Abstract
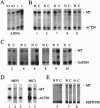
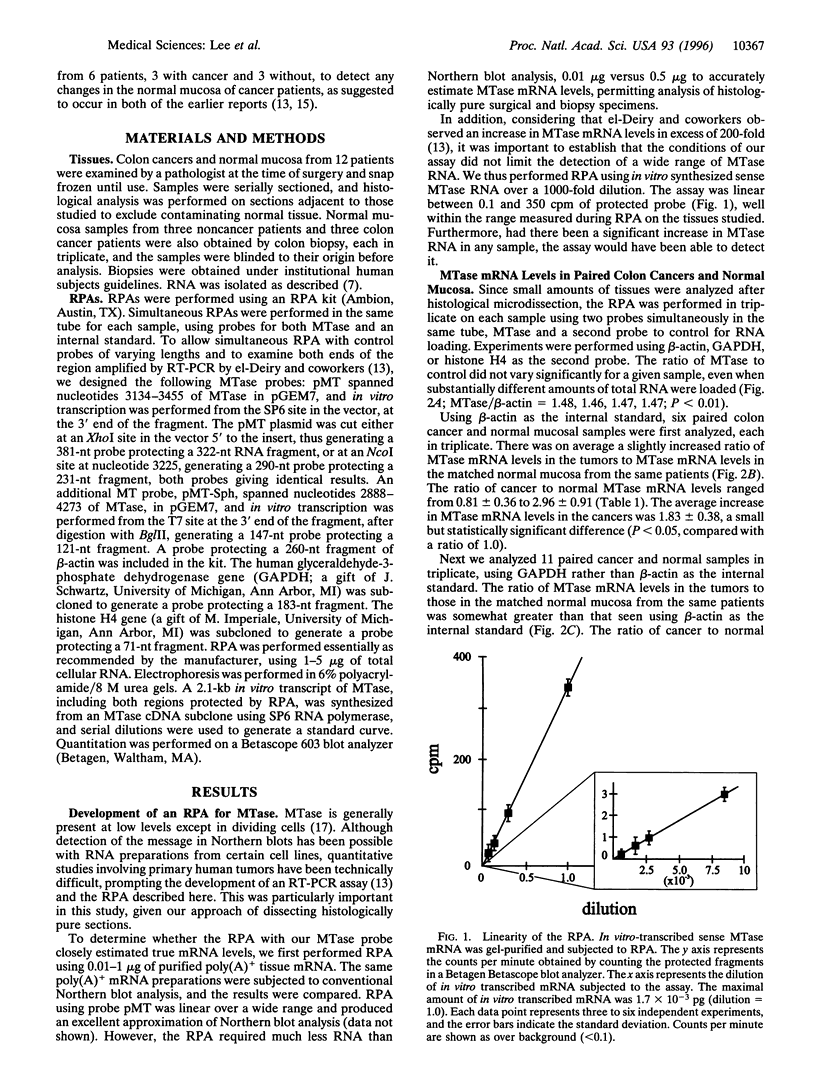
Epigenetic alterations in the genome of tumor cells have attracted considerable attention since the discovery of widespread alterations in DNA methylation of colorectal cancers over 10 years ago. However, the mechanism of these changes has remained obscure. el-Deiry and coworkers [el-Deiry, W. S., Nelkin, B. D., Celano, P., Yen, R. C., Falco, J. P., Hamilton, S. R. & Baylin, S. B. (1991) Proc. Natl. Acad. Sci. USA 88, 3470-3474], using a quantitative reverse transcription-PCR assay, reported 15-fold increased expression of DNA methyltransferase (MTase) in colon cancer, compared with matched normal colon mucosa, and a 200-fold increase in MTase mRNA levels compared with mucosa of unaffected patients. These authors suggested that increases in MTase mRNA levels play a direct pathogenetic role in colon carcinogenesis. To test this hypothesis, we developed a sensitive quantitative RNase protection assay of MTase, linear over three orders of magnitude. Using this assay on 12 colorectal carcinomas and matched normal mucosal specimens, we observed a 1.8- to 2.5-fold increase in MTase mRNA levels in colon carcinoma compared with levels in normal mucosa from the same patients. There was no significant difference between the normal mucosa of affected and unaffected patients. Furthermore, when the assay was normalized to histone H4 expression, a measure of S-phase-specific expression, the moderate increase in tumor MTase mRNA levels was no longer observed. These data are in contrast to the previously reported results, and they indicate that changes in MTase mRNA levels in colon cancer are nonspecific and compatible with other markers of cell proliferation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antequera F., Boyes J., Bird A. High levels of de novo methylation and altered chromatin structure at CpG islands in cell lines. Cell. 1990 Aug 10;62(3):503–514. doi: 10.1016/0092-8674(90)90015-7. [DOI] [PubMed] [Google Scholar]
- Baylin S. B., Fearon E. R., Vogelstein B., de Bustros A., Sharkis S. J., Burke P. J., Staal S. P., Nelkin B. D. Hypermethylation of the 5' region of the calcitonin gene is a property of human lymphoid and acute myeloid malignancies. Blood. 1987 Aug;70(2):412–417. [PubMed] [Google Scholar]
- Carlson L. L., Page A. W., Bestor T. H. Properties and localization of DNA methyltransferase in preimplantation mouse embryos: implications for genomic imprinting. Genes Dev. 1992 Dec;6(12B):2536–2541. doi: 10.1101/gad.6.12b.2536. [DOI] [PubMed] [Google Scholar]
- Cedar H., Razin A. DNA methylation and development. Biochim Biophys Acta. 1990 May 24;1049(1):1–8. doi: 10.1016/0167-4781(90)90076-e. [DOI] [PubMed] [Google Scholar]
- Ehrlich M., Gama-Sosa M. A., Huang L. H., Midgett R. M., Kuo K. C., McCune R. A., Gehrke C. Amount and distribution of 5-methylcytosine in human DNA from different types of tissues of cells. Nucleic Acids Res. 1982 Apr 24;10(8):2709–2721. doi: 10.1093/nar/10.8.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Kalikin L. M., Johnson L. A., Thompson J. S. Loss of imprinting in human cancer. Cold Spring Harb Symp Quant Biol. 1994;59:357–364. doi: 10.1101/sqb.1994.059.01.040. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. Hypomethylation distinguishes genes of some human cancers from their normal counterparts. Nature. 1983 Jan 6;301(5895):89–92. doi: 10.1038/301089a0. [DOI] [PubMed] [Google Scholar]
- Gause W. C., Adamovicz J. The use of the PCR to quantitate gene expression. PCR Methods Appl. 1994 Jun;3(6):S123–S135. doi: 10.1101/gr.3.6.s123. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Zulueta M., Bender C. M., Yang A. S., Nguyen T., Beart R. W., Van Tornout J. M., Jones P. A. Methylation of the 5' CpG island of the p16/CDKN2 tumor suppressor gene in normal and transformed human tissues correlates with gene silencing. Cancer Res. 1995 Oct 15;55(20):4531–4535. [PubMed] [Google Scholar]
- Heintz N., Sive H. L., Roeder R. G. Regulation of human histone gene expression: kinetics of accumulation and changes in the rate of synthesis and in the half-lives of individual histone mRNAs during the HeLa cell cycle. Mol Cell Biol. 1983 Apr;3(4):539–550. doi: 10.1128/mcb.3.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa J. P., Vertino P. M., Wu J., Sazawal S., Celano P., Nelkin B. D., Hamilton S. R., Baylin S. B. Increased cytosine DNA-methyltransferase activity during colon cancer progression. J Natl Cancer Inst. 1993 Aug 4;85(15):1235–1240. doi: 10.1093/jnci/85.15.1235. [DOI] [PubMed] [Google Scholar]
- Jones P. A., Buckley J. D. The role of DNA methylation in cancer. Adv Cancer Res. 1990;54:1–23. doi: 10.1016/s0065-230x(08)60806-4. [DOI] [PubMed] [Google Scholar]
- Jones P. A., Wolkowicz M. J., Rideout W. M., 3rd, Gonzales F. A., Marziasz C. M., Coetzee G. A., Tapscott S. J. De novo methylation of the MyoD1 CpG island during the establishment of immortal cell lines. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6117–6121. doi: 10.1073/pnas.87.16.6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kautiainen T. L., Jones P. A. DNA methyltransferase levels in tumorigenic and nontumorigenic cells in culture. J Biol Chem. 1986 Feb 5;261(4):1594–1598. [PubMed] [Google Scholar]
- Laird P. W., Jackson-Grusby L., Fazeli A., Dickinson S. L., Jung W. E., Li E., Weinberg R. A., Jaenisch R. Suppression of intestinal neoplasia by DNA hypomethylation. Cell. 1995 Apr 21;81(2):197–205. doi: 10.1016/0092-8674(95)90329-1. [DOI] [PubMed] [Google Scholar]
- Li E., Beard C., Jaenisch R. Role for DNA methylation in genomic imprinting. Nature. 1993 Nov 25;366(6453):362–365. doi: 10.1038/366362a0. [DOI] [PubMed] [Google Scholar]
- Merlo A., Herman J. G., Mao L., Lee D. J., Gabrielson E., Burger P. C., Baylin S. B., Sidransky D. 5' CpG island methylation is associated with transcriptional silencing of the tumour suppressor p16/CDKN2/MTS1 in human cancers. Nat Med. 1995 Jul;1(7):686–692. doi: 10.1038/nm0795-686. [DOI] [PubMed] [Google Scholar]
- Moulton T., Crenshaw T., Hao Y., Moosikasuwan J., Lin N., Dembitzer F., Hensle T., Weiss L., McMorrow L., Loew T. Epigenetic lesions at the H19 locus in Wilms' tumour patients. Nat Genet. 1994 Jul;7(3):440–447. doi: 10.1038/ng0794-440. [DOI] [PubMed] [Google Scholar]
- Rideout W. M., 3rd, Coetzee G. A., Olumi A. F., Jones P. A. 5-Methylcytosine as an endogenous mutagen in the human LDL receptor and p53 genes. Science. 1990 Sep 14;249(4974):1288–1290. doi: 10.1126/science.1697983. [DOI] [PubMed] [Google Scholar]
- Schmutte C., Yang A. S., Nguyen T. T., Beart R. W., Jones P. A. Mechanisms for the involvement of DNA methylation in colon carcinogenesis. Cancer Res. 1996 May 15;56(10):2375–2381. [PubMed] [Google Scholar]
- Steenman M. J., Rainier S., Dobry C. J., Grundy P., Horon I. L., Feinberg A. P. Loss of imprinting of IGF2 is linked to reduced expression and abnormal methylation of H19 in Wilms' tumour. Nat Genet. 1994 Jul;7(3):433–439. doi: 10.1038/ng0794-433. [DOI] [PubMed] [Google Scholar]
- Szyf M., Kaplan F., Mann V., Giloh H., Kedar E., Razin A. Cell cycle-dependent regulation of eukaryotic DNA methylase level. J Biol Chem. 1985 Jul 25;260(15):8653–8656. [PubMed] [Google Scholar]
- el-Deiry W. S., Nelkin B. D., Celano P., Yen R. W., Falco J. P., Hamilton S. R., Baylin S. B. High expression of the DNA methyltransferase gene characterizes human neoplastic cells and progression stages of colon cancer. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3470–3474. doi: 10.1073/pnas.88.8.3470. [DOI] [PMC free article] [PubMed] [Google Scholar]