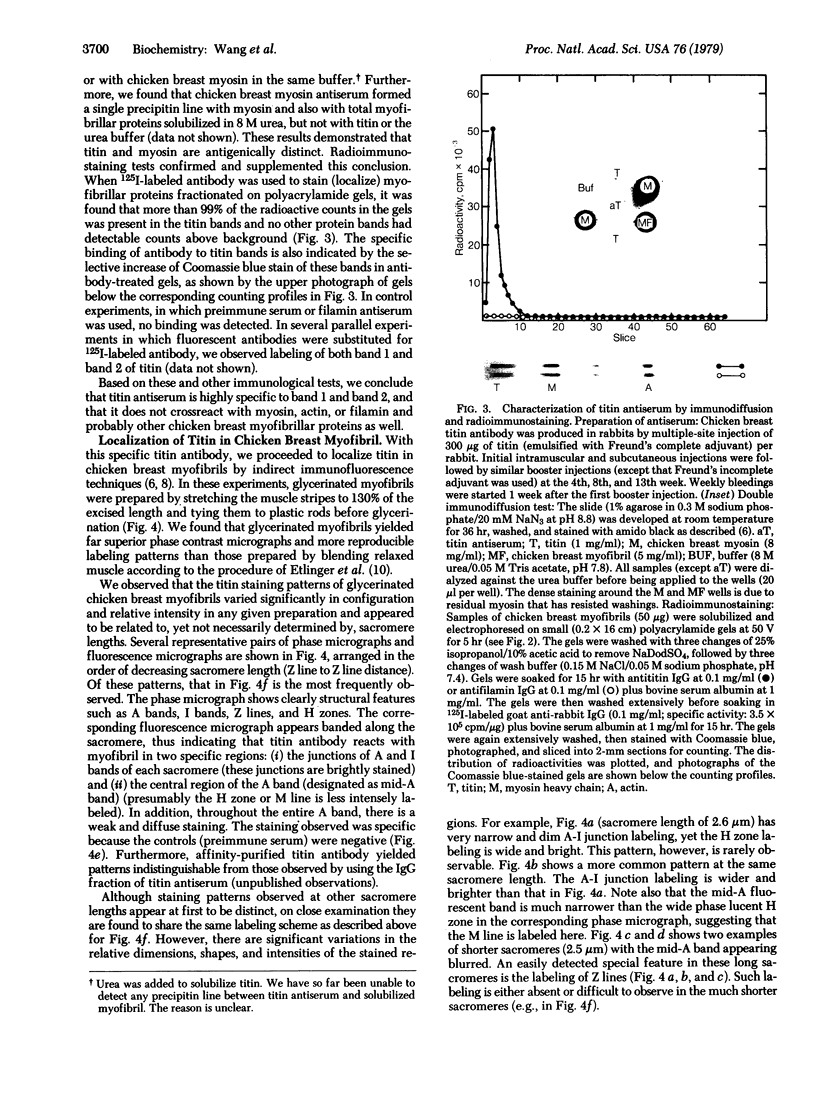
Abstract
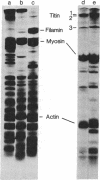
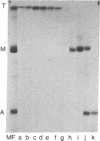
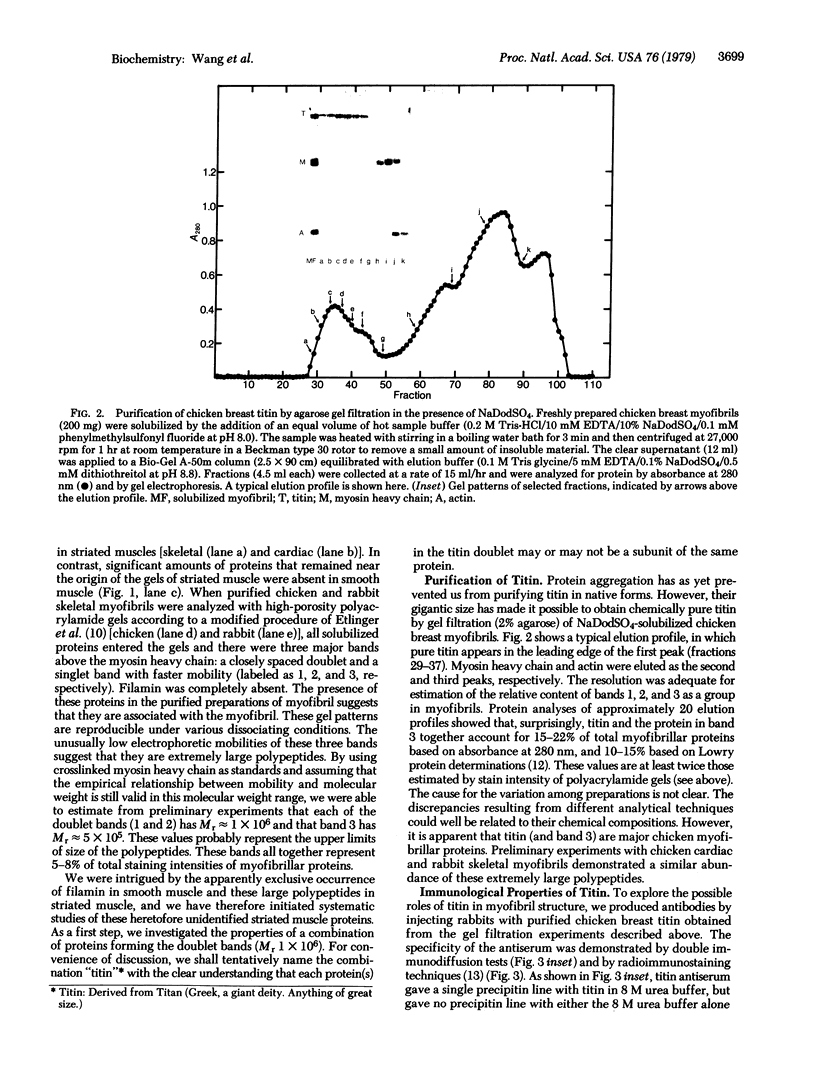
Electrophoretic analyses of protein components of striated muscle myofibril purified from various vertebrate and invertebrate species revealed that proteins much larger than myosin heavy chain are present in significant amounts. To define possible roles of these heretofore unidentified proteins, we purified a combination of two uncommonly large proteins, designated as titin, from chicken breast myofibrils. Chemical and immunological studies indicated that titin is distinct from myosin, actin, and filamin. Specific titin anti body crossreacts with similar protein in both skeletal and cardiac myofibrils of many vertebrate and invertebrate species. Immunofluorescent staining of glycerinated chicken breast myofibrils indicated that titin is present in M lines, Z lines, the junctions of A and I bands, and perhaps throughout the entire A bands. Similar staining studies of myofibrils from other species suggest that titinlike proteins may be organized in all myofibrils according to a common architectural plan. We conclude that titin is a structurally conserved myofibrillar component of vertebrate and invertebrate striated muscles.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burridge K. Changes in cellular glycoproteins after transformation: identification of specific glycoproteins and antigens in sodium dodecyl sulfate gels. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4457–4461. doi: 10.1073/pnas.73.12.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen F., Fuchs F., Knappeis G. G. Contractility and ultrastructure in glycerol--extracted muscle fibers. II. Ultrastructure in resting and shortened fibers. J Cell Biol. 1965 Oct;27(1):35–46. doi: 10.1083/jcb.27.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke M., Spudich J. A. Nonmuscle contractile proteins: the role of actin and myosin in cell motility and shape determination. Annu Rev Biochem. 1977;46:797–822. doi: 10.1146/annurev.bi.46.070177.004053. [DOI] [PubMed] [Google Scholar]
- Etlinger J. D., Zak R., Fischman D. A. Compositional studies of myofibrils from rabbit striated muscle. J Cell Biol. 1976 Jan;68(1):123–141. doi: 10.1083/jcb.68.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- HUXLEY H., HANSON J. Changes in the cross-striations of muscle during contraction and stretch and their structural interpretation. Nature. 1954 May 22;173(4412):973–976. doi: 10.1038/173973a0. [DOI] [PubMed] [Google Scholar]
- Heggeness M. H., Wang K., Singer S. J. Intracellular distributions of mechanochemical proteins in cultured fibroblasts. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3883–3887. doi: 10.1073/pnas.74.9.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle G. Diversity of striated muscle. Am Zool. 1967 Aug;7(3):435–449. doi: 10.1093/icb/7.3.435. [DOI] [PubMed] [Google Scholar]
- Knappeis G. G., Carlsen F. The ultrastructure of the M line in skeletal muscle. J Cell Biol. 1968 Jul;38(1):202–211. doi: 10.1083/jcb.38.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn E. D. Biochemistry of actomyosin-dependent cell motility (a review). Proc Natl Acad Sci U S A. 1978 Feb;75(2):588–599. doi: 10.1073/pnas.75.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Locker R. H., Leet N. G. Histology of highly-stretched beef muscle. I. The fine structure of grossly stretched single fibers. J Ultrastruct Res. 1975 Jul;52(1):64–75. doi: 10.1016/s0022-5320(75)80022-0. [DOI] [PubMed] [Google Scholar]
- Lowey S., Steiner L. A. An immunochemical approach to the structure of myosin and the thick filament. J Mol Biol. 1972 Mar 14;65(1):111–126. doi: 10.1016/0022-2836(72)90495-0. [DOI] [PubMed] [Google Scholar]
- Luther P., Squire J. Three-dimensional structure of the vertebrate muscle M-region. J Mol Biol. 1978 Nov 5;125(3):313–324. doi: 10.1016/0022-2836(78)90405-9. [DOI] [PubMed] [Google Scholar]
- Mannherz H. G., Goody R. S. Proteins of contractile systems. Annu Rev Biochem. 1976;45:427–465. doi: 10.1146/annurev.bi.45.070176.002235. [DOI] [PubMed] [Google Scholar]
- Morimoto K., Harrington W. F. Isolation and composition of thick filaments from rabbit skeletal muscle. J Mol Biol. 1973 Jun 15;77(1):165–175. doi: 10.1016/0022-2836(73)90370-7. [DOI] [PubMed] [Google Scholar]
- Offer G., Moos C., Starr R. A new protein of the thick filaments of vertebrate skeletal myofibrils. Extractions, purification and characterization. J Mol Biol. 1973 Mar 15;74(4):653–676. doi: 10.1016/0022-2836(73)90055-7. [DOI] [PubMed] [Google Scholar]
- Pepe F. A. Structure of muscle filaments from immunohistochemical and ultrastructural studies. J Histochem Cytochem. 1975 Jul;23(7):543–562. doi: 10.1177/23.7.1095653. [DOI] [PubMed] [Google Scholar]
- Porzio M. A., Pearson A. M. Improved resolution of myofibrillar proteins with sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Biochim Biophys Acta. 1977 Jan 25;490(1):27–34. doi: 10.1016/0005-2795(77)90102-7. [DOI] [PubMed] [Google Scholar]
- SZENT-GYORGYI A. G. A new method for the preparation of actin. J Biol Chem. 1951 Sep;192(1):361–369. [PubMed] [Google Scholar]
- Sjöstrand F. S., Jagendorf-Elfvin M. Ultrastructure studies of the contraction-relaxation cycle of glycerinated rabbit psoas muscle. I. The ultrastructure of glycerinated fibers contracted by treatment with ATP. J Ultrastruct Res. 1967 Feb;17(3):348–378. doi: 10.1016/s0022-5320(67)80054-6. [DOI] [PubMed] [Google Scholar]
- Squire J. M. Muscle filament structure and muscle contraction. Annu Rev Biophys Bioeng. 1975;4(00):137–163. doi: 10.1146/annurev.bb.04.060175.001033. [DOI] [PubMed] [Google Scholar]
- Ullrick W. C., Toselli P. A., Chase D., Dasse K. Are there extensions of thick filaments to the Z line in vertebrate and invertebrate striated muscle? J Ultrastruct Res. 1977 Aug;60(2):263–271. doi: 10.1016/s0022-5320(77)80070-1. [DOI] [PubMed] [Google Scholar]
- Wang K., Ash J. F., Singer S. J. Filamin, a new high-molecular-weight protein found in smooth muscle and non-muscle cells. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4483–4486. doi: 10.1073/pnas.72.11.4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K. Filamin, a new high-molecular-weight protein found in smooth muscle and nonmuscle cells. Purification and properties of chicken gizzard filamin. Biochemistry. 1977 May 3;16(9):1857–1865. doi: 10.1021/bi00628a015. [DOI] [PubMed] [Google Scholar]
- Weber K., Rathke P. C., Osborn M. Cytoplasmic microtubular images in glutaraldehyde-fixed tissue culture cells by electron microscopy and by immunofluorescence microscopy. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1820–1824. doi: 10.1073/pnas.75.4.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]