Abstract
The N-methyl-d-aspartate (NMDA) receptor antagonist ketamine produces episodic memory deficits. We used functional magnetic resonance imaging to characterize the effects of ketamine on frontal and hippocampal responses to memory encoding and retrieval in healthy volunteers using a double-blind, placebo-controlled, randomized, within-subjects comparison of two doses of intravenous ketamine. Dissociation of the effects of ketamine on encoding and retrieval processes was achieved using two study-test cycles: in the first, items were encoded prior to drug infusion and retrieval tested, during scanning, on drug; in the second, encoding was scanned on drug, and retrieval tested once ketamine plasma levels had declined. We additionally determined the interaction of ketamine with the depth of processing that occurred at encoding. A number of effects upon task-dependent activations were seen. Overall, our results suggest that left frontal activation is augmented by ketamine when elaborative semantic processing is required at encoding. In addition, successful encoding on ketamine is supplemented by additional non-verbal processing that is incidental to task demands. The effects of ketamine at retrieval are consistent with impaired access to accompanying contextual features of studied items. Our findings show that, even when overt behaviour is unimpaired, ketamine has an impact upon the recruitment of key regions in episodic memory task performance.
Keywords: encoding, episodic memory, hippocampus, ketamine, NMDA antagonist, retrieval
Introduction
Exposure to N-methyl-d-aspartate (NMDA) receptor antagonists, such as phencyclidine and ketamine, evokes a range of symptoms and cognitive deficits that are redolent of schizophrenia (Krystal et al., 1994). Ketamine is therefore increasingly used as a human pharmacological model to investigate the hypothesis that schizophrenia is characterized by glutamatergic hypofunction (Olney and Farber, 1995; Tamminga et al., 1995). A critical area of investigation in this respect is episodic memory: a cognitive domain that has been repeatedly shown to be impaired under ketamine administration (e.g. Krystal et al., 1994; Malhotra et al., 1996; Newcomer et al., 1999; Radant et al., 1998; Hetem et al., 2000). One way of specifying this impairment more clearly is through ever more precise memory manipulations (Morgan et al., 2004). Another approach is to explore the modulations of memory-related brain activations that accompany ketamine administration. Functional imaging studies have shown that encoding and retrieval of episodic information involves frontal and hippocampal regions, which contain dense populations of NMDA receptors (Lepage et al., 1998; Fletcher and Henson, 2001). Engagement of this system is sensitive to the ‘depth of processing’ (Craik and Lockhart, 1972) at encoding (Otten et al., 2001) and is associated with subsequent memory (Brewer et al., 1998; Wagner et al., 1998b; Fletcher et al., 2003). Glutamate plays a critical role in this system: long-term potentiation in hippocampus and neocortex are blocked by NMDA antagonists (Morris et al., 1986) and episodic memory disruption is a prominent feature of ketamine administration. The goal of the current study was to specify the regional effects of ketamine upon brain responses in human episodic memory.
The study also has implications for schizophrenia. Episodic memory represents a core and enduring cognitive deficit in schizophrenic patients (Calev et al., 1983; McKenna et al., 1990; Heinrichs and Zakzanis, 1998). Fronto-hippocampal dysfunction has been demonstrated in schizophrenia during both encoding (Leube and Erb, 2002; Eyler Zorrilla et al., 2003) and retrieval (Heckers et al., 1998; Weiss et al., 2003) of episodic information. A specific advantage of the ketamine model is that it allows the dissociation of impairments in encoding from retrieval processes, clearly not feasible within the context of the disease itself. This is particularly advantageous, as deficits in episodic memory in schizophrenia may be principally related to an impairment of encoding processes, due to a failure of semantic strategies (Calev et al., 1983; Gold et al., 1992; Brebion et al., 1997). In accordance with this, the deleterious effects of ketamine on episodic memory have been related to a disruption of encoding new information, rather than its retrieval from information stores (Hetem et al., 2000; Honey et al., 2005).
Our experimental design was motivated by a belief that functional neuroimaging representations of pharmacological effects demand careful matching of performance such that regional differences between on- and off-drug conditions are not interpretable in terms of overt performance differences. Of course, the implications of a functional neuroimaging difference in the absence of an overt behavioural difference may be debatable (for discussion, see Wilkinson and Halligan, 2004). We suggest, however, that functional magnetic resonance imaging (fMRI) may provide an index of the drug effects that are hidden from measures such as performance accuracy. In this sense our fMRI observations are intended as a complement to, rather than a replication of, a behavioural psychopharmacology study. The current experiment was therefore designed on the basis of a previous purely behavioural study of the effects of ketamine on a related episodic memory task in which we made more wide-ranging manipulations in our encoding tasks (Honey et al., 2005). This prior study showed that ketamine, at the doses used here, does indeed have an impact upon episodic memory and, on the basis of this study, we selected encoding conditions in which there was no overt impairment in order to characterize the sub-behavioural effects on frontal and hippocampal activity.
We used an event-related fMRI design to establish ketamine’s effects upon encoding and retrieval-related brain activation. At encoding, we were interested in the drug’s effects upon frontal and hippocampal activity in response to a depth of processing manipulation, and also whether it influences the extent to which activation in these areas is predictive of subsequent memory performance. In so doing, we aimed to identify the effects of NMDA blockade upon the cognitive processes that are intimately related to memory encoding. Our interest in retrieval was more exploratory and primarily attempted to establish whether, despite the behavioural evidence that the drug’s deleterious effects are confined to encoding (Hetem et al., 2000; Honey et al., 2005), it has any influence upon the brain systems engaged during recognition.
Materials and Methods
Subjects
Twelve right-handed healthy volunteers (six males) were recruited by advertisement. All subjects gave written informed consent prior to involvement and received an honorarium for participation. The research protocol was approved by the Addenbrooke’s NHS Trust research ethics committee. No subject had a history of psychiatric disorders, serious medical illness or drug abuse in the last 12 months. Subjects had a mean age of 31.17 years (range 18-49), mean NART IQ of 104.67 (± 12.71) (Nelson, 1982), were within 10% of ideal body mass index and spoke English as their primary language.
Subjects undergoing this study also carried out a working memory study: these data are reported elsewhere (Honey et al., 2004)
Experimental Design
The study was a double-blind, placebo-controlled, randomized, within-subjects comparison of fixed steady-state doses of intravenous ketamine. An unblinded clinician prepared and administered the infusions, but was not involved in clinical or cognitive testing. Subjects attended on three occasions receiving a different infusion on each occasion (saline, 50 ng/ml plasma ketamine and 100 ng/ml plasma ketamine). Order of infusion was counterbalanced across subjects. The use of a within-subjects design could lead to contamination of results by longer-lasting effects of ketamine. Scanning sessions for each subject were 7 days apart in order to minimize this.
Infusion Protocol
Prior to testing, bilateral upper extremity intravenous catheters were inserted, one for ketamine infusion, the other for serial blood sampling for plasma ketamine levels. Racemic ketamine (1 mg/ml solution) was administered by bolus and continuous infusion using a computerized pump (Graseby 3500, Graseby Medical Ltd, UK). The pump was programmed (Anaetech Ltd, UK) to deliver a rapid bolus dose (< 60 s) followed by varying infusion rates to achieve constant estimated target plasma concentrations of 50 or 100 ng/ml, using pharmacokinetic parameters of a three-compartment model described by Domino et al. (1982). At the conclusion of testing (~1 h later) target plasma levels were reduced such that estimated plasma ketamine levels 90 min following behavior testing would be ~28 ng/ml in both dosing groups (see below). Blinding of each subject and the investigator performing the clinical assessments was assured by use of the same infusion pump and pump adjustment schedule on all three study days programmed by a single investigator not involved in cognitive assessment.
Peripheral venous blood samples were drawn on three occasions: 10 minutes after the infusion began, at the completion of the study phase for list 2, and at the end of the test phase for list 2 (~60 min post-encoding list 1; see Fig. 1). The latter two measures only are relevant to the testing reported here and we will present results from these only. In total, subjects were maintained on ketamine for ~1 h. Blood samples were placed on ice, plasma obtained by centrifugation, and plasma samples stored at −20°C. Ketamine plasma levels were measured by gas chromatography-mass spectrometry (Kharasch and Labroo, 1992).
Figure 1. Diagrammatic representation of experimental protocol.
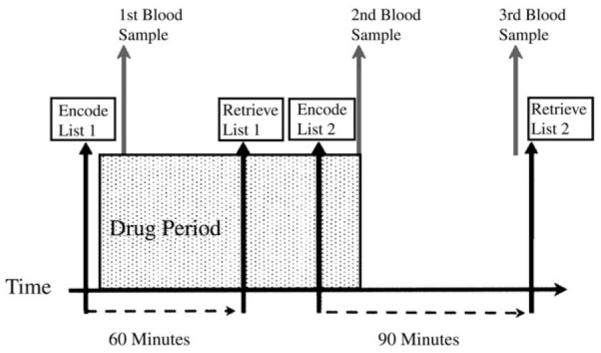
The period of drug exposure, shown in red, included the retrieval of the first study list, and encoding of the second study list.
Episodic Memory Task
Study items consisted of 720 words selected from the MRC psycholinguistic database (www.psy.uwa.edu.au/mrcdatabase/uwa_mrc.htm), and divided into three sets used for each of the three study visits, matched for frequency of occurrence in written English (Francis and Kucera, 1982). Items were counterbalanced across foil and targets, as well as across tasks and drug dosage. Words were visually presented on a computer screen, using DMDX (Forster and Forster: www.u.arizona.edu/~jforster/dmdx/official.htm). The two experimental manipulations (encoding versus retrieval and depth of processing) were made as follows:
(i) Exploring Separately the Effects of Ketamine on Encoding and Retrieval
During each visit, subjects were presented with two lists, each consisting of 90 words, constituting two separate study phases; the two lists were used to assess separately effects of ketamine on encoding and retrieval. The design is summarized in Figure 1. The first encoded list (list 1) was presented prior to drug infusion. Recognition of these items was then tested ~60 min after drug administration was initiated. A second study list (list 2) was presented to subjects for encoding while steady-state infusion was maintained. The estimated plasma ketamine level was then allowed to decline and retrieval was tested 1 h later.
Encoding and retrieval each occurred in the presence and absence of ketamine. fMRI data were acquired during the recognition phase of list 1, and during the encoding phase of list 2. We will refer to these as the Retrieval and Encoding conditions respectively. Observed disruption of neurophysiological responses induced by ketamine during retrieval of list 1 items could be confidently attributed to a direct effect on retrieval processes, given that encoding occurred prior to drug treatment. The success of encoding of list 2 items under ketamine was measured by testing subsequent retrieval after the infusion had been stopped and the ketamine plasma level had significantly reduced.
(ii) Depth of Processing Manipulation
Each of the 90 item lists comprised 45 items for each of two study tasks, corresponding to subjective binary judgements of pleasantness (pleasant/unpleasant; deep encoding) or number of syllables (even/odd); shallow encoding). Each item was preceded by an instruction (‘Pleasant?’ or ‘Syllables?’) to specify the corresponding decision required, and to further dissociate the task requirements, the deeply encoded items were presented at the top of the screen, while shallowly encoded items were presented at the bottom (in half the subjects, the positions were reversed). Subjects indicated a yes/no (‘yes’ if pleasant/even number of syllables) response via a keyboard. Items were presented in a randomized order for 3.5 s, with an interstimulus interval (ISI) of 2 s. These two levels of encoding were selected on the basis of findings from our prior study, indicating that ketamine disrupts recognition of items within an intermediate window of depth, whereas very deeply encoded items and items recognized on a sense of familiarity, rather than recollection of contextual detail, are not affected (Honey et al., 2005). We therefore intended that item recognition would not be disrupted at the behavioural level in this study, in order to avoid the issue of interpretation of drug-related changes in the context of confounding behavioural deficits.
Recognition memory was tested for each of the study lists ~1 h subsequent to the corresponding study phase. For each list, subjects were presented with 135 sequentially presented items, comprising the 90 items presented at study, and an additional 45 new items which the subject had not previously seen. Items were presented in the centre of the screen for a duration of 3.5 ms (ISI = 2.5 ms). Participants were instructed to press one of two buttons on a keypad to indicate whether each item had been previously presented during the study phase.
fMRI Data Acquisition
Imaging data were collected using a Bruker Medspec (Ettlingen, Germany) scanner operating at 3 T. T2*-weighted echo-planar images, depicting BOLD contrast, were acquired in each session (TE, 27.5 ms; TR, 1.1 s). 21 slices (each of 4 mm thickness; interslice gap, 1 mm; matrix size, 65 × 64) per image were acquired. For the Retrieval condition, 530 whole-brain volumes were acquired; for the Encoding condition, 360 volumes were acquired. The first six EPI images in each session were subsequently discarded to avoid T1 equilibration effects.
fMRI Data Analysis
fMRI data were analysed using statistical parametric mapping (SPM2; Wellcome Department of Cognitive Neurology, London, UK). This included slice acquisition time correction, within-subject image realignment, spatial normalization to a standard template (Friston et al., 1995) and spatial smoothing using a Gaussian kernel (8 mm full width at half-maximum). The time series in each session was high-pass filtered (to a maximum of 1/120 Hz).
Events were specified as occurring at the presentation of the verbal stimulus. For the Encoding condition, events were designated both according to the task instruction at presentation (deep versus shallow) and according to subsequent performance (post-scanning) on the recognition memory test (subsequently remembered versus subsequently forgotten). Four event types were therefore modelled: ‘deep encoding/remembered, ‘deep encoding/forgotten, ‘shallow encoding/remembered’ and ‘shallow encoding/forgotten’. For the Retrieval condition, events were identified according to whether or not the word had been previously presented during the study phase (old versus new); whether or not the word was correctly identified as old or new and, if the word was old, whether prior encoding had been deep or shallow six event types were therefore modeled: ‘new item/correct’, ‘new item/incorrect’, ‘deep encoding/correct’, ‘deep encoding/incorrect’, ‘shallow encoding/correct’ and ‘shallow encoding/incorrect’. Each of these event types were separately modelled using a canonical, synthetic haemodynamic response form (Friston et al., 1998). This function was used as a covariate in a general linear model in order to generate a parameter estimate for each voxel for each event type.
fMRI Comparisons
Encoding
Ketamine Effects on ‘Depth of Processing’ Activations
We contrasted brain responses to deeply encoded items with responses to shallowly encoded items. We were then able to explore the influence of ketamine on these activations.
Ketamine Effects on Brain Activations Predictive of Subsequent Memory (Dm)
In order to estimate brain responses signifying successful encoding we sorted trials according to whether words were subsequently recognized or not [the ‘difference due to subsequent memory’ (Dm) effect]. We were then able to compare successful with unsuccessful encoding trials and to determine the effects of ketamine upon these activations
Retrieval
Ketamine Effects on Activations Reflecting Depth of Prior Encoding
For old items only, we compared the brain activations for those items that had been subject to the deep encoding task with those that had been subject to the shallow task.
Ketamine Effects on Correctly Identified Compared to Incorrectly Identified Items
Items which were correctly identified as either old or new items were contrasted with those which were incorrectly identified as old or new.
Ketamine Effects on Old versus New Items
Items which had been previously presented at encoding were contrasted with new items which had not previously been seen.
Identifying Task Effects and Drug-by-Task Interactions
The linear combinations of parameter estimates for each contrast were stored as separate images for each subject. These contrast images were entered into a second level one-sample t-test to permit inferences about condition effects within the placebo group, treating inter-subject variability as a random effect. Based on findings from a number of similar previous studies we restricted our analyses to regions that have shown task related activation during episodic memory encoding and retrieval (for review, see Fletcher and Henson, 2001). We therefore constructed a region of interest (ROI) involving bilateral frontal cortex, incorporating Brodmann regions 9, 10, 11, 44, 45, 46 and 47, and bilateral hippocampi and parahippocampal gyri (areas 28, 35 and 36) (Maldjian et al., 2003). For these a priori regions of interest, a threshold of P < 0.005, uncorrected for multiple comparisons, was set for these analyses. In order to reduce type I error, a limit was set of >5 voxels for activated foci.
Analysis of drug effects was conducted using a within-subjects repeated-measures analysis of variance (ANOVA) model. We identified and explored dose-dependent drug-task interactions using a linked series contrasts. First, we established regions showing drug effects on task-related activations for each of the contrasts described above using voxel-wise F-tests (confined to the frontal and medial temporal masks described above). Second, we excluded regions in this system in which there was no significant effect of the higher dose of ketamine compared to placebo. We note that this strategy of analysis will not identify regions for which observed differences at the repeated measures ANOVA are due to differences between the 50 ng/ml ketamine condition and placebo, where no differences are evident between placebo and the 100 ng/ml condition. We accept this limitation on the basis that such effects were not predicted a priori, and have not been previously reported in the literature. We therefore constrain our analysis to regions demonstrating a significant effect of the 100ng/ml condition, and examine dose-related effects only in these regions. Third, for those regions satisfying these two criteria (in which there was a significant drug by task interaction and an effect produced by the higher dose of the drug alone), we plotted parameter estimates across the three conditions (placebo, 50 ng/ml ketamine and 100 ng/ml ketamine) in order to identify the profile and dose-dependence of the drug effect.
A final stage of the analysis sought to determine whether observed treatment effects represented a modulation of normal task-related activation/deactivation, or an effect outside this network. That is, we determined whether the drug-by-task interaction occurred in a region that was sensitive to the task under placebo only or whether it occurred in a region that was not activated (or deactivated) under placebo conditions. We used a masking procedure to determine areas of overlap between placebo and drug effect. As this stage was not inferential, our primary concern was to avoid type II error, therefore these analyses were thresholded at P < 0.05 uncorrected, in order to detect activation in the placebo data that may be evident below conventionally accepted statistical thresholds. In reporting each of the drug effects, we report also whether the effect occurred within system activated under placebo, or outside it.
Behavioural Data Analysis
Accuracy of recognition was indexed by the discrimination accuracy measure Pr, [probability of a hit (Phit) minus probability of a false alarm (Pfalse alarm)]. Discrimination accuracy was then compared separately under each of the drug treatments using a repeated-measures 3 (drug) × 2 (level of processing) ANOVA model, applied separately to data from list 1 (Retrieval Effect Condition) and list 2 (Encoding Effect Condition).
Results
Plasma Levels
Plasma level sampled during the infusion closely reflected those predicted by the pharmacokinetic model, with a mean of 47.7 ng/ml in the 50 ng/ml dose condition and 102.3 ng/ml in the 100 ng/ml dose condition.
For the 50 ng/ml condition, the mean plasma level sampled 1 h after infusion had ceased, subsequent to the test phase for list 2, was also in line with the predicted reduction in plasma level to a target of 28 ng/ml (29.6 ± 8.3 ng/ml); this was slightly higher in the 100 ng/ml condition (41.6 ± 7.6 ng/ml). It is important to note that the effect of ketamine upon the activation during encoding and retrieval reported in this study are unaffected by the slightly increased residual levels of drug measured 1 h subsequent to the end of the scanning session.
Behavioural Results
For the encoding condition, there was a significant main effect of depth on reaction time, with slower responses for the syllables judgment (shallow encoding condition) compared to the pleasant/unpleasant judgment (deep encoding condition) [repeated-measures ANOVA; F(1,11) = 19.469, P < 0.001] (see Table 1). This is in line with a large number of previous studies, and indicates that the mnemonic advantage associated with deep encoding is not simply attributable to increased time on task. There was no main effect of drug or drug × depth interaction on reaction time (P > 0.05).
Table 1. Reaction times (seconds) for the rncoding task across drug treatments with standard deviations shown in parentheses.
| Depth | Encoding |
||
|---|---|---|---|
| Placebo | Ketamine 50 ng/ml | Ketamine 100 ng/ml | |
| Deep | 1.94 (0.19) | 1.9 (0.21) | 1.89 (0.21) |
| Shallow | 2.09 (0.21) | 2.09 (0.24) | 2.14 (0.18) |
Item recognition (Pr) scores are shown in Table 2. There was significant effect of depth for both lists 1 and list 2, with item recognition greater for deep compared to shallow items under all treatment conditions [repeated-measures ANOVA; list 1: F(1,11) = 6.287, P = 0.029; list 2: F(1,11) = 6.74, P = 0.025]. There was no significant effect of drug on item recognition for either list (P > 0.05).
Table 2. Discrimation index (Pr) scores across drug treatments and levels of processing at encoding for list 1 (effect of drug on retrieval) and list 2 (effects of drug on encoding).
| Depth | List 1 (retrieval) |
List 2 (encoding) |
||||
|---|---|---|---|---|---|---|
| Placebo | Ketamine 50 ng/ml |
Ketamine 100 ng/ml |
Placebo | Ketamine 50 ng/ml |
Ketamine 100 ng/ml |
|
| Deep | 0.6 (0.13) | 0.59 (0.18) | 0.62 (0.13) | 0.57 (0.14) | 0.6 (0.12) | 0.53 (0.14) |
| Shallow | 0.34 (0.14) | 0.4 (0.14) | 0.29 (0.17) | 0.31 (0.14) | 0.27 (0.19) | 0.3 (0.14) |
| Total | 0.48 (0.12) | 0.5 (0.14) | 0.46 (0.13) | 0.44 (0.1) | 0.44 (0.14) | 0.43 (0.11) |
fMRI Results: Effects of Ketamine on Encoding
Placebo
Depth of Encoding
Regions within the fronto-hippocampal ROI that showed greater activity during the deep relative to the shallow encoding task, and survived the set threshold of P < 0.005, are shown in Figure 2A and detailed in Table 3A. These regions included bilateral (predominantly left) inferior frontal gyrus, medial prefrontal cortex and bilateral hippocampus.
Figure 2. Activation associated with episodic encoding during placebo treatment.
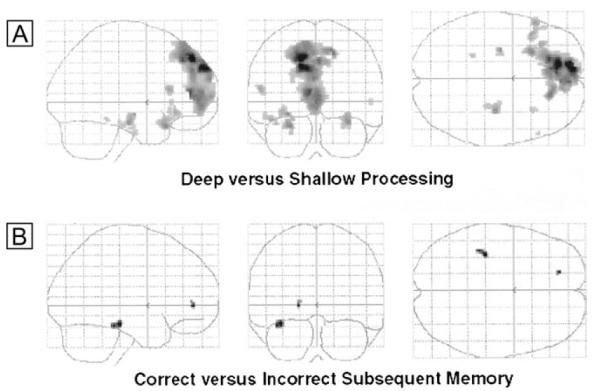
The maximum intensity projections or ‘glass brain’ figures (viewed from the right, from behind and from above, thresholded at P < 0.005, uncorrected) show activations associated with the deep versus shallow (A) and difference due to subsequent memory (Dm) (B) contrasts. Details of the activations are given in Table 3.
Table 3. Regions of increased activation during encoding under placebo.
| Region | MNI coordinates | Z-score | ||
|---|---|---|---|---|
| (A) Deep versus shallow encoding | ||||
| Medial prefrontal cortex | −14 | 46 | 44 | 4.35 |
| Hippocampal gyrus | −26 | 20 | −24 | 3.5 |
| Hippocampus | −28 | −12 | −12 | 3.43 |
| 32 | −18 | −20 | 3.42 | |
| Dorsolateral prefrontal cortex | −56 | 22 | 14 | 3.2 |
| Ventrolateral prefrontal cortex | −44 | 18 | −18 | 3.18 |
| 32 | 20 | −22 | 2.84 | |
| (B) Subsequent correct versus subsequent incorrect | ||||
| Hippocampus | −36 | −28 | −18 | 3.27 |
| Anterior cingulate | −18 | 46 | 0 | 3.09 |
Coordinates refer to the position (x, y and z mm) according to the Montreal Neurological Institute (MNI) template.
Subsequent Memory Effect
Items that were subsequently remembered at test were associated with increased activity in left hippocampus and anterior cingulate compared to items that were subsequently forgotten (see Fig. 2B and Table 3B).
Drug Effects
Depth of Encoding
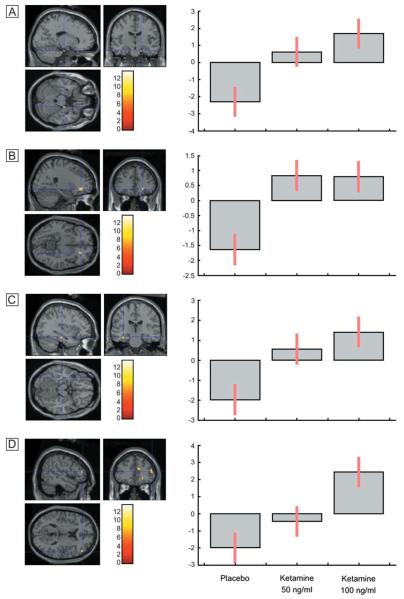
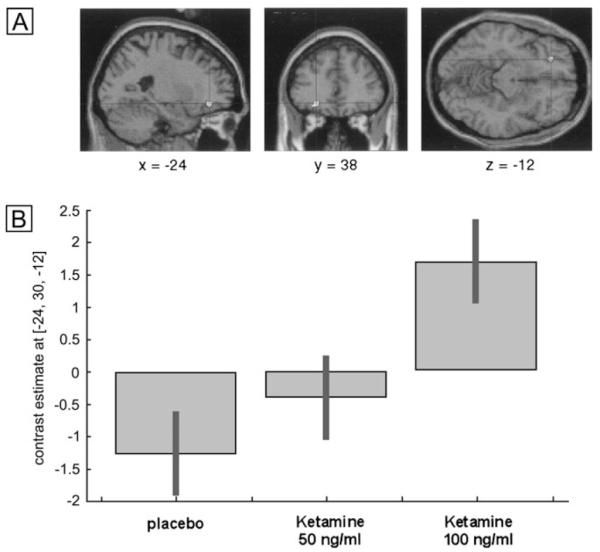
An effect of drug treatment on the depth of processing was observed in left ventrolateral prefrontal cortex (MNI x,y,z coordinates: −24 38 −12) [repeated-measures ANOVA; F(2,22) = 9.76; Z = 3.11; P = 0.001] (see Fig. 3A). Post hoc paired t-tests between placebo and the 100 ng/ml ketamine condition indicated that this represented an increased response under ketamine. Inspection of the depth of processing contrasts for the placebo data and ketamine data indicated that this region was active for shallow compared to deep items under placebo and deep compared to shallow items under ketamine. It is evident from the plot of the parameter estimates across conditions that this represented a linear dose-dependent increase across the three treatments (see Fig. 3B).
Figure 3. Dose-dependent increase in a region of left prefrontal cortex for deep compared to shallowly encoded items under ketamine compared to placebo.
Subsequent Memory
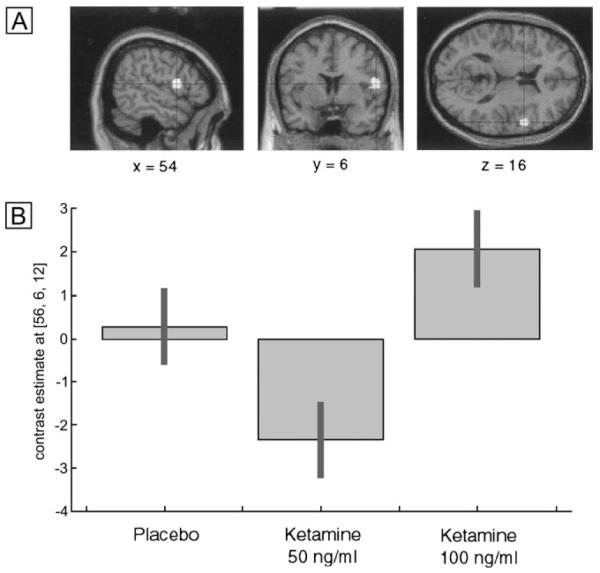
An effect of drug treatment for items subsequently correctly identified compared to items subsequently forgotten was observed in the right inferior frontal gyrus [MNI: 54 6 16; F(2,22) = 13.79; Z = 3.65; P = 0.0001]. Post hoc comparisons showed that activation was significantly increased under the 100 ng/ml ketamine condition compared to placebo (see Fig. 4A). Inspection of the within-treatment contrast between correct and incorrect items indicates that this region was not activated under placebo, and was increased for correct items under ketamine. The plot of the parameter estimates indicates that while increased activation of this region was evident for the 100 ng/ml condition, the 50 ng/ml condition was associated with reduced activity (see Fig. 4B).
Figure 4. Increased activation of right prefrontal cortex during encoding under ketamine compared to placebo, associated with items which were subsequently correctly identified, compared to incorrectly identified items.
Activation was reduced for the 50 ng/ml condition.
fMRI Results: Effects of Ketamine on Retrieval
Placebo
Depth of Prior Encoding
Bilateral regions of dorsal and ventral lateral prefrontal cortex showed greater activity during retrieval for items that had previously been deeply encoded compared to shallowly encoded items (see Table 4A and Fig. 5A).
Table 4. Regions of increased activation during retrieval under placebo.
| Region | MNI coordinates | Z-score | ||
|---|---|---|---|---|
| (A) Retrieval of deep versus shallow items | ||||
| Middle frontal gyrus | −42 | 38 | 16 | 3.79 |
| 48 | 38 | 22 | 3.02 | |
| Superior frontal gyrus | −38 | 44 | 38 | 3.14 |
| 42 | 18 | 50 | 3.41 | |
| (B) Incorrect versus correct retrieval | ||||
| Anterior cingulate/medial frontal cortex | 8 | 40 | 18 | 3.45 |
| −16 | 42 | 20 | 3.07 | |
| (C) Retrieval of new versus old items | ||||
| Middle frontal gyrus | −42 | 30 | 24 | 4.45 |
| 54 | 10 | 42 | 4.32 | |
| Hippocampus/hippocampal gyrus | 30 | −34 | −20 | 3.9 |
| −38 | −32 | −28 | ||
| −16 | −2 | −18 | 3.64 | |
| 14 | 0 | −28 | 3.36 | |
| −14 | −2 | −26 | 3.03 | |
| Medial frontal cortex | −6 | 60 | 10 | 3.85 |
| −4 | 56 | −14 | 3.12 | |
| Superior frontal gyrus | 28 | 56 | 18 | 3.45 |
| −22 | 48 | −12 | 3.29 | |
Coordinates refer to the position (x, y and z mm) according to the Montreal Neurological Institute (MNI) template.
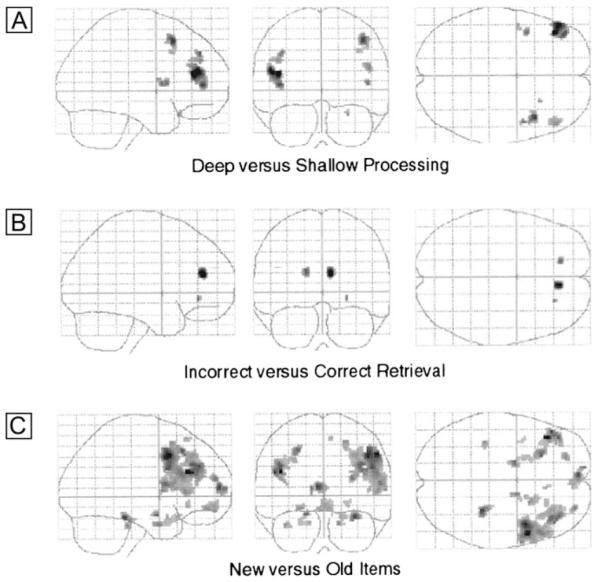
Figure 5. Activation associated with episodic retrieval during placebo treatment.
Glass brain images (threshold at P < 0.005, uncorrected). (A) Activation associated with retrieval of deep, compared to shallowly encoded items. (B) Activation associated with items which were incorrectly identified at test compared to correct responses. (C) Activation associated with items which had not been presented at encoding, compared to items previously encountered.
Correct versus Incorrect Item Recognition
There were no regions that demonstrated increased activation for items which were correctly identified, at the threshold of P < 0.005. However, incorrectly identified items were associated with increased activation in bilateral anterior cingulate cortex (see Fig. 5B and Table 4B).
Old versus New Items
Response to previously presented items was not associated with increased activation in comparison to new items. However, increased activation for new compared to old items was observed in extensive regions of bilateral medial and lateral frontal cortex, and rostral and posterior aspects of bilateral hippocampus (see Fig. 5A and Table 4C).
Drug Effects
Depth of Prior Encoding
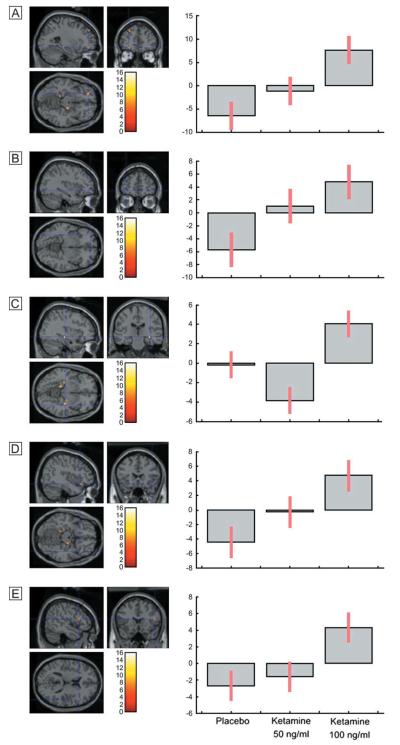
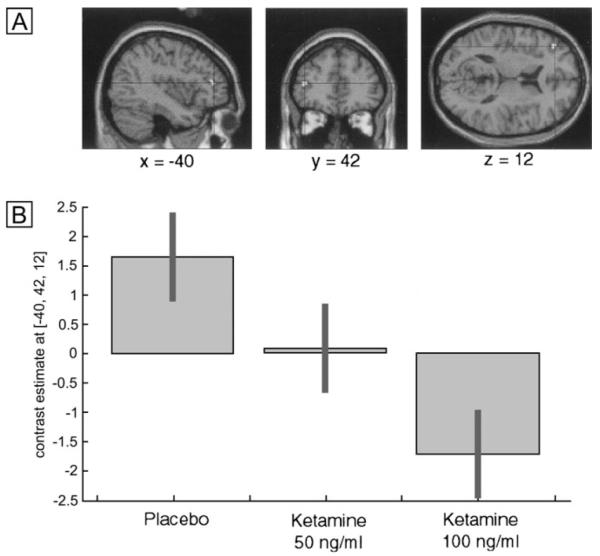
An effect of drug treatment on the contrast between retrieval of items that had previously been deeply encoded, compared to shallowly encoded items, was observed in the left middle frontal gyrus [MNI: −40 42 12; F (2,22) = 9.10; Z = 3.02; P = 0.001] (see Fig 6A). Post hoc paired t-tests between placebo and the 100 ng/ml ketamine condition indicated that this represented a reduced response under ketamine. Inspection of the within-treatment depth of processing contrasts indicated that this region was active for retrieval of deeply compared to shallowly encoded items under placebo, and no activation was evident under ketamine treatment. It is evident from the plot of the parameter estimates across conditions that this represented a linear dose-dependent reduction across the three treatments (see Fig. 6B).
Figure 6. Dose-dependent decrease of left frontal activation for deep compared to shallowly encoded items under ketamine compared to placebo.
Correct versus Incorrect Item Recognition
Items which were successfully identified, compared to incorrectly identified items, were associated with an effect of ketamine in anterior cingulate [MNI: 24 36 −8; F(2,22) = 13.61, Z = 3.63, P = 0.0001], right inferior frontal gyrus [MNI: 52 40 12; F(2,22) = 12.07, Z = 3.44, P = 0.0001] left hippocampus [MNI: −34 −22 −16; F(2,22) = 9.72; Z = 3.11, P = 0.001] and right hippocampal gyrus [MNI: 18 −14 −24; F(2,22) = 10.46, Z = 3.22, P = 0.001] (see Fig. 7). Post hoc t-tests indicated that this represented an increased response under ketamine. The foci in the anterior cingulate was also observed under placebo treatment, with an increased response to incorrectly identified items, compared to correct identification, and an increased response to incorrectly identified items under ketamine. The other regions for which an effect of drug modulation were observed were outside the network identified under placebo, but were evident for incorrect compared to correct items under ketamine. Inspection of the parameter estimates across treatments shows that an increased response was also observed in the 50 ng/ml condition in all regions, with the exception of the right inferior frontal foci (see Fig. 7).
Figure 7. Regions demonstrating increased activation for correct compared to incorrectly identified items under ketamine compared to placebo.
Old versus New Items
The contrast between recognition of items presented during encoding compared to new items was associated with an effect of drug treatment in foci in ventrolateral frontal regions on the left [MNI: −26 46 −12; F(2,22) = 10.55, Z = 3.23, P = 0.001; MNI: −34 52 −6; F(2,22) = 7.72, Z = 2.76 P = 0.003] and right hemispheres [MNI: 42 22 −14; F(2,22) = 8.52, Z = 2.91, P = 0.002; MNI: 50 18 14; F(2,22) = 8.2, Z = 2.25, P = 0.002], and right hippocampus [MNI: 36 −22 −8; F(2,22) = 15.9, Z = 3.87, P = 0.0001] (see Fig. 8). Post-hoc t-tests indicated that this represented an increased response under ketamine compared to placebo. All of these regions also showed increased activation under placebo to new items, compared to those presented at encoding, but an increased response to old compared to new items under ketamine. Inspection of the parameter estimates across treatments shows that a linear dose response was observed across conditions for the bilateral frontal foci. In the right hippocampus, activation was increased under 100 ng/ml ketamine, but was reduced for the 50 ng/ml condition (see Fig. 8).
Figure 8. Regions demonstrating increased activation for old compared to new items under ketamine compared to placebo.
Summary of Results
There were two notable effects of ketamine on encoding. Firstly, increased activation of a region of left prefrontal cortex was observed under ketamine treatment to deeply encoded items (Fig. 3). This region showed relatively greater activation for shallowly encoded items under placebo. Secondly, items which were subsequently correctly identified under ketamine were associated with increased activation of right prefrontal cortex during encoding compared to incorrectly identified items (Fig. 4). This region was not a predictor of subsequent memory under placebo.
The effect of ketamine on retrieval was to reduce activation of a region of left prefrontal cortex observed under placebo for items which had previously been deeply encoded, compared to shallowly encoded items (Fig. 6). Items which were incorrectly identified at retrieval were associated with increased activation of right prefrontal cortex and left hippocampus, which was not evident under placebo, and increased activation of the anterior cingulate which was evident for incorrectly identified items under placebo (Fig. 7). Finally, increased activation was observed in bilateral prefrontal cortex and left hippocampus under ketamine for items which had previously been presented at encoding, compared to new items (Fig. 8); conversely, these regions were increased for new items under placebo compared to old items.
Discussion
The current study demonstrates modulation of task-dependent frontal and hippocampal activation during episodic memory following NMDA receptor blockade via administration of sub-dissociative doses of ketamine. The pattern of changes may provide some indication of the cognitive nature of mnemonic changes produced by ketamine and we consider these below. Furthermore, while existing behavioural studies have proven sensitive to the effects of ketamine at encoding (Oye et al., 1992; Hetem et al., 2000; Honey et al., 2005), using fMRI we have shown that there are nevertheless profound effects of the drug when encoding occurred prior to drug administration. These effects on episodic retrieval are consistent with reduced access to contextual features of studied items. Such a reduction would be associated with, for example, changes in source memory ability, an effect that we have indeed observed in our previous work on the behavioural effects of ketamine (Honey et al., 2005).
The Effects of Ketamine at Encoding
In brief, ketamine produced two significant effects on encoding: an increased left frontal activation associated with the deep (compared to the shallow) encoding task and an additional subsequent memory effect in right lateral prefrontal cortex. While we provide some tentative explanations for these findings in the following two paragraphs, we do so with caution since the observations were not predicted and since the pattern is difficult to explain parsimoniously.
Under placebo, activation in the left hippocampus at encoding predicted subsequent memory, as expected (Brewer et al., 1998; Wagner et al., 1998b; Henson et al., 1999b; Kirchhoff et al., 2000; Otten et al., 2001). Under ketamine administration, right lateral prefrontal cortex also predicted subsequent successful recognition. Otten et al. (2001) also showed a subsequent memory effect in right prefrontal cortex and suggested that such activation, also observed in non-verbal episodic memory tasks (Brewer et al., 1998; Wagner et al., 1998a; McDermott et al., 1999), could reflect incidental non-verbal strategies. Our observation would be consistent with an increase in such incidental non-verbal processing under ketamine, a phenomenon that, at higher doses than those used here, is associated with thought disorder (Malhotra et al., 1996; Adler et al., 1999; Duncan et al., 2001). In the current study, though this processing is incidental to task demands, it may have beneficial effects on episodic memory, as indicated by the fact that activity in right prefrontal cortex is predictive of subsequent memory under ketamine.
Ketamine also produced a dose-dependent increase in the left frontal response to the deep encoding task. There was no evidence for increased activation in this area of left prefrontal cortex for deeply encoded items on placebo (indeed there was trend towards increased activation for the shallow condition; P < 0.05, uncorrected). The precise nature of this effect is presently unclear. Left inferior frontal gyrus activation during semantic processing tasks has been discussed in terms of controlled retrieval (Wagner et al., 2001), selection (Thompson-Schill et al., 1997) and sculpting operations (Fletcher et al., 2000), among others (Fletcher and Henson, 2001). While our data are unable to specify the effects of ketamine more fully, it is possible that increased activation of this region in response to the pleasant/unpleasant judgement may reflect difficulties in identifying and selecting the semantic attributes that are relevant to such a judgement. This effect may underlie findings reported at higher doses and with greater task demands, for which performance is measurably impaired and is accompanied by disordered thinking (Adler et al., 1998).
The Effects of Ketamine at Retrieval
Ketamine also had significant effects on the frontal and hippocampal activity during retrieval of episodic information. This is a novel finding given the existing behavioural psychopharmacological data (Oye et al., 1992; Hetem et al., 2000; Honey et al., 2005) and highlights the complementarity of the psychopharmacological fMRI approach to delineating the cognitive effects of the drug. We have shown that ketamine was associated with an attenuation of the left prefrontal response to items that had been deeply encoded. This left frontal response under placebo is consistent with previous data suggesting that retrieval based upon semantic features is associated with greater left prefrontal cortex activation (Fletcher et al., 1996; Cabeza et al., 2003). It is also consistent with studies suggesting left prefrontal cortex involvement when retrieval is accompanied by memory for source (Nolde et al., 1998; Henson et al., 1999a; Rugg et al., 1999). The reduced activation under ketamine may indicate a failure to retrieve the contextual detail that would normally accompany recollective processing.
If this conclusion — that accompanying contextual details may be attenuated when ketamine is present at retrieval — is correct, then it suggests that retrieval would become more demanding under drug administration. Our further analyses are consistent with this suggestion: ketamine was associated with increased activation of bilateral prefrontal cortex and right hippocampus in association with studied compared to new items. Henson and colleagues have shown that lateral prefrontal cortex activation is greater for items in which the memory trace is reduced (Henson et al., 1999b), suggesting the importance of this region in monitoring the products of retrieval. Clearly, if there is less access to contextual detail then increased monitoring and hence prefrontal cortex activation would be expected under ketamine. The opposite relationship was observed under placebo, with these regions responsive to new compared to studied items. Increased activation of these regions under placebo presumably reflects an increased search of memory stores for which the subject could have had no memory trace (Schacter et al., 1996).
Interestingly, we also found an effect of ketamine upon error-related activation. Anterior cingulate activation was greater in response to incorrect trials, both misses and false positives. This is consistent with the findings of Carter et al. (1998) that anterior cingulate cortex contributes to performance monitoring by detecting errors and response conflict, and shows increased activation to erroneous responses. Under ketamine, anterior cingulate activity was actually reduced for incorrect compared to correct responses: a further suggestion that recognition under the drug may be impoverished.
One cautionary note is important: it might be argued that characterizing the effects of ketamine, or any drug, upon task-specific brain activity, is ultimately confounded by the possibility that the drug has an impact upon baseline activation. That is, the changes in activation are actually produced by a shifting baseline rather than by a change in the brain response to the task itself. With this in mind, we chose not to use ‘baseline’ events in the current study. Consequently, all comparisons that we report are related to differentiations within the memory tasks rather than to changes relative to a low level baseline or resting condition. Thus, we have avoided relying upon an under-specified baseline condition in the hope that our interpretations of the drug effects may stand on firmer ground.
Ketamine as a Model for Schizophrenia?
Overall, the findings in this study have implications for the ketamine model of schizophrenia. Of course, the comparison of the current data with previous studies involving patients is complicated by several considerations. First, the present study necessarily involved acute infusion, however one might conjecture, although it would be almost impossible to test, that chronic administration of PCP and ketamine could provide a more valid model of schizophrenia (Tsai and Coyle, 2002). Second, a key aim in this study was to dissociate the effects of ketamine on encoding and retrieval processes. Clearly, such a dissociation would be impossible in people with schizophrenia. Finally, the comparison to studies with schizophrenia is complicated by the use of resting baseline measures in patient studies of episodic memory; this was avoided in the present study, as there is substantial evidence that baseline measures of metabolism are disrupted in frontal and hippocampal regions at rest following ketamine treatment (Lahti et al., 1995; Breier et al., 1997; Vollenweider et al., 1997a,b; Holcomb et al., 2001). Nevertheless, our observation of regional changes during episodic memory following ketamine treatment resemble those reported in patients with schizophrenia. Studies of verbal encoding have shown that patients with schizophrenia show reduced activation of right (Hofer et al., 2003) and left (Ragland et al., 2001) prefrontal cortex and left hippocampus (Jessen et al., 2003) during verbal encoding, and reduced right hippocampal activation during encoding of visual information (Leube and Erb, 2002; Eyler Zorrilla et al., 2003). Frontal and hippcampal deficits have also been reported during episodic retrieval (Heckers et al., 1998; Ragland et al., 2001; Weiss et al., 2003), and are dependent on the depth of processing at encoding (Heckers et al., 1998).
The mechanism by which ketamine disrupts frontal and hippocampal function during episodic memory remains unknown. Ketamine administration leads to a blockade of NMDA receptors, and consequent hypoactivity. NMDA receptors are located throughout the brain, but the highest densities are found in the cortex, limbic system and striatum (Monaghan and Buller, 1995). This mechanism may therefore relate to our finding of reduced frontal activation during retrieval of deeply encoded items. However, acute administration of ketamine also results in increases in both cortical glutamate and dopamine (Moghaddam et al., 1997) most likely via stimulation of non-NMDA glutamate receptors. Alternatively, NMDA receptor antagonists also modulate GABAergic transmission, resulting in a disinhibition of cortical GABAergic activity on monoaminergic neurons (Yonezawa et al., 1998). These effects may relate to increased cortical metabolism at rest (Lahti et al., 1995; Breier et al., 1997; Vollenweider et al., 1997a,b; Holcomb et al., 2001), and the increased activation under cognitive challenge described in the present data.
In conclusion, we have demonstrated that, even in the absence of measurable behavioural deficits, ketamine has a modulatory effect on task-specific frontal and hippocampal activations. These changes occurred at both the encoding and retrieval of episodic memory. Our findings are consistent with studies reported using animal models, and with the suggested role for NMDA receptor function in this episodic memory system. This work provides insights into the influence of ketamine upon the cognitive processes that underpin episodic memory. Moreover, these are important observations with regard to future evaluations of ketamine as a model for schizophrenia.
Acknowledgments
We would like to thank Vicky Lupson, Ruth Bisbrown-Chippendale and Gloria Gee and other members of the Wolfson Brain Imaging Centre. This work was supported by the Wellcome Trust.
References
- Adler CM, Goldberg TE, Malhotra AK, Pickar D, Breier A. Effects of ketamine on thought disorder, working memory, and semantic memory in healthy volunteers. Biol Psychiatry. 1998;43:811–816. doi: 10.1016/s0006-3223(97)00556-8. [DOI] [PubMed] [Google Scholar]
- Adler CM, Malhotra AK, Elman I, Goldberg T, Egan M, Pickar D, Breier A. Comparison of ketamine-induced thought disorder in healthy volunteers and thought disorder in schizophrenia. Am J Psychiatry. 1999;156:1646–1649. doi: 10.1176/ajp.156.10.1646. [DOI] [PubMed] [Google Scholar]
- Brebion G, Smith MJ, Widlocher D. Discrimination and response bias in memory: effects of depression severity and psychomotor retardation. Psychiatry Res. 1997;70:95–103. doi: 10.1016/s0165-1781(97)03098-9. [DOI] [PubMed] [Google Scholar]
- Breier A, Malhotra AK, Pinals DA, Weisenfeld NI, Pickar D. Association of ketamine-induced psychosis with focal activation of the prefrontal cortex in healthy volunteers. Am J Psychiatry. 1997;154:805–811. doi: 10.1176/ajp.154.6.805. [DOI] [PubMed] [Google Scholar]
- Brewer JB, Zhao Z, Desmond JE, Glover GH, Gabrieli JD. Making memories: brain activity that predicts how well visual experience will be remembered. Science. 1998;281:1185–1187. doi: 10.1126/science.281.5380.1185. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Locantore JK, Anderson ND. Lateralization of prefrontal activity during episodic memory retrieval: evidence for the production-monitoring hypothesis. J Cogn Neurosci. 2003;15:249–259. doi: 10.1162/089892903321208187. [DOI] [PubMed] [Google Scholar]
- Calev A, Venables PH, Monk AF. Evidence for distinct verbal memory pathologies in severely and mildly disturbed schizophrenics. Schizophr Bull. 1983;9:247–264. doi: 10.1093/schbul/9.2.247. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Lockhart RS. Levels of processing: A framework for memory research. J Verb Learn Verb Behav. 1972;11:671–684. [Google Scholar]
- Domino EF, Zsigmond EK, Domino LE, Domino KE, Kothary SP, Domino SE. Plasma levels of ketamine and two of its metabolites in surgical patients using a gas chromatographic mass fragmentographic assay. Anesth Analg. 1982;61:87–92. [PubMed] [Google Scholar]
- Duncan EJ, Madonick SH, Parwani A, Angrist B, Rajan R, Chakravorty S, Efferen TR, Szilagyi S, Stephanides M, Chappell PB, Gonzenbach S, Ko GN, Rotrosen JP. Clinical and sensorimotor gating effects of ketamine in normals. Neuropsychopharmacology. 2001;25:72–83. doi: 10.1016/S0893-133X(00)00240-2. [DOI] [PubMed] [Google Scholar]
- Eyler Zorrilla LT, Jeste DV, Paulus M, Brown GG. Functional abnormalities of medial temporal cortex during novel picture learning among patients with chronic schizophrenia. Schizophr Res. 2003;59:187–198. doi: 10.1016/s0920-9964(01)00340-1. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Henson RN. Frontal lobes and human memory: insights from functional neuroimaging. Brain. 2001;124:849–881. doi: 10.1093/brain/124.5.849. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Shallice T, Frith CD, Frackowiak RS, Dolan RJ. Brain activity during memory retrieval. The influence of imagery and semantic cueing. Brain. 1996;119:1587–1596. doi: 10.1093/brain/119.5.1587. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Shallice T, Dolan RJ. ‘Sculpting the response space’ — an account of left prefrontal activation at encoding. Neuroimage. 2000;12:404–417. doi: 10.1006/nimg.2000.0633. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Stephenson CM, Carpenter TA, Donovan T, Bullmorel ET. Regional brain activations predicting subsequent memory success: an event-related fMRI study of the influence of encoding tasks. Cortex. 2003;39:1009–1026. doi: 10.1016/s0010-9452(08)70875-x. [DOI] [PubMed] [Google Scholar]
- Francis WN, Kucera H. Frequency analysis of English usage: lexicon and grammar. Houghton Mifflin; Boston, MA: 1982. [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R. Event-related fMRI: characterizing differential responses. Neuroimage. 1998;7:30–40. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- Gold JM, Randolph C, Carpenter CJ, Goldberg TE, Weinberger DR. Forms of memory failure in schizophrenia. J Abnorm Psychol. 1992;101:487–494. doi: 10.1037//0021-843x.101.3.487. [DOI] [PubMed] [Google Scholar]
- Heckers S, Rauch SL, Goff D, Savage CR, Schacter DL, Fischman AJ, Alpert NM. Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nat Neurosci. 1998;1:318–323. doi: 10.1038/1137. [DOI] [PubMed] [Google Scholar]
- Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- Henson RN, Shallice T, Dolan RJ. Right prefrontal cortex and episodic memory retrieval: a functional MRI test of the monitoring hypothesis. Brain. 1999a;122:1367–1381. doi: 10.1093/brain/122.7.1367. [DOI] [PubMed] [Google Scholar]
- Henson RN, Rugg MD, Shallice T, Josephs O, Dolan RJ. Recollection and familiarity in recognition memory: an event-related functional magnetic resonance imaging study. J Neurosci. 1999b;19:3962–3972. doi: 10.1523/JNEUROSCI.19-10-03962.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetem LA, Danion JM, Diemunsch P, Brandt C. Effect of a subanesthetic dose of ketamine on memory and conscious awareness in healthy volunteers. Psychopharmacology (Berl) 2000;152:283–288. doi: 10.1007/s002130000511. [DOI] [PubMed] [Google Scholar]
- Hofer A, Weiss EM, Golaszewski SM, Siedentopf CM, Brinkhoff C, Kremser C, Felber S, Fleischhacker WW. An FMRI study of episodic encoding and recognition of words in patients with schizophrenia in remission. Am J Psychiatry. 2003;160:911–918. doi: 10.1176/appi.ajp.160.5.911. [DOI] [PubMed] [Google Scholar]
- Holcomb HH, Lahti AC, Medoff DR, Weiler M, Tamminga CA. Sequential regional cerebral blood flow brain scans using PET with H2(15)O demonstrate ketamine actions in CNS dynamically. Neuropsychopharmacology. 2001;25:165–172. doi: 10.1016/S0893-133X(01)00229-9. [DOI] [PubMed] [Google Scholar]
- Honey RAE, Honey GD, O’Loughlin C, Sharar SR, Kumaran D, Bullmore ET, Menon DK, Donovan T, Lupson VC, Bisbrown-Chippendale R, Fletcher PC. Acute ketamine administration alters the brain responses to executive demands in a verbal working memory task: an fMRI study. Neuropsychopharmacology. 2004;29:1203–1214. doi: 10.1038/sj.npp.1300438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey GD, Honey RAE, Sharar SR, Turner DC, Pomarol-Clotet E, Kumaran D, Simons JS, Hu X, Rugg MD, Bullmore ET, Fletcher PC. Impairment of specific episodic memory processes by subpsychotic doses of ketamine: the effects of levels of processing at encoding and of the subsequent retrieval task. Psychopharmacology. 2005 doi: 10.1007/s00213-005-0001-z. in press. [DOI] [PubMed] [Google Scholar]
- Jessen F, Scheef L, Germeshausen L, Tawo Y, Kockler M, Kuhn KU, Maier W, Schild HH, Heun R. Reduced hippocampal activation during encoding and recognition of words in schizophrenia patients. Am J Psychiatry. 2003;160:1305–1312. doi: 10.1176/appi.ajp.160.7.1305. [DOI] [PubMed] [Google Scholar]
- Kharasch ED, Labroo R. Metabolism of ketamine stereoisomers by human liver microsomes. Anesthesiology. 1992;77:1201–1207. doi: 10.1097/00000542-199212000-00022. [DOI] [PubMed] [Google Scholar]
- Kirchhoff BA, Wagner AD, Maril A, Stern CE. Prefrontal-temporal circuitry for episodic encoding and subsequent memory. J Neurosci. 2000;20:6173–6180. doi: 10.1523/JNEUROSCI.20-16-06173.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Jr., Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Holcomb HH, Medoff DR, Tamminga CA. Ketamine activates psychosis and alters limbic blood flow in schizophrenia. Neuroreport. 1995;6:869–872. doi: 10.1097/00001756-199504190-00011. [DOI] [PubMed] [Google Scholar]
- Lepage M, Habib R, Tulving E. Hippocampal PET activations of memory encoding and retrieval: the HIPER model. Hippocampus. 1998;8:313–322. doi: 10.1002/(SICI)1098-1063(1998)8:4<313::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Leube DT, Erb M. Hippocampal dysfunction during episodic memory encoding in patients with schizophrenia — an fMRI study. Schizophr Res. 2002;1877:1–3. doi: 10.1016/s0920-9964(02)00503-0. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Malhotra AK, Pinals DA, Weingartner H, Sirocco K, Missar CD, Pickar D, Breier A. NMDA receptor function and human cognition: the effects of ketamine in healthy volunteers. Neuropsychopharmacology. 1996;14:301–307. doi: 10.1016/0893-133X(95)00137-3. [DOI] [PubMed] [Google Scholar]
- McDermott KB, Buckner RL, Petersen SE, Kelley WM, Sanders AL. Set- and code-specific activation in frontal cortex: an fMRI study of encoding and retrieval of faces and words. J Cogn Neurosci. 1999;11:631–640. doi: 10.1162/089892999563698. [DOI] [PubMed] [Google Scholar]
- McKenna PJ, Tamlyn D, Lund CE, Mortimer AM, Hammond S, Baddeley AD. Amnesic syndrome in schizophrenia. Psychol Med. 1990;20:967–972. doi: 10.1017/s0033291700036667. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan DT, Buller AL. Anatomical, pharmacological and molecular diversity of native NMDA receptor subtypes. In: Collingridge JL, Watkins JC, editors. The NMDA receptor. 2nd edn Oxford University Press; New York: 1995. pp. 158–176. [Google Scholar]
- Morgan CJA, Mofeez A, Brandner B, Bromley L, Curran HV. Acute effects of ketamine on memory systems and psychotic symptoms in healthy volunteers. Neuropychopharmacology. 2004;29:208–218. doi: 10.1038/sj.npp.1300342. [DOI] [PubMed] [Google Scholar]
- Morris RG, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Nelson H. National Adult Reading Test. NFER-Nelson; New York: 1982. [Google Scholar]
- Newcomer JW, Farber NB, Jevtovic-Todorovic V, Selke G, Melson AK, Hershey T, et al. Ketamine-induced NMDA receptor hypofunction as a model of memory impairment and psychosis. Neuropsychopharmacology. 1999;20:106–118. doi: 10.1016/S0893-133X(98)00067-0. [DOI] [PubMed] [Google Scholar]
- Nolde SF, Johnson MK, D’Esposito M. Left prefrontal activation during episodic remembering: an event-related fMRI study. Neuroreport. 1998;9:3509–3514. doi: 10.1097/00001756-199810260-00032. [DOI] [PubMed] [Google Scholar]
- Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry. 1995;52:998–1007. doi: 10.1001/archpsyc.1995.03950240016004. [DOI] [PubMed] [Google Scholar]
- Otten LJ, Henson RN, Rugg MD. Depth of processing effects on neural correlates of memory encoding: relationship between findings from across- and within-task comparisons. Brain. 2001;124:399–412. doi: 10.1093/brain/124.2.399. [DOI] [PubMed] [Google Scholar]
- Oye I, Paulsen O, Maurset A. Effects of ketamine on sensory perception: evidence for a role of N-methyl-D-aspartate receptors. J Pharmacol Exp Ther. 1992;260:1209–1213. [PubMed] [Google Scholar]
- Radant AD, Bowdle TA, Cowley DS, Kharasch ED, Roy-Byrne PP. Does ketamine-mediated N-methyl-D-aspartate receptor antagonism cause schizophrenia-like oculomotor abnormalities? Neuropsychopharmacology. 1998;19:434–444. doi: 10.1016/S0893-133X(98)00030-X. [DOI] [PubMed] [Google Scholar]
- Ragland JD, Gur RC, Raz J, Schroeder L, Kohler CG, Smith RJ, Alavi A, Gur RE. Effect of schizophrenia on frontotemporal activity during word encoding and recognition: a PET cerebral blood flow study. Am J Psychiatry. 2001;158:1114–1125. doi: 10.1176/appi.ajp.158.7.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugg MD, Fletcher PC, Chua PM, Dolan RJ. The role of the prefrontal cortex in recognition memory and memory for source: an fMRI study. Neuroimage. 1999;10:520–529. doi: 10.1006/nimg.1999.0488. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Alpert NM, Savage CR, Rauch SL, Albert MS. Conscious recollection and the human hippocampal formation: evidence from positron emission tomography. Proc Natl Acad Sci USA. 1996;93:321–325. doi: 10.1073/pnas.93.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamminga CA, Holcomb HH, Gao XM, Lahti AC. Glutamate pharmacology and the treatment of schizophrenia: current status and future directions. Int Clin Psychopharmacol. 1995;10:29–37. [PubMed] [Google Scholar]
- Thompson-Schill SL, D’Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proc Natl Acad Sci USA. 1997;94:14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai G, Coyle JT. Glutamatergic mechanisms in schizophrenia. Annu Rev Pharmacol Toxicol. 2002;42:165–179. doi: 10.1146/annurev.pharmtox.42.082701.160735. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Leenders KL, Oye I, Hell D, Angst J. Differential psychopathology and patterns of cerebral glucose utilisation produced by (S)- and (R)-ketamine in healthy volunteers using positron emission tomography (PET) Eur Neuropsychopharmacol. 1997a;7:25–38. doi: 10.1016/s0924-977x(96)00042-9. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Leenders KL, Scharfetter C, Antonini A, Maguire P, Missimer J, Angst J. Metabolic hyperfrontality and psychopathology in the ketamine model of psychosis using positron emission tomography (PET) and [18F]fluorodeoxyglucose (FDG) Eur Neuropsychopharmacol. 1997b;7:9–24. doi: 10.1016/s0924-977x(96)00039-9. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Poldrack RA, Eldridge LL, Desmond JE, Glover GH, Gabrieli JD. Material-specific lateralization of prefrontal activation during episodic encoding and retrieval. Neuroreport. 1998a;9:3711–3717. doi: 10.1097/00001756-199811160-00026. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, Rosen BR, Buckner RL. Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998b;281:1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Pare-Blagoev EJ, Clark J, Poldrack RA. Recovering meaning: left prefrontal cortex guides controlled semantic retrieval. Neuron. 2001;31:329–338. doi: 10.1016/s0896-6273(01)00359-2. [DOI] [PubMed] [Google Scholar]
- Weiss AP, Schacter DL, D CG, Rauch SL, Alpert NM, Fischman AJ, Heckers S. Impaired hippocampal recruitment during normal modulation of memory performance in schizophrenia. Biol Psychiatry. 2003;53:48–55. doi: 10.1016/s0006-3223(02)01541-x. [DOI] [PubMed] [Google Scholar]
- Wilkinson D, Halligan P. Opinion: The relevance of behavioural measures for functional-imaging studies of cognition. Nat Rev Neurosci. 2004;5:67–73. doi: 10.1038/nrn1302. [DOI] [PubMed] [Google Scholar]
- Yonezawa Y, Kuroki T, Kawahara T, Tashiro N, Uchimura H. Involvement of gamma-aminobutyric acid neurotransmission in phencyclidine-induced dopamine release in the medial prefrontal cortex. Eur J Pharmacol. 1998;341:45–56. doi: 10.1016/s0014-2999(97)01435-0. [DOI] [PubMed] [Google Scholar]