Abstract
Problem
There are many silver-containing wound dressings available for managing and preventing wound infection. Each claims to provide effective antimicrobial activity due to the presence of silver in the dressing. However, assuming effectiveness on the basis of an antimicrobial alone ignores the importance of the carrier dressing and its overall role in wound healing.
Solution
Choice of a silver-containing wound dressing should include consideration of the impact that dressing technology has on the effectiveness of antimicrobial activity as well as its influence on other factors essential for wound healing.
New Technology
AQUACEL® Ag dressing combines patented Hydrofiber® Technology with ionic silver, a proven broad-spectrum antimicrobial. Recent in vitro testing suggests that dressing technology may impact the antimicrobial effectiveness of silver-containing dressings as well as other factors relevant to wound healing. Specifically, a comparative in vitro study demonstrated how the ability of a dressing to micro-contour to the wound bed is critical to antimicrobial effectiveness. Compared with other silver dressings tested, AQUACEL Ag dressing minimized voids and spaces where bacteria can thrive, allowing the silver within the dressing to be in contact with the wound. AQUACEL Ag dressing was observed to kill more bacteria and control the spread of pathogens.
Indications for Use
AQUACEL Ag dressing is indicated for the management of a variety of at risk/infected chronic and acute wounds.
Caution
Not all silver dressings are created equal. Selection of an antimicrobial dressing should take into account in vitro data regarding antimicrobial efficacy of a dressing in addition to its ability to promote wound healing.

Jenny Hurlow, GNP-BC, CWCN
Unmet Need
Wound infection delays healing, impacts patient quality of life, and contributes to healthcare costs. Therefore, infection prevention and management are important clinical priorities to be considered when selecting a dressing. However, guidance for appropriate dressing selection is lacking, and although a proven antimicrobial agent is important, its mere presence in a dressing may not be sufficient to ensure optimal wound healing. To help guide the clinician in making informed decisions on dressing selection, a greater understanding is needed of the impact that dressing technology may have on clinical outcomes.
Product Technology
Hydrofiber® Technology is based on an innovative sodium carboxymethylcellulose hydrocolloid fiber material. The physical properties of Hydrofiber Technology are designed to promote the benefits of moist wound healing by forming a gel on contact with exudate, providing effective fluid management, removing harmful bacteria and enzymes, and providing conformability of the dressing to the wound bed, reducing dead space where bacteria can thrive.
AQUACEL® Ag dressing combines the benefits of Hydrofiber Technology with ionic silver, a proven broad-spectrum antimicrobial. AQUACEL Ag dressing provides rapid and sustained antimicrobial activity for up to 14 days by responding to changes in the components of the wound fluid with increased silver ion availability as needed.1,2 In addition, its ability to micro-contour to the wound bed maximizes the availability of the antimicrobial agent.3 In this manner, the unique properties of AQUACEL Ag dressing allow it to promote wound healing while effectively preventing and managing infection, actions that may be attributed to the combination of the antimicrobial agent and the dressing technology.
Innovation
Hydrofiber Technology is an innovative technology with regard to its mechanism of action, as its physicochemical structure ensures that fluid is absorbed directly into the fibers, which coalesce to form a cohesive gel.4 This gel formation locks in fluid and its harmful components (including bacteria and proteases).5,6 In addition, this gel formation provides intimate contact with the wound bed and prevents lateral spread of fluid.3,5
The clinical implications of this dressing's unique mechanism of action include prevention of voids or spaces where fluid and bacteria can collect while maximizing exposure of superficial bioburden to the ionic silver. The vertical wicking prevents lateral spread of fluid to surrounding skin, promotes optimal exudate management, and minimizes the risk of periwound maceration. The gel supports a moist wound healing environment conducive to optimal healing and formation of granulation tissue, whereas the fluid handling properties allow for a longer wear time, minimizing the need for frequent dressing changes. Overall, for AQUACEL Ag dressing, the combination of dressing technology and the antimicrobial agent may lead to clinical benefits that help manage and prevent infection, promote wound healing, and maximize patient's comfort.
Peer-Reviewed Data
A recent publication7 suggested that viewing silver dressings as passive delivery vehicles/carriers for silver may disregard contributions of the carrier dressing. A broader perspective considers the influence of the carrier dressing on wound progression by managing factors important to wound healing, including exudate management, conformability to the wound bed, and bacterial sequestration.
Numerous in vitro studies have demonstrated the unique fluid-handling properties of Hydrofiber Technology,4–6 as well as the rapid and sustained antimicrobial efficacy of AQUACEL Ag dressing.1,2
Additionally, multiple clinical studies have demonstrated the efficacy of AQUACEL Ag dressing in a variety of chronic and acute wounds.8–10 These studies reported positive clinical outcomes related to control of bacterial balance, wound healing, pain at dressing changes, cost-effectiveness, time to healing, frequency of dressing changes, and nursing time.
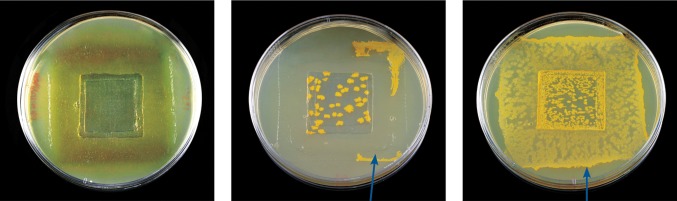
The importance of the combination of dressing technology and antimicrobial agent was illustrated recently in a study by Bowler et al.3 comparing different silver-containing dressings (AQUACEL Ag and certain silver-containing foam dressings) using a shallow wound antimicrobial model to calculate the area of bacterial growth beneath each dressing. The study suggested that a dressing's ability to micro-contour to the wound bed may be important to achieving antimicrobial effectiveness as it minimizes the voids and spaces where bacteria can thrive and allows the silver within the dressing to be in direct contact with wound pathogens. Specifically, AQUACEL Ag dressing was found to conform better to a simulated wound bed compared with competitor foam dressings. With the competitor dressings, fluid accumulation was observed in voids where the dressing did not conform to the wound bed leading to significantly greater bacterial growth beneath the dressing. The results suggest that dressing technology contributes to antimicrobial efficacy.
Non-Peer-Reviewed Observations
Note that we have chosen to only include peer-reviewed data.
Summary Illustrations
| AQUACEL Ag dressing covered by DuoDERM® extra thin dressing, applied to the simulated wound surface. | Gelling commences as AQUACEL Ag dressing absorbs exudate. | AQUACEL Ag dressing forms an intimate contact with the simulated wound surface, limiting spaces where bacteria can thrive. |

Conformability of Allevyn™ Ag adhesive dressing to an uneven tissue surface.

Conformability of Mepilex® Ag dressing to an uneven tissue surface.

In these figures, pieces of dressing were placed on simulated wound tissue (pork belly). A needle and syringe containing dyed physiological saline was then inserted through the base of the tissue, and the solution was then inoculated into the wound space to simulate an exuding wound. Arrows indicate voids.
Staphylococcus aureus
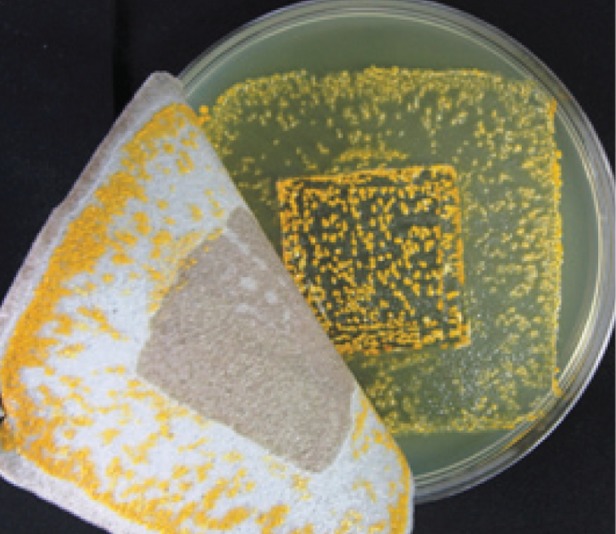
Additionally, AQUACEL Ag dressing was observed to control the spread of bacteria under the dressing better than Allevyn Ag adhesive dressing and Mepilex Ag dressing. The arrows indicate bacteria growth outside of the inoculated area (indented square).
S. aureus observed growing on Mepilex Ag dressing. Photo is representative example of the results.
In an in vitro study that simulated a shallow wound model (indented agar square) for S. aureus and Pseudomonas aeruginosa for a 48 h contact period, the testing of all products was performed three times. The photos are representative examples of the results. Dressing test ranges:
AQUACEL Ag covered by Versiva XC adhesive (S. aureus, 0.0%–1.2%; P. aeruginosa, 2.7%–20.8%).
Allevyn Ag adhesive (S. aureus, 21.0%–30.8%; P. aeruginosa, all 100%).
Mepilex Ag (S. aureus, 65.9%–80.6%; P. aeruginosa, all 100%).
| AQUACEL Ag dressing covered by | ||
| Versiva® XC® adhesive dressing. | Allevyn Ag adhesive dressing. | Mepilex Ag dressing. |
Caution, Critical Remarks, and Recommendations
When selecting an antimicrobial dressing, it is recommended to incorporate it into an appropriate protocol of care, including wound assessment, cleansing, debridement, and infection management strategies, as clinically needed. Always consult the manufacturer's product insert for information on the indications, contraindications, and precautions for use.
In conclusion, not all silver-containing wound dressings are created equal. When choosing a silver dressing, it is important to take into account the impact that dressing technology may have on antimicrobial effectiveness.
Acknowledgment and Funding Source
None to report/not applicable.
Author Disclosure and Ghostwriting
Editorial assistance was provided by Lynssay McGargle and Global Medical Communications at ConvaTec Inc.
References
- 1.Parsons D. Bowler PG. Myles V. Jones SA. Silver antimicrobial dressings in wound management: a comparison of antibacterial, physical and chemical characteristics. Wounds. 2005;17:222. [Google Scholar]
- 2.Bowler PG. Jones SA. Walker M. Parsons D. Microbicidal properties of a silver-containing hydrofiber dressing against a variety of burn wound pathogens. J Burn Care Rehabil. 2004;25:192. doi: 10.1097/01.bcr.0000112331.72232.1b. [DOI] [PubMed] [Google Scholar]
- 3.Bowler P. Jones S. Towers V. Booth R. Parsons D. Walker M. Dressing conformability and silver-containing wound dressings. Wounds UK. 2010;6:14. [Google Scholar]
- 4.Waring MJ. Parsons D. Physico-chemical characterisation of carboxymethylated spun cellulose fibres. Biomaterials. 2001;22:903. doi: 10.1016/s0142-9612(00)00254-4. [DOI] [PubMed] [Google Scholar]
- 5.Walker M. Parsons D. Hydrofiber technology: its role in exudate management. Wounds UK. 2010;6:31. [Google Scholar]
- 6.Walker M. Hobot JA. Newman GR. Bowler PG. Scanning electron microscopic examination of bacterial immobilisation in a carboxymethylcellulose (Aquacel) and alginate dressings. Biomaterials. 2003;24:883. doi: 10.1016/s0142-9612(02)00414-3. [DOI] [PubMed] [Google Scholar]
- 7.Cutting K. White R. Hoekstra H. Topical silver-impregnated dressings and the importance of the dressing technology. Int Wound J. 2009;6:396. doi: 10.1111/j.1742-481X.2009.00635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caruso DM. Foster KN. Blome-Eberwein SA. Twomey JA. Herndon DN. Luterman A, et al. Randomized clinical study of Hydrofiber dressing with silver or silver sulfadiazine in the management of partial-thickness burns. J Burn Care Res. 2006;27:298. doi: 10.1097/01.BCR.0000216741.21433.66. [DOI] [PubMed] [Google Scholar]
- 9.Jurczak F. Dugre T. Johnstone A. Offori T. Vujovic Z. Hollander D. Randomised clinical trial of Hydrofiber dressing with silver versus povidone-iodine gauze in the management of open surgical and traumatic wounds. Int Wound J. 2007;4:66. doi: 10.1111/j.1742-481X.2006.00276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coutts P. Sibbald RG. The effect of a silver-containing Hydrofiber dressing on superficial wound bed and bacterial balance of chronic wounds. Int Wound J. 2005;2:348. doi: 10.1111/j.1742-4801.2005.00150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


