 s condition instruction (Fig. 2). Each condition was presented four times in pseudorandom order. Lure trials (repetitions that did not correspond with the current condition) discouraged simple visual matching. Subjects responded on all trials, indicating target identification by right middle finger button press, and nontrials with right index finger.
Figure 2.
Cognitive tasks. Four cognitive tasks were administered. For details, see Materials and Methods.
Attention (continuous performance test).
The task has been fully described previously (Honey et al., 2005). Briefly, 28 stimuli (even numerals) were presented for 42 ms each (958 ms ISI) over a period of 30 s and were either degraded (40% pixel inversion) or undegraded. Subjects were required to indicate the presentation of an infrequently presented target (number 0, presented pseudorandomly on 25% of trials); no response was required to nontargets. Each 30 s condition was repeated five times. Visual baseline conditions consisting of noise pixels, in which no digits were shown, matched the luminance of task conditions, and no response was required.
Sentence completion and verbal self-monitoring tasks.
The two language tasks were presented in a 2 × 2 factorial design. Sentences were presented, half of which were complete, and half had the final word replaced by an underscore. Completed sentences were read subvocally (“READ” condition) or for incomplete sentences (“GENERATE” condition), subjects were required to read the sentence and generate a word that appropriately completed a meaningful sentence. Subjects were required to press a button with their right index finger to indicate task completion. The predicted mean increase in reaction time for the GENERATE compared with READ condition served to indicate that subjects were appropriately engaged in the task.
To engage verbal self-monitoring, for both complete and incomplete sentences described above, subjects were required to subvocalize the sentence in either their own voice or one of two robotic voices. Before the study, samples of the two robotic voices were played to subjects, using example sentences not used in the experiment, to familiarize subjects with the robotic voices and to practice subvocally reproducing the voices until confident in doing this. The two voices were played to the subject again immediately before beginning the task in the scanner. To provide an overt behavioral indicator that subjects were performing the self-monitoring task appropriately, we used two robotic voices with slow or fast rates of speech production. The robotic voices were further distinguished as male and female computerized voices. Subjects were expected to take longer to reproduce the sentence in the slow-speaking robotic voice and therefore show longer reaction times (as measured by the button press to indicate completion of the task) than when reproducing the fast-speaking voice or when using their own voice. We used this reaction time difference as a measure to indicate that subjects were appropriately engaged in the self-monitoring task. Half of the sample heard a fast-speaking male robotic voice and a slow female voice, and this was reversed for the other half of the sample. Two hundred twenty-five sentences were selected from a normative database (Bloom and Fischler, 1980). Of the 329 sentences in this database, 104 with a probability of >0.8 for a given response were discarded, to avoid the task being insufficiently demanding on semantic generation. An additional 75 sentences were constructed de novo by the authors, producing 300 sentences in total. To minimize the potentially confounding effects of differences between sentences (e.g., imagibility, syntactic complexity, etc.), sentences were randomized across drug and task conditions and over subjects.
Sentences were grouped in blocks of five sentences. Before each block, an instruction was presented for 1 s, indicating whether the subject should read/complete the sentence in either their own voice (“YOUR VOICE” presented on screen), the male (“MALE ROBOT” presented on screen), or the female robotic voice (“FEMALE ROBOT” presented). Each block consisted of either five complete sentences or five incomplete sentences. Sentences were presented for 7 s each, and subjects were required to press a button after each trial to indicate completion of the task. Twenty-four blocks were presented, with four repetitions of each of the six conditions (read/complete sentence in own voice/male robotic voice/female robotic voice).
Clinical interview
Symptoms during the ketamine infusion were evaluated using the Brief Psychiatric Rating Scale (BPRS) (Ventura et al., 1993), the Comprehensive Assessment of Symptoms and History (CASH) (Andreasen et al., 1992), and the Clinician Administered Dissociative States Scale (CADSS) (Bremner et al., 1998). Interviews were video recorded and rated jointly by two experienced psychiatrists. Negative symptom scores were identified on the basis of summed scores for three key BPRS items, blunted affect, emotional withdrawal, and motor retardation, based on their reliability and validity (Thiemann et al., 1987) and as reported in similar previous studies (Krystal et al., 1998, 1999). Thought disorder was assessed by a combined score of items from the CASH (derailment, tangentiality, incoherence, illogicality, circumstantiality, pressure of speech, distractible speech) and BPRS (unusual thought content, conceptual disorganization). Auditory illusory experiences were rated subjectively using responses to the item from the CADSS: “Do sounds almost disappear or become much stronger than you would have expected?” This relates to our proposition, outlined previously, that perceptual changes described in the prodromal phase (McGhie and Chapman, 1961) (for which acute ketamine is likely to be a more appropriate model than in relation to chronic illness) may be precursory to symptom development (Chapman, 1966). Clinical ratings were compared across drug and placebo conditions using paired t tests.
Behavioral data analysis
Reaction time (RT) and target detection (d′) were compared across the four levels of load using a one-way ANOVA for the n-back task and using paired t tests for the degraded versus undegraded conditions for the continuous performance test (CPT). Paired t tests were used to test for the expected increase in latency for sentence completion compared with sentence reading and to compare the expected increased latency in reproducing the slow robotic voice compared with reading in the subject's own voice.
fMRI data acquisition
fMRI data were acquired using a Bruker MedSpec 30/100 operating at 3 tesla. Gradient-echo echo planar T2*-weighted images depicting blood oxygenation level-dependent (BOLD) contrast were acquired from 21 noncontiguous near axial planes: repetition time, 1.1 s; echo time, 27.5 ms; flip angle, 66°; in-plane resolution, 3.1 × 3.1 mm; matrix size, 64 × 64; field of view, 20 × 20 cm; bandwidth, 100 kHz. A total of 640 volumes per subject were acquired (21 slices each of 4 mm thickness, interslice gap of 1 mm). The first six volumes were discarded to allow for T1 equilibration effects, leaving 634 volumes.
fMRI data analysis
SPM2 (Wellcome Department of Cognitive Neurology, London, UK) was used to analyze the fMRI data using statistical parametric mapping. Images were realigned, spatially normalized to a standard template, and spatially smoothed with a Gaussian kernel (8 mm). The time series in each session were high-pass filtered (to a maximum of Hz), and serial autocorrelations were estimated using an autoregressive (1) model.
Modeling BOLD responses.
Blocks of stimuli were modeled using a boxcar function incorporating a delay appropriate to the hemodynamic response. This function was used as a covariate in a general linear model to generate a parameter estimate for each voxel for the contrasts listed below in reference to the control condition. Individuals' contrast images, derived from the pairwise comparisons between task blocks and the corresponding control task, were then entered into a second-level group analysis using an ANOVA model with nonsphericity correction, to permit inference about the effects of task, treating intersubject variability as a random effect.
Type I error control.
To maximize sensitivity but minimize the risk of type I error, we confined the critical analyses to a number of regions of interest (ROIs) using anatomical masking software (Maldjian et al., 2003), based on reported regional activation in previous related studies. For the working memory task, analyses were constrained to the lateral prefrontal cortex (including inferior and middle frontal gyri), parietal cortex, basal ganglia, and thalamus on the basis of increased activity being consistently reported in previous data (for review, see Cabeza and Nyberg, 2000). For the attention task, we selected a cortico-striato-cerebello-thalamic network that we have shown previously to be responsive to this task (Honey et al., 2005). Cortical regions included anterior cingulate and lateral prefrontal cortex. For the sentence completion task, a bilateral fronto-temporo-parietal cortical ROI was constructed based on a similar previous study (Kircher et al., 2001). In all cases, the activations occurring in these volumes of interest were thresholded at a false discovery rate (FDR) of p < 0.05 (Genovese et al., 2002).
Planned comparisons.
The linear combinations of parameter estimates for each contrast described below were stored as separate images for each subject. These contrast images were entered into a second-level one-sample t test. (1) For working memory, contrast images for all working memory load conditions were compared with the 0-back control task. To examine the effect of working memory load, a contrast image was computed on the basis of a linear weighting of each of the four conditions (0-back to 3-back). (2) For attention, contrast images for the two task conditions (degraded and undegraded stimuli) were compared with the visual baseline condition. To examine the effect of stimulus degradation, the two task conditions were compared, each contrasted to a baseline condition matched for luminosity. (3) For verbal self-monitoring, self-monitoring conditions (fast/slow robot combined) were compared with the inner speech condition. (4) For sentence completion, the word generation condition was contrasted to the sentence reading condition.
Effects of low-dose ketamine on task-specific activation.
The linear combinations of parameter estimates for each contrast above were stored as separate images for each subject. These contrast images were entered into a second-level one-sample t test to permit inferences about condition effects within the placebo group, treating intersubject variability as a random effect. Analysis of drug effects was conducted using a within-subjects repeated-measures ANOVA model (n-back task) or paired t tests for all other tasks involving only two conditions. All effects of interest were thresholded using the FDR at p < 0.05
Task-specific fMRI/symptom correlations.
A regression model, including linear and quadratic terms, was applied separately to each of the contrast images above, incorporating symptom ratings under drug. That is, we identified regions in which the magnitude of response across individuals was significantly correlated with symptom scores under ketamine. All effects of interest were thresholded using the FDR at p < 0.05. To determine the degree of overlap between regions for which there was an effect of low-dose ketamine and those regions that under placebo correlated with symptom expression, we conducted the correlational analysis first masked by the task × drug interaction analysis and separately, without this mask (to identify regions for which placebo-related activity was associated with symptom expression under high dose but for which there was no effect of drug at the low dose used). To determine the specificity of observed correlations between regional activation and symptom scores for each task, we used a step-forward multiple regression procedure, incorporating each of the symptom ratings and ketamine plasma values. The purpose of this was to identify whether the target symptom for each task was found to predict a significant amount of the variance of the regional activation and whether this model could be improved by the inclusion of other explanatory variables.
Task-independent correlations.
To determine whether task-specific effects were independent, we compared regional activation across each of the four tasks. Using a series of inclusive masks, we constructed a map that identified regions that were significantly responsive under each of the task conditions compared with its corresponding baseline. For the regions identified, we calculated the correlation between fMRI response observed across each task to establish whether some subjects showed consistently increased activation in these regions, regardless of task.
Results
Clinical observations
The observed drug plasma level for the target of 200 ng/ml was 209.6 ± 13 ng/ml (based on 14 of the 15 subjects, because we were unable to draw blood samples from one subject). Subjects experienced symptoms under ketamine that were qualitatively similar to those of schizophrenia, as confirmed by the symptom ratings measured using psychiatric rating scales. Ketamine produced significant increases in negative symptoms (t (13) = −4.48, p = 0.001), thought disorder (t (13) = −3.71, p = 0.003), and auditory illusions (t (13) = −3.46, p = 0.004). Symptom patterns were variable in quality and severity across individuals, as expected, and were qualitatively similar to previous reports of perceptual disturbances in patients in the prodromal phase of the illness.
Behavioral results
Data was unavailable for one subject because of malfunction of the recording equipment. Repeated-measures ANOVA revealed a main effect of load in the working memory task on both accuracy (d′) (F (3,39) = 10.919, p < 0.001) and RT (F (3,39) = 41.543, p < 0.001). The effect of ketamine was not significant for accuracy (mean of 2.97 ± 0.42 placebo; 2.97 ± 0.39 ketamine; F (1,13) = 0.0001, p = 0.995) but was significant for reaction time (mean of 0.66 ± 0.16 placebo; 0.73 ± 0.16; ketamine; F (1,13) = 14.267, p = 0.002). The interaction between ketamine and working memory load was not significant for accuracy (F (3,39) = 1.039, p = 0.386) or reaction time (F (3,39) = 1.437, p = 0.247). Subjects less accurately rejected distractors [letters that were presented earlier in the block but not the precise number of trials back specified by the current condition, e.g., a 1-back repeat in the context of a 3-back task compared with nontargets as load increased (F (3,42) = 16.405, p < 0.001)] and were slower to do so (F (3,39) = 20.03, p < 0.001). Under ketamine, subjects were slower to reject distractors than under placebo (F (3,39) = 5.528, p = 0.003) but were not less accurate (F (3,39) = 0.511, p = 0.677).
The effect of stimulus degradation in the attention task was significant for accuracy (F (1,14) = 13.952, p = 0.002) and RT (F (1,14) = 77.232, p < 0.0001). Subjects were slower under ketamine (mean of 0.38 ± 0.03 placebo; 0.4 ± 0.03 ketamine; F (1,14) = 8.509, p = 0.011) but were not less accurate (mean of 4.41 ± 0.31 placebo; 4.28 ± 0.27 ketamine; F (1,14) = 1.635, p = 0.222). There was no interaction between drug and stimulus degradation for accuracy (F (1,14) = 0.145, p = 0.709) or RT (F (1,14) = 0.486, p = 0.497).
Data from two subjects were unavailable for the language processing tasks attributable to technical error. Subjects' RTs were significantly slower for the sentence completion task when required to generate a word to complete a sentence compared with simply reading the sentence (F (1,12) = 32.277, p < 0.0001), indicating that subjects were appropriately engaged in performance of the task. There was no effect of ketamine on this response (F (1,12) = 0.305, p < 0.591).
For the self-monitoring task, there was a significant difference between RT for the sentences reproduced in the subjects' own voices and the fast/slow robotic voices (F (1,12) = 42.745, p < 0.0001). Post hoc t tests showed that this was attributable to a significantly slower response for the slow robotic voice compared with both the fast robot (t (14) = −6.26, p < 0.0001) and subjects' own voices (t (14) = −9.29, p < 0.0001). This also demonstrated that subjects were appropriately engaged in the self-monitoring task. There was no effect of ketamine on this measure (F (1,12) = 0.558, p < 0.579).
fMRI results
Working memory
For the combined n-back task relative to the 0-back baseline, a regression model incorporating linear and quadratic terms revealed a striking association between activation in left thalamus (r 2 = 0.79), right putamen (r 2 = 0.68), and bilateral foci in prefrontal cortex (right, r 2 = 0.64; left, r 2 = 0.71) under placebo and BPRS negative symptom scores under ketamine (Fig. 3 B; Table 1, Working memory).
Figure 3.
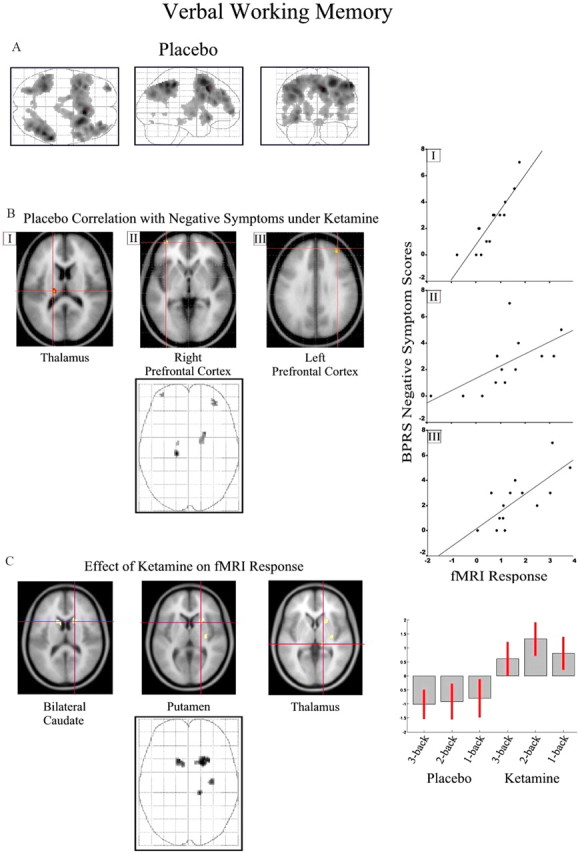
Verbal working memory task. See Results and Table 1 (Continuous performance test) for coordinates and model statistics. A, Task-related responses for the working memory task (combined across 1-, 2-, and 3-back conditions compared with 0-back baseline) under placebo are presented on maximum intensity projections from sagittal, coronal, and axial perspectives. B, Association with negative symptoms for three selected regions is superimposed on a representative T1-weighted image rendered into the same anatomical space and illustrated on a three-dimensional “glass brain.” fMRI activity is thresholded at p < 0.05 corrected for multiple comparisons using false discovery rate. Scatter plots showing the association between parameter estimates from the selected regions (I–III) and negative symptom score are presented on the right. C, Effects of a subpsychotic dose of ketamine (100 ng/ml plasma) on brain activation during the working memory task.
Table 1.
Montreal Neurological Institute coordinates, corrected p values, and Z scores for regions demonstrating effects of low-dose ketamine (I) and regions for which activity under placebo correlated with symptom severity under the high dose of ketamine
| Region | Laterality | Corrected p value | Z score | MNI coordinates |
||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Working memory | ||||||
| Effect of drug | ||||||
| Caudate | R | 0.034 | 3.82 | 18 | 14 | 14 |
| Putamen | R | 0.034 | 3.69 | 22 | 12 | 6 |
| Caudate | L | 0.034 | 3.66 | −12 | 16 | 14 |
| Thalamus | R | 0.034 | 3.65 | 16 | −28 | 0 |
| Putamen | R | 0.034 | 3.54 | 32 | −14 | 8 |
| Symptom correlation | ||||||
| Thalamus | L | 0.032 | 4.62 | −16 | −22 | 16 |
| Caudate | R | 0.032 | 4.08 | 22 | 6 | 20 |
| Middle frontal gyrus | R | 0.032 | 3.99 | 36 | 46 | 28 |
| Middle frontal gyrus | L | 0.032 | 3.79 | −36 | 58 | −2 |
| Thalamus | L | 0.044 | 3.43 | −14 | −6 | 4 |
| Continuous performance test a | ||||||
| Symptom correlation | ||||||
| Inferior frontal gyrus | L | 0.048 | 3.99 | −56 | 10 | 24 |
| Middle frontal gyrus | R | 0.048 | 3.93 | 50 | 6 | 46 |
| Middle frontal gyrus | R | 0.048 | 3.74 | 38 | 14 | 52 |
| Inferior frontal gyrus | R | 0.048 | 3.7 | 46 | 6 | 24 |
| Semantic processing a | ||||||
| Symptom correlation | ||||||
| Middle temporal gyrus | L | 0.0001 | 5.51 | −64 | −14 | −8 |
| Inferior frontal gyrus | L | 0.012 | 4.56 | −50 | 12 | 28 |
| Superior temporal gyrus | L | 0.004 | 4.2 | −50 | −56 | 10 |
| Inferior frontal gyrus | L | 0.029 | 3.5 | −58 | 26 | 4 |
| Inferior frontal gyrus | L | 0.029 | 3.49 | −46 | 18 | −2 |
| Verbal self-monitoring a | ||||||
| Symptom correlation | ||||||
| Inferior frontal gyrus | R | 0.028 | 4.01 | 54 | 14 | 20 |
| Anterior cingulate | L | 0.028 | 3.7 | −10 | 22 | 60 |
| Middle temporal gyrus | L | 0.028 | 3.69 | −52 | −30 | −8 |
MNI, Montreal Neurological Institute; R, right; L, left.
aEffect of drug: no suprathreshold voxels.
Increased activation of basal ganglia and thalamus was observed after administration of the low dose of ketamine (Fig. 3 C; Table 1, Working memory). This effect was observed across working memory load conditions under ketamine, with some indication of a pronounced effect for the 2-back condition.
Continuous performance test
No association between cingulate activation (that showed increased activation for the degraded compared with the undegraded condition) and symptomatology was observed. For the combined task versus fixation baseline, a regression model incorporating linear and quadratic terms showed a strong association between task-related activation under placebo and negative symptoms in bilateral inferior frontal gyri (right, r 2 = 0.37; left, r 2 = 0.6) and right middle frontal gyrus (r 2 = 0.37) (Fig. 4 B; Table 1, Continuous performance test). No effect of the low-dose treatment was observed at the selected threshold.
Figure 4.
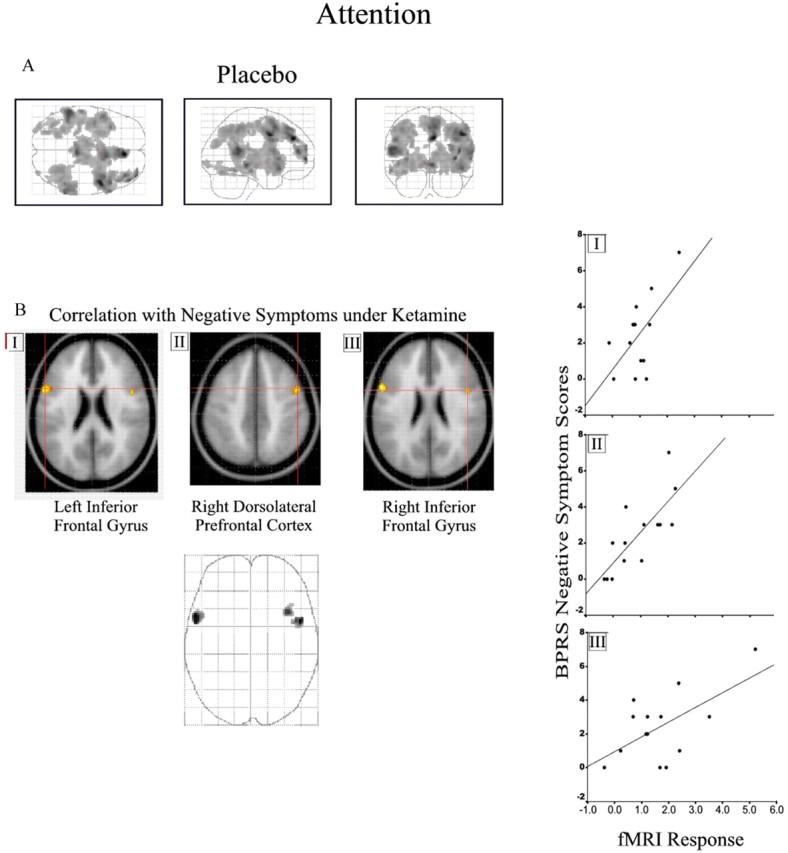
Continuous performance task. See Results and Table 1 (Continuous performance test) for coordinates and model statistics. A, Task-related response for the CPT compared with baseline under placebo. B, Association with negative symptoms for three selected regions (I–III) is superimposed on a T1-weighted image and illustrated as a three-dimensional glass brain. Scatter plots showing the association between parameter estimates from the selected regions (I–III) and negative symptom score are presented on the right. The figure is presented as described for Figure 3.
Sentence completion
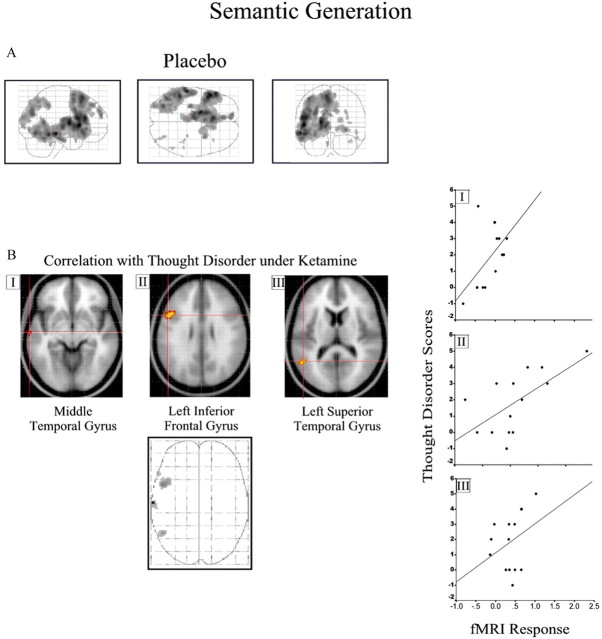
A significant positive association between activation under placebo in left middle (r 2 = 0.38) and superior temporal (r 2 = 0.29) and left inferior frontal gyri (r 2 = 0.41) was observed with the severity of thought disorder (Fig. 5 B; Table 1, Semantic processing). No effect of the low-dose treatment was observed at the selected threshold.
Figure 5.
Semantic generation task. See Results and Table 1 (Semantic processing) for coordinates and model statistics. A, Task-related response for word generation compared with word reading under placebo. B, Association with thought disorder scores for three selected regions (I–III) is superimposed on a T1-weighted image and illustrated as a three-dimensional glass brain. Scatter plots showing the association between parameter estimates from the selected regions (I–III) and the thought disorder symptom score are presented on the right. The figure is presented as described for Figure 3.
Verbal self-monitoring
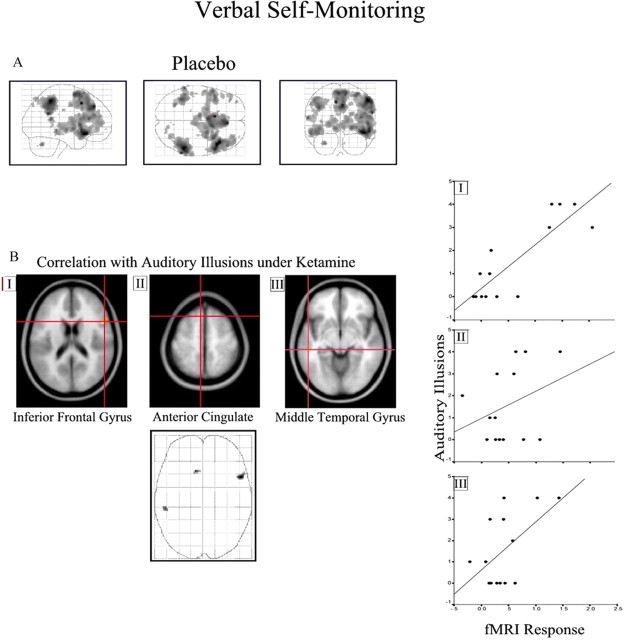
We observed a positive correlation between activation of the left middle temporal gyrus (r 2 = 0.3), anterior cingulate (r 2 = 0.11), and right inferior frontal gyri (r 2 = 0.73) under placebo with the degree of auditory illusions experienced after ketamine administration (Fig. 6 B; Table 1, Verbal self-monitoring). No effect of the low-dose treatment was observed at the selected threshold.
Figure 6.
Verbal self-monitoring. See Results and Table 1 (Continuous performance test) for coordinates and model statistics. A, Task-related response in bilateral frontal cortex under placebo. B, Association with auditory illusion scores for three selected regions (I–III) is superimposed on a T1-weighted image and illustrated as a three-dimensional glass brain. Scatter plots showing the association between parameter estimates from the selected regions (I–III) and the auditory illusions score are presented on the right. The figure is presented as described for Figure 3.
Task specificity
We examined the correlation of activation over subjects in the following seven regions, found to be coactivated across all tasks: anterior cingulate, left inferior frontal gyrus, left middle frontal gyrus, left inferior parietal cortex, left thalamus, and bilateral caudate. None of the pairwise correlations between regions were significant across tasks, indicating that the amplitude of subjects' regional response was indeed task dependent.
Symptom specificity
Each of the correlations between task-specific regional activity and predicted symptom was subjected to step-forward regression analysis incorporating all symptoms and ketamine plasma level. In each case, the model fit was not significantly improved by inclusion in the model of other symptoms or plasma ketamine levels.
Discussion
These findings show, first, that cognitive processes may be related to symptoms of drug-related psychosis via the identification of brain markers for task-specific activation. This study may contribute to a framework that unites theoretical models of cognitive deficits with symptoms characterizing schizophrenia. In this respect, there is a compelling consistency between the task/region/symptom associations and those reported in schizophrenic patients. We also observed a ketamine-induced increase in working memory activation comparable with that reported in patients with schizophrenia, providing some support for the validity of the ketamine model.
In relation to the associations observed between physiological responses to symptom-related cognitive processes under placebo and the expression of those symptoms under drug, it is worth noting that these associations do not of course imply any risk of disease onset in this sample of healthy volunteers. Rather, we are suggesting that individuals vary in their susceptibility to particular symptoms, whether these symptoms are exogenously induced by administration of a psychotomimetic drug, as in this study, or whether the symptoms occur endogenously as a result of a pathophysiological insult, as in patients with schizophrenia. This suggestion is supported by the colocalization of task/region/symptom associations observed in the present data with symptom-related physiological abnormalities reported in patients. Because the patterns we observed were task/symptom specific, it is unlikely that our findings reflect any global or nonspecific effect such as individual variability in cerebrovascular dynamics or stress responsivity.
Negative symptoms were predicted by frontal responses to working memory and attentional tasks
Negative symptoms, such as social withdrawal, apathy, and unresponsiveness, are particularly disabling features of schizophrenia. Influential models implicate attention and working memory deficits in the disruption of goal-directed motivated behavior, manifesting clinically as negative symptoms (Nuechterlein et al., 1986). The co-occurrence of these deficits (Barch and Carter, 1998) and their relationship to negative symptoms (Silver and Feldman, 2005) suggests a common mechanism. Here we show that increased response in frontal, thalamic, and caudate regions to a working memory task and increased frontal response to an attentional task predict increased vulnerability to negative symptoms under ketamine. It has been suggested that limbic projections to the cortex via the thalamus play a key role in motivated action and goal-directed behavior and that pathology of this system may contribute to negative symptoms not only in schizophrenia but also Parkinson's and Alzheimer's disease (Brown and Pluck, 2000). Negative symptoms may therefore emerge when working memory and attention are compromised, rendering the subject unable to contend with ongoing demands within their environment, leading ultimately to disengagement and unresponsiveness. The current data indicate that individual differences in physiological efficiency in cortico-striato-thalamic loops may explain why vulnerability to this process is amplified in some subjects.
Thought disorder and auditory perceptual changes were predicted by frontal and temporal responses to language and monitoring tasks
Thought disorder, an impairment in arranging and communicating thoughts, is also characteristic of schizophrenia. Models of thought disorder focus on a failure to use semantic information to constrain thought and language (Goldberg et al., 1998), manifest clinically as impairments in semantic generation, as measured, for example, by semantic association and sentence completion tasks (Kuperberg et al., 1998). Our sentence completion task required use of semantic information to constrain the selection of an appropriate word. The level of frontal and temporal activation during task performance was predictive of thought disorder experienced under ketamine. Schizophrenic patients with thought disorder show abnormal frontal and temporal response to this task compared with patients without thought disorder and healthy controls (Kircher et al., 2001). Together, these findings suggest that frontotemporal activity during semantic processing could represent a vulnerability marker of this symptom in schizophrenia.
Patients with schizophrenia frequently experience auditory hallucinations, perhaps reflecting an inability to monitor the agency of inner speech (Frith, 1992). The tendency to experience auditory illusory experiences under ketamine in this study was predicted by activity in medial and inferior frontal and left temporal regions during an auditory imagery task involving verbal self-monitoring (Fig. 6). Frontotemporal deficits have been implicated in auditory hallucinations (Frith, 1996) and elicited when patients with hallucinations imagine another person's speech (McGuire et al., 1995). The auditory perceptual changes under ketamine are compellingly similar to those reported during the earliest stages of schizophrenia (McGhie and Chapman, 1961) and may contribute to the development of symptoms (Chapman, 1966): such changes could be a prelude to more severe disruptions of auditory processing that are experienced as hallucinations. The magnitude (McGuire et al., 1995) and integration (Lawrie et al., 2002) of frontal and temporal lobe response is abnormal in schizophrenic patients with auditory hallucinations. The association between frontotemporal activity during verbal self-monitoring and auditory perceptual changes under ketamine suggests that individual variability in frontotemporal function may confer a vulnerability to auditory hallucinations.
Possible implications of task- and region-specific variability in individual responses
The association between regionally specific increases in brain response and subsequent drug-induced psychotic symptoms was observed in the absence of a behavioral advantage. That is, those subjects who showed increased task-related activation did so without any measurable increase in concurrent behavioral performance. This implies physiological inefficiency, because additional physiological expenditure was not manifest in improved performance. This may relate to the symptom-specific vulnerability observed and indeed parallels previous observations in patients with schizophrenia (Callicott et al., 2000). It may also underpin the nonlinear pattern of brain response seen in such patients. Inefficiency may relate to symptom vulnerability, such that functioning in inefficient regions becomes compromised by drug challenge, leading to the emergence of symptoms related to these specific processes. The neurobiological underpinning of our observed relationship, however, is unclear. Genetic variability of the NMDA system, which has been identified as a susceptibility factor for schizophrenia (Coyle et al., 2003; Harrison and Law, 2006), may be important. Blockade of NMDA receptors has been linked to the psychotomimetic effects of ketamine (Krystal et al., 1994; Tsai and Coyle, 2002), and NMDA receptor occupancy predicts the degree to which an individual will experience ketamine-induced negative symptoms (Stone et al., 2008). However, such variability is likely to be complex: NMDA receptor function may promote sustained neural activity (Castner and Williams, 2007) but also contribute to GABA-mediated collateral inhibition. One might speculate that task-related overactivation in a given individual reflects a downregulation of such inhibition, one that could portend a more profound effect on that cognitive system when that individual's NMDA function is subsequently compromised under ketamine. However, this is highly speculative, and the possibility is not addressed by our findings. Moreover, we cannot ignore interactions of NMDA with dopamine and GABA systems (Seamans and Yang, 2004). Clearly, measurement of the BOLD response alone will elucidate individual variability in responses to specific tasks, but alternative approaches will be required to establish the neurobiological underpinnings of this variability.
The influence of ketamine on brain activations
In parallel with the above observations, we investigated the effects of a lower, subpsychotic dose of ketamine on brain responses to the tasks used. A significant effect of drug was only observed during the working memory task during which subjects showed increased activation of basal ganglia and thalamus under low-dose ketamine. This is strikingly similar to the observations of Manoach et al. (2000) who compared schizophrenic patients with healthy volunteers. The increased activation of basal ganglia and thalamus in these patients could be associated with attendant psychopathology but equally may reflect compensatory changes required to maintain task performance. That similar observations are evident in our data, in the absence of psychopathology, may support the latter. Practice-related decreases in basal ganglia and thalamic activation during working memory tasks and the involvement of these regions in the formation of arbitrary visuomotor associations and abstract rules has been suggested to reflect the automation of this aspect of performance (Landau et al., 2004). Failure of this automation could therefore underlie increased responding of these regions under ketamine and perhaps in patients with schizophrenia.
We did not observe an effect of ketamine on fMRI response to the other three tasks in the study, and indeed the effect of the drug on behavioral performance was minimal across tasks. The lack of a physiological effect of ketamine on the attention and language tasks perhaps reflects the fact that these tasks were less challenging. In contrast to the working memory task, in which increased response times under drug may suggest use of compensatory responses, these may not have been required to maintain behavioral performance in these tasks. If the physiological changes observed under ketamine do indeed represent compensatory responses to aid task performance at the subpsychotic dose of the drug, then this may explain why fMRI response during the subpsychotic dose of ketamine was not directly related to symptom severity under the higher dose, because these effects do not necessarily pertain to the psychopathological features of the drug.
Conclusion
Our findings indicate a strong link between individual variability in brain responses and subsequent psychopathology. They may therefore provide a vulnerability marker to predict psychotic symptoms emerging due to drug or, potentially, due to schizophrenia. This perhaps raises the prospect of early intervention strategies targeted toward patients' individual patterns of symptom vulnerability.
Footnotes
This work was supported by a grant from The Wellcome Trust (P.C.F.). P.C.F. is Bernard Wolfe Professor of Health Neuroscience (supported by the Bernard Wolfe Health Neuroscience Fund). This work was performed within the Behavioural and Clinical Neurosciences Institute, jointly supported by the Medical Research Council and The Wellcome Trust. We thank the radiography team at the Wolfson Brain Imaging Centre for support in acquisition of the fMRI data.
References
- Adler CM, Malhotra AK, Elman I, Goldberg T, Egan M, Pickar D, Breier A. Comparison of ketamine-induced thought disorder in healthy volunteers and thought disorder in schizophrenia. Am J Psychiatry. 1999;156:1646–1649. doi: 10.1176/ajp.156.10.1646. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Flaum M, Arndt S. The comprehensive assessment of symptoms and history (CASH). An instrument for assessing diagnosis and psychopathology. Arch Gen Psychiatry. 1992;49:615–623. doi: 10.1001/archpsyc.1992.01820080023004. [DOI] [PubMed] [Google Scholar]
- Barch DM, Carter CS. Selective attention in schizophrenia: relationship to verbal working memory. Schizophr Res. 1998;33:53–61. doi: 10.1016/s0920-9964(98)00064-4. [DOI] [PubMed] [Google Scholar]
- Bloom PA, Fischler I. Completion norms for 329 sentence contexts. Mem Cognit. 1980;8:631–642. doi: 10.3758/bf03213783. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Putnam FW, Southwick SM, Marmar C, Charney DS, Mazure CM. Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS) J Trauma Stress. 1998;11:125–136. doi: 10.1023/A:1024465317902. [DOI] [PubMed] [Google Scholar]
- Brown RG, Pluck G. Negative symptoms: the “pathology” of motivation and goal-directed behaviour. Trends Neurosci. 2000;23:412–417. doi: 10.1016/s0166-2236(00)01626-x. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition. II. An empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Bertolino A, Mattay VS, Langheim FJ, Duyn J, Coppola R, Goldberg TE, Weinberger DR. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex. 2000;10:1078–1092. doi: 10.1093/cercor/10.11.1078. [DOI] [PubMed] [Google Scholar]
- Castner SA, Williams GV. Tuning the engine of cognition: a focus on NMDA/D1 receptor interactions in prefrontal cortex. Brain Cogn. 2007;63:94–122. doi: 10.1016/j.bandc.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Chapman J. The early symptoms of schizophrenia. Br J Psychiatry. 1966;112:225–251. doi: 10.1192/bjp.112.484.225. [DOI] [PubMed] [Google Scholar]
- Corlett PR, Honey GD, Aitken MR, Dickinson A, Shanks DR, Absalom AR, Lee M, Pomarol-Clotet E, Murray GK, McKenna PJ, Robbins TW, Bullmore ET, Fletcher PC. Frontal responses during learning predict vulnerability to the psychotogenic effects of ketamine: linking cognition, brain activity, and psychosis. Arch Gen Psychiatry. 2006;63:611–621. doi: 10.1001/archpsyc.63.6.611. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Tsai G, Goff D. Converging evidence of NMDA receptor hypofunction in the pathophysiology of schizophrenia. Ann NY Acad Sci. 2003;1003:318–327. doi: 10.1196/annals.1300.020. [DOI] [PubMed] [Google Scholar]
- Domino EF, Zsigmond EK, Domino LE, Domino KE, Kothary SP, Domino SE. Plasma levels of ketamine and two of its metabolites in surgical patients using a gas chromatographic mass fragmentographic assay. Anesth Analg. 1982;61:87–92. [PubMed] [Google Scholar]
- Frith C. The cognitive neuropsychology of schizophrenia. Hove, UK: Erlbaum; 1992. [Google Scholar]
- Frith C. The role of the prefrontal cortex in self-consciousness: the case of auditory hallucinations. Philos Trans R Soc Lond B Biol Sci. 1996;351:1505–1512. doi: 10.1098/rstb.1996.0136. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Aloia MS, Gourovitch ML, Missar D, Pickar D, Weinberger DR. Cognitive substrates of thought disorder. I. The semantic system. Am J Psychiatry. 1998;155:1671–1676. doi: 10.1176/ajp.155.12.1671. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Law AJ. Neuregulin 1 and schizophrenia: genetics, gene expression, and neurobiology. Biol Psychiatry. 2006;60:132–140. doi: 10.1016/j.biopsych.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Honey GD, Pomarol-Clotet E, Corlett PR, Honey RA, McKenna PJ, Bullmore ET, Fletcher PC. Functional dysconnectivity in schizophrenia associated with attentional modulation of motor function. Brain. 2005;128:2597–2611. doi: 10.1093/brain/awh632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160:13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Berenbaum H. Cognitive impairments associated with formal thought disorder in people with schizophrenia. J Abnorm Psychol. 2002;111:211–224. [PubMed] [Google Scholar]
- Kircher TT, Bullmore ET, Brammer MJ, Williams SC, Broome MR, Murray RM, McGuire PK. Differential activation of temporal cortex during sentence completion in schizophrenic patients with and without formal thought disorder. Schizophr Res. 2001;50:27–40. doi: 10.1016/s0920-9964(00)00042-6. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Jr, Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Bennett A, D'Souza DC, Abi-Dargham A, Morrissey K, Abi-Saab D, Bremner JD, Bowers MB, Jr, Suckow RF, Stetson P, Heninger GR, Charney DS. Interactive effects of subanesthetic ketamine and subhypnotic lorazepam in humans. Psychopharmacology (Berl) 1998;135:213–229. doi: 10.1007/s002130050503. [DOI] [PubMed] [Google Scholar]
- Krystal JH, D'Souza DC, Karper LP, Bennett A, Abi-Dargham A, Abi-Saab D, Cassello K, Bowers MB, Jr, Vegso S, Heninger GR, Charney DS. Interactive effects of subanesthetic ketamine and haloperidol in healthy humans. Psychopharmacology (Berl) 1999;145:193–204. doi: 10.1007/s002130051049. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, McGuire PK, David AS. Reduced sensitivity to linguistic context in schizophrenic thought disorder: evidence from on-line monitoring for words in linguistically anomalous sentences. J Abnorm Psychol. 1998;107:423–434. doi: 10.1037//0021-843x.107.3.423. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Koffel B, LaPorte D, Tamminga CA. Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology. 1995;13:9–19. doi: 10.1016/0893-133X(94)00131-I. [DOI] [PubMed] [Google Scholar]
- Landau SM, Schumacher EH, Garavan H, Druzgal TJ, D'Esposito M. A functional MRI study of the influence of practice on component processes of working memory. NeuroImage. 2004;22:211–221. doi: 10.1016/j.neuroimage.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Lawrie SM, Buechel C, Whalley HC, Frith CD, Friston KJ, Johnstone EC. Reduced frontotemporal functional connectivity in schizophrenia associated with auditory hallucinations. Biol Psychiatry. 2002;51:1008–1011. doi: 10.1016/s0006-3223(02)01316-1. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Gollub RL, Benson ES, Searl MM, Goff DC, Halpern E, Saper CB, Rauch SL. Schizophrenic subjects show aberrant fMRI activation of dorsolateral prefrontal cortex and basal ganglia during working memory performance. Biol Psychiatry. 2000;48:99–109. doi: 10.1016/s0006-3223(00)00227-4. [DOI] [PubMed] [Google Scholar]
- McGhie A, Chapman J. Disorders of attention and perception in early schizophrenia. Br J Med Psychol. 1961;34:103–116. doi: 10.1111/j.2044-8341.1961.tb00936.x. [DOI] [PubMed] [Google Scholar]
- McGuire PK, Silbersweig DA, Wright I, Murray RM, David AS, Frackowiak RS, Frith CD. Abnormal monitoring of inner speech: a physiological basis for auditory hallucinations. Lancet. 1995;346:596–600. doi: 10.1016/s0140-6736(95)91435-8. [DOI] [PubMed] [Google Scholar]
- Morgan CJ, Curran HV. Acute and chronic effects of ketamine upon human memory: a review. Psychopharmacology (Berl) 2006;188:408–424. doi: 10.1007/s00213-006-0572-3. [DOI] [PubMed] [Google Scholar]
- Nelson H. National adult reading test. New York: NFER-Nelson Publishing Company; 1982. [Google Scholar]
- Nuechterlein KH, Edell WS, Norris M, Dawson ME. Attentional vulnerability indicators, thought disorder, and negative symptoms. Schizophr Bull. 1986;12:408–426. doi: 10.1093/schbul/12.3.408. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Silver H, Feldman P. Evidence for sustained attention and working memory in schizophrenia sharing a common mechanism. J Neuropsychiatry Clin Neurosci. 2005;17:391–398. doi: 10.1176/jnp.17.3.391. [DOI] [PubMed] [Google Scholar]
- Stone JM, Erlandsson K, Arstad E, Squassante L, Teneggi V, Bressan RA, Krystal JH, Ell PJ, Pilowsky LS. Relationship between ketamine-induced psychotic symptoms and NMDA receptor occupancy-a [123I]CNS-1261 SPET study. Psychopharmacology. 2008;197:401–408. doi: 10.1007/s00213-007-1047-x. [DOI] [PubMed] [Google Scholar]
- Thiemann S, Csernansky JG, Berger PA. Rating scales in research: the case of negative symptoms. Psychiatry Res. 1987;20:47–55. doi: 10.1016/0165-1781(87)90122-3. [DOI] [PubMed] [Google Scholar]
- Tsai G, Coyle JT. Glutamatergic mechanisms in schizophrenia. Annu Rev Pharmacol Toxicol. 2002;42:165–179. doi: 10.1146/annurev.pharmtox.42.082701.160735. [DOI] [PubMed] [Google Scholar]
- Ventura J, Green MF, Shaner A, Liberman RP. Training and quality assurance with the brief psychiatric rating scale: “the drift buster.”. Int J Methods Psychiatr Res. 1993;3:221–244. [Google Scholar]