Abstract
Regioselective methods for allene hydrosilylation have been developed, with regioselectivity being governed primarily by choice of metal. Alkenylsilanes are produced via nickel catalysis with larger N-heterocyclic carbene ligands, and allylsilanes are produced via palladium catalysis with smaller N-heterocyclic carbene ligands. These complementary methods allow either regioisomeric product to be obtained with exceptional regiocontrol.
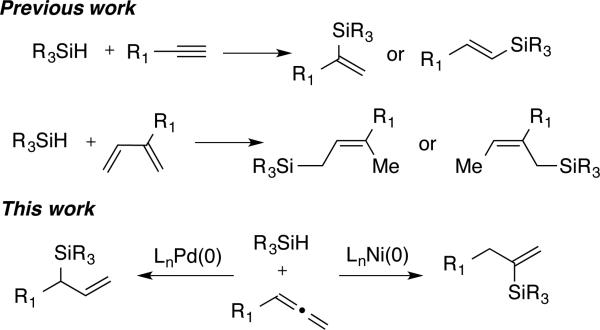
Alkenylsilanes and allylsilanes are useful reagent classes that are employed in numerous synthetic transformations.1 For example, the Hiyama cross-coupling2 and Sakurai-type allylation and crotylation reactions3 are widely used methods for C-C bond formation. Currently, the most direct and atom-economical routes to vinyl- and allylsilanes proceed via metal-catalyzed hydrosilylations of π-components (Scheme 1). The hydrosilylation of alkynes to afford alkenylsilanes4 and the hydrosilylation of 1,3-dienes to afford allylsilanes5 are versatile methods that have been widely employed. In both instances, control of regiochemistry is required with unsymmetrical substrates, and even the most efficient hydrosilylation procedures are often plagued by the lack of complete regioselectivity for a variety of substrates without directing group effects. Compounding these challenges is the difficulty typically seen in the separation of regioisomeric allyl- or vinylsilanes produced by these methods.
Scheme 1.
In contrast to the significant developments that have been reported in hydrosilylations of alkynes and 1,3-dienes, the hydrosilylation of allenes has rarely been examined.6 The hydrometallation of allenes presents considerable challenges in selectivity since 1,2- or 2,1-addition to either of the two contiguous π-systems can occur, potentially providing two regioisomeric allylsilanes and two regioisomeric vinylsilanes, in some cases as mixtures of alkene stereoisomers. The hydroboration of allenes has been widely developed, but these processes are typically uncatalyzed, thus providing a single allylborane product governed by substrate steric and electronic characteristics.7,8 Significant advances have appeared in the regioselective bis-metallation of allenes, through catalytic reactions such as diboration9 and silaboration,10 but regiodivergent processes in simpler hydrometallations of allenes are lacking. Given the synthetic utility of allyl- and vinylsilanes and the potential to provide regiocontrolled access to both structure classes from a common allene substrate, we report herein our efforts to develop regioselective, catalyst-controlled methods for the hydrosilylation of allenes.
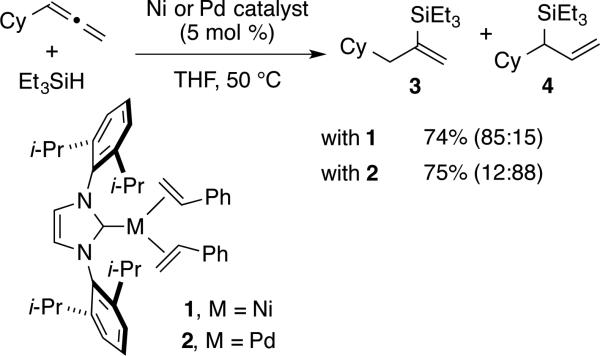
As part of our laboratory's broader interest in the development of regioselective catalytic processes,11 we initially hoped to develop ligand-controlled regioselectivity reversal in the nickel-catalyzed hydrosilylation of allenes. However, upon screening a broad range of nickel catalysts modified with N-heterocyclic carbene or phosphine ligands, combinations that allowed high-yielding and highly regioselective access to both alkenylsilane and allylsilane products were not identified. However, in the course of these exploratory studies, we were attracted to the recent disclosures of well-defined N-heterocyclic carbene complexes of palladium and nickel.12 To our surprise, simple modification of the metal, while leaving the ligand and reaction conditions unchanged, provided an encouraging lead towards identifying routes to both product regioisomers. As illustrated in the hydrosilylation of cyclohexylallene with triethylsilane, the use of nickel complex 1 provided the vinylsilane 3 with 85:15 regioselectivity, whereas exactly the same procedure and same ligand, but employing the palladium complex 2 instead, provided the allylsilane 4 with 88:12 regioselectivity. Reactions involving the pre-synthesized nickel complex 1 required elevated temperature, likely due to the need to dissociate styrene to provide the active catalyst form, whereas palladium complex 2 was catalytically active at rt or elevated temperature. This unusual example of regiochemistry reversal with a common ligand scaffold and within a single metal triad presented an unexpected and effective strategy for accessing both allylsilanes and alkenylsilanes from a common allene precursor.
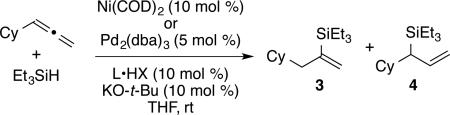
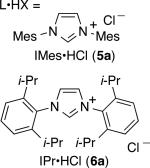
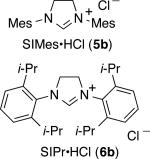
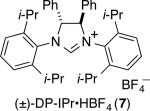
To further boost the regioselectivity of these complementary processes, we examined ligand variation for both nickel- and palladium-catalyzed hydrosilylations. In comparing catalytic reactions of 1 and 2 with the corresponding complexes prepared in situ from Ni(COD)2 or Pd2(dba)3, the chemical yields are similar and regioselectivities are identical in comparing the catalysts pre-synthesized or prepared in situ (comparing Scheme 2 to Table 1, entries 3 and 8). The use of the pre-synthesized complexes comes with the advantage of higher catalyst turnover and ease of accurate weighing on small scale that becomes difficult for the in situ protocol. However, to facilitate more rapid screening of a variety of ligands, catalysts for the optimization studies were prepared in situ by treatment of Ni(COD)2 or Pd2(dba)3 with the HCl or HBF4 salt of the desired NHC ligands.
Scheme 2.
Table 1.
| ||||
|---|---|---|---|---|
| entry | precatalyst | L•HX | % yield | regiosel. (3:4) |
| 1 | Ni(COD)2 | 5a | 22 | 33:67 |
| 2 | Ni(COD)2 | 5b | 15 | 40:60 |
| S | Ni(COD)2 | 6a | 58 | 85:15 |
| 4 | Ni(COD)2 | 6b | 47 | 81:19 |
| 5 | Ni(COD)2 | 7 | 84 | >9B:<2 |
| 6 | Pd2(dba)3 | 7 | trace | not determined |
| 7 | Pd2(dba)3 | 6b | 56 | 14:86 |
| 8 | Pd2(dba)3 | 6a | 75 | 12:88 |
| 9 | Pd2(dba)3 | 5b | 74 | 2:98 |
| 10 | Pd2(dba)3 | 5a | 80 | <2:>9B |
|
|
|
||
From the ligand variation studies, an interesting trend emerged that regioselectivities for alkenylsilane products using nickel were enhanced with larger ligands, whereas regioselectivities for allylsilanes using palladium were enhanced with smaller ligands. To illustrate this trend using Ni(COD)2 as pre-catalyst, IMes and SIMes slightly favored allylsilane production (Table 1, entries 1 and 2), IPr and SIPr favored alkenylsilane production with good selectivity (Table 1, entries 3 and 4), and the very bulky ligand DP-IPr afforded the alkenylsilane in 84% isolated yield with >98:2 regioselectivity (Table 1, entry 5). In comparison, using Pd2(dba)3 as the pre-catalyst, the use of DP-IPr afforded a low-yielding mixture of regioisomers (Table 1, entry 6), IPr and SIPr favored allylsilane production with good selectivity (Table 1, entries 7 and 8), and IMes and SIMes provided exceptional regioselectivities (Table 1, entries 9 and 10), with IMes providing an 80% isolated yield of the allylsilane with >98:2 regioselectivity.
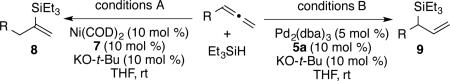
The above guidelines for the in situ-generated Ni(0) and Pd(0) hydrosilylation catalysts were utilized in the reactions for a variety of allene substrate classes (Table 2). In addition to the use of cyclohexyl allene (entries 1 and 2), allenes containing less hindered aliphatic groups (Table 2, entries 3 and 4), aromatic groups (Table 2, entries 5 and 6), unprotected hydroxyls (Table 2, entries 7 and 8) and silyloxy groups (Table 2, entries 9 and 10) were successfully hydrosilylated using either Ni(COD)2 with ligand 7 (DP-IPr; conditions A) or Pd2(dba)3 with ligand 5a (IMes; conditions B) with consistently excellent regioselectivities (>98:2) and in high chemical yields. In addition, an allene containing a phthalimido moiety underwent smooth hydrosilylation to afford either regioisomeric product (Table 2, entries 11 and 12). To further explore the scaleup and utility in reactions not using glove-box manipulations, the palladium-catalyzed procedure with cyclohexyl allene was optimized on a 6 mmol scale, which proceeded in 98% isolated yield in >98:2 regioselectivity (Table 2, entry 13). Additionally, a procedure conducted with a standard bench-top assembly purged with a nitrogen line proceed in 74% isolated yield with slight eroded (96:4) regioselectivity (Table 2, entry 14). Finally, an experiment with reduced catalyst loading (2.5 mol % Pd2(dba)3) proceeded in 72% isolated yield (Table 2, entry 15), whereas attempts to lower the catalyst loading further resulted in more significant drops in chemical yield.
Table 2.
| ||||
|---|---|---|---|---|
| entry | R | conditionsa | product (% yield) | regiosel. (8:9) |
| 1 | Cy | A | 3 (84) | >98:2 |
| 2 | B | 4 (80) | <2:98 | |
| 3 | n-Oct | A | 8a (80) | >98:2 |
| 4 | B | 9a (98) | <2:98 | |
| 5 | Ph | A | 8b (78) | >98:2 |
| 6 | B | 9b (94) | <2:98 | |
| 7 |
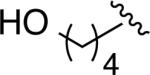
|
A | 8c (62) | >98:2 |
| 8 | B | 9c (88) | <2:98 | |
| 9 |
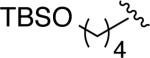
|
A | 8d (73) | >98:2 |
| 10 | B | 9d (97) | <2:98 | |
| 11 |
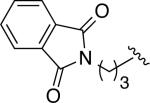
|
A | 8e (78) | >98:2 |
| 12 | B | 9e (97) | <2:98 | |
| 13 | Cy | Bb | 4 (98) | >98:2 |
| 14 | Cy | Bc | 4 (74) | 96:4 |
| 15 | Cy | Bd | 4 (72) | >98:2 |
Unless otherwise noted, reactions were conducted on a 0.3 mmol scale.
Conducted on 6.0 mmol scale.
Conducted with benchtop assembly of the catalyst and ligand followed by three vacuum/purge cycles before allene and silane introduction.
Conducted at 2.5 mol % loading of Pd2(dba)3.
In order to evaluate the effects of introducing allene 1,1-disubstitution, we subjected vinylidene cyclohexane to both nickel- and palladium-catalyzed reaction conditions (Scheme 3). The nickel-catalyzed reaction resulted in an outcome consistent with the previous observations for monosubstituted allenes, where the silyl group is installed at the central allenic carbon to afford vinylsilane 10. Reactions with palladium, however, afforded a new observed allylsilane isomer 11, with the C-Si bond forming at the least substituted allenic carbon. This observation offers a new entry to a useful allylsilane traditionally accessed via the hydrosilylation of 1,3-dienes.
Scheme 3.
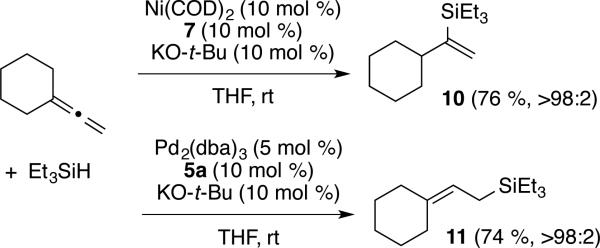
The palladium-based procedure tolerates variation of silane structure with minimal change in regioselectivity and yield. For example, additions to cyclohexyl allene cleanly proceed with a range of synthetically versatile silane structures (eq 1).13 Alternatively, the nickel-based procedure is currently more limited with silane variation leading to a drop in yield and erosion of regioselectivy.
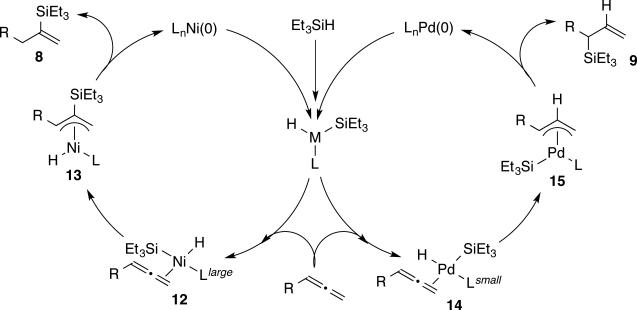
While the origin of regiocontrol is at present uncertain, we propose that a likely mechanism involves a change from a silylmetallation pathway with nickel to a hydrometallation pathway with palladium (Scheme 4). Migratory insertions to allenes typically favor addition to the central allene carbon with the formation of a metal π-allyl complex.9g,10g Through this common mechanistic feature, silylmetallation of nickel allene complex 12 to generate π-allyl intermediate 13, followed by C-H reductive elimination, would afford vinylsilane product 8. Alternatively, hydrometallation of palladium allene complex 14 to generate π-allyl intermediate 15, followed by C-Si reductive elimination, would afford allylsilane product 9. By utilizing a large ligand with nickel, disposition of the silyl group trans to the ligand in intermediate 12 reinforces the preference for silylmetallation. However, by utilizing a small ligand with palladium, disposition of the silyl group cis to the ligand in 14 reinforces the preference for hydrometallation. Detailed studies from Morken and Suginome in diborations and silaborations of allenes provide significant insight into the factors that control regiochemistry in these related processes, and the mechanistic model described in Scheme 4 draws from the insights of those studies.9g,10g Future work will be devoted to evaluating the intriguing mechanistic basis of these regiodivergent hydrosilylation processes.
Scheme 4.
In summary, the complementary use of metals (Ni vs Pd) results in regiochemical reversals in allene hydrosilylations. Alterations in NHC ligand structure can further improve the regioselectivities to provide regiodivergent access to a wide range of alkenylsilanes or allylsilanes in high yields and exceptional regiocontrol. The above study demonstrates the special role that metal identity can play in governing regioselective catalytic reactions. Future studies will explore these effects in other classes of catalytic reactions.
Supplementary Material
Table 3.
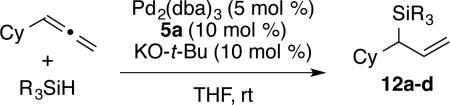
| |||
|---|---|---|---|
| entry | silane | product (% yield) | regiosel. |
| 1 | HSiMe2Ph | 12a (91) | >98:2 |
| 2 | HSiMe2Bn | 12b (93) | >98:2 |
| 3 | HSiMe(OSiMe3)2 | 12c (82) | >98:2 |
| 4 | H2Si(t-Bu)2 | 12d (75) | >98:2 |
ACKNOWLEDGMENT
Professor Pedro Pérez (Universidad de Huelva) is thanked for encouragement and helpful discussions.
Funding Sources
We thank the National Science Foundation (CHE-1265491), the National Institutes of Health (GM57014) and MINECO (CTQ2011-24502) for support of this research. NSF supports a program including a comparative analysis of base metal and precious metal catalysis. NIH supports a program in the design of NHC ligands for regioselective transformations.
Footnotes
Supporting Information. Experimental details and analytical data. This material is available free of charge via the Internet at http://pubs.acs.org.
No competing financial interests have been declared.
REFERENCES
- 1.a Brook MA. Silicon in Organic, Organometallic, and Polymer Chemistry. Wiley; Chichester: 2000. [Google Scholar]; b Fleming I, Barbero A, Walter D. Chem. Rev. 1997;97:2063–2192. doi: 10.1021/cr941074u. [DOI] [PubMed] [Google Scholar]; c Fleming I, Dunoguès J, Smithers R. Org. React. 1989;37:57. [Google Scholar]; d Ojima I, Li ZY, Zhu JW. In: In Chemistry of Organic Silicon Compounds. Rappoport Z, Apeloig Y, editors. John Wiley & Sons Ltd; Chichester, England: 1998. [Google Scholar]
- 2.a Nakao Y, Hiyama T. Chem. Soc. Rev. 2011;40:4893. doi: 10.1039/c1cs15122c. [DOI] [PubMed] [Google Scholar]; b Chang W-TT, Smith RC, Regens CS, Bailey AD, Werner NS, Denmark SE. Org. React. 2011;75:213. [Google Scholar]
- 3.a Hosomi A, Endo M, Sakurai H. Chem. Lett. 1976:941. [Google Scholar]; b Masse CE, Panek JS. Chem. Rev. 1995;95:1293. [Google Scholar]
- 4.a Rooke DA, Ferreira EM. Angew. Chem. Int. Ed. 2012;51:3225. doi: 10.1002/anie.201108714. [DOI] [PubMed] [Google Scholar]; b Trost BM, Ball ZT. J. Am. Chem. Soc. 2001;123:12726. doi: 10.1021/ja0121033. [DOI] [PubMed] [Google Scholar]; c Ball ZT, Trost BM. J. Am. Chem. Soc. 2005;127:17644. doi: 10.1021/ja0528580. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Berthon-Gelloz G, Schumers J, De Bo G, Markó IE. J. Org. Chem. 2008;73:4190. doi: 10.1021/jo800411e. [DOI] [PubMed] [Google Scholar]; e De Bo G, Berthon-Gelloz G, Tinant B, Markó IE. Organometallics. 2006;25:1881. [Google Scholar]; f Denmark SE, Wang ZG. Org. Lett. 2001;3:1073. doi: 10.1021/ol0156751. [DOI] [PubMed] [Google Scholar]; g Kawasaki Y, Ishikawa Y, Igawa K, Tomooka K. J. Am. Chem. Soc. 2011;133:20712. doi: 10.1021/ja209553f. [DOI] [PubMed] [Google Scholar]; h Chaulagain MR, Mahandru GM, Montgomery J. Tetrahe dron. 2006;62:7560. [Google Scholar]; i Lappert MF, Nile TA, Takahashi S. J. Organomet. Chem. 1974;72:425. [Google Scholar]
- 5.a Ojima I, Kumagai M. J. Organomet. Chem. 1978;157:359. [Google Scholar]; b Wu JY, Stanzl BN, Ritter T. J. Am. Chem. Soc. 2010;132:13214. doi: 10.1021/ja106853y. [DOI] [PubMed] [Google Scholar]; c Onozawa S, Sakakura T, Tanaka M. Tetrahedron Lett. 1994;35:8177. [Google Scholar]; d Tsuji J, Hara M, Ohno K. Tetrahedron. 1974;30:2143. [Google Scholar]
- 6.a Sudo T, Asao N, Gevorgyan V, Yamamoto Y. J. Org. Chem. 1999;64:2494. [Google Scholar]; b Huang G, Isobe M. Tetrahedron. 2001;57:10241. [Google Scholar]
- 7.For uncatalyzed hydroborations: Kister J, DeBaillie AC, Lira R, Roush WR. J. Am. Chem. Soc. 2009;131:14174. doi: 10.1021/ja905494c.Chen M, Handa M, Roush WR. J. Am. Chem. Soc. 2009;131:14602. doi: 10.1021/ja904599h.Ess DH, Kister J, Chen M, Roush WR. Org. Lett. 2009;11:5538. doi: 10.1021/ol902364d.Gonzalez AZ, Roman JG, Alicea E, Canales E, Soderquist JA. J. Am. Chem. Soc. 2009;131:1269. doi: 10.1021/ja808360z.Brown HC, Liotta R, Kramer GW. J. Am. Chem. Soc. 1979;101:2966.
- 8.For catalyzed hydroborations: Semba K, Shinomiya M, Fujihara T, Terao J, Tsuji Y. Chem.-Eur. J. 2013;19:7125. doi: 10.1002/chem.201300443.Yamamoto Y, Fujikawa R, Yamada A, Miyaura N. Chem. Lett. 1999:1069.
- 9.a Ishiyama T, Kitano T, Miyaura N. Tetrahedron Lett. 1998;39:2357. [Google Scholar]; b Yang FY, Cheng CH. J. Am. Chem. Soc. 2001;123:761. doi: 10.1021/ja005589g. [DOI] [PubMed] [Google Scholar]; c Pelz NF, Woodward AR, Burks HE, Sieber JD, Morken JP. J. Am. Chem. Soc. 2004;126:16328. doi: 10.1021/ja044167u. [DOI] [PubMed] [Google Scholar]; d Woodward AR, Burks HE, Chan LM, Morken JP. Org. Lett. 2005;7:5505. doi: 10.1021/ol052312i. [DOI] [PubMed] [Google Scholar]; e Sieber JD, Morken JP. J. Am. Chem. Soc. 2006;128:74. doi: 10.1021/ja057020r. [DOI] [PubMed] [Google Scholar]; f Pelz NF, Morken JP. Org. Lett. 2006;8:4557. doi: 10.1021/ol0616891. [DOI] [PubMed] [Google Scholar]; g Burks HE, Liu SB, Morken JP. J. Am. Chem. Soc. 2007;129:8766. doi: 10.1021/ja070572k. [DOI] [PubMed] [Google Scholar]
- 10.a Suginome M, Ohmori Y, Ito Y. Synlett. 1999:1567. [Google Scholar]; b Suginome M, Ohmori Y, Ito Y. J. Organomet. Chem. 2000;611:403. [Google Scholar]; c Suginome M, Ohmura T, Miyake Y, Mitani S, Ito Y, Murakami M. J. Am. Chem. Soc. 2003;125:11174. doi: 10.1021/ja0368958. [DOI] [PubMed] [Google Scholar]; d Ohmura T, Suginome M. Org. Lett. 2006;8:2503. doi: 10.1021/ol060666j. [DOI] [PubMed] [Google Scholar]; e Ohmura T, Taniguchi H, Suginome M. J. Am. Chem. Soc. 2006;128:13682. doi: 10.1021/ja063934h. [DOI] [PubMed] [Google Scholar]; f Chang KJ, Rayabarapu DK, Yang FY, Cheng CH. J. Am. Chem. Soc. 2005;127:126. doi: 10.1021/ja044662q. [DOI] [PubMed] [Google Scholar]; g Abe Y, Kuramoto K, Ehara M, Nakatsuji H, Suginome M, Murakami M, Ito Y. Organometallics. 2008;27:1736. [Google Scholar]; h Ohmura T, Oshima K, Taniguchi H, Suginome M. J. Am. Chem. Soc. 2010;132:12194. doi: 10.1021/ja105096r. [DOI] [PubMed] [Google Scholar]
- 11.a Malik HA, Sormunen GJ, Montgomery J. J. Am. Chem. Soc. 2010;132:6304. doi: 10.1021/ja102262v. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Liu P, Montgomery J, Houk KN. J. Am. Chem. Soc. 2011;133:6956. doi: 10.1021/ja202007s. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Li W, Chen N, Montgomery J. Angew. Chem. Int. Ed. 2010;49:8712. doi: 10.1002/anie.201004740. [DOI] [PubMed] [Google Scholar]
- 12.a Martín C, Molina F, Alvarez E, Belderrain TR. Chem. Eur. J. 2011;17:14885. doi: 10.1002/chem.201102900. [DOI] [PubMed] [Google Scholar]; b Iglesias MJ, Blandez JF, Fructos MR, Prieto A, Álvarez E, Belderrain TR, Nicasio MC. Organometallics. 2012;31:6312. [Google Scholar]
- 13.a Roberson CW, Woerpel KA. J. Am. Chem. Soc. 2002;124:11342. doi: 10.1021/ja012152f. [DOI] [PubMed] [Google Scholar]; b Tinsley JM, Roush WR. J. Am. Chem. Soc. 2005;127:10818. doi: 10.1021/ja051986l. [DOI] [PubMed] [Google Scholar]; c Tanino K, Yoshitani N, Moriyama F, Kuwajima I. J. Org. Chem. 1997;62:4206. doi: 10.1021/jo9703515. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.