Abstract
Background
A chronic wound is a wound that is arrested in the inflammatory phase of wound healing and cannot progress further. Over 90% of chronic wounds contain bacteria and fungi living within a biofilm construct.
The Problem
Each aggregation of microbes creates a distinct biofilm with differing characteristics so that a clinical approach has to be tailored to the specifics of a given biofilm. Defining the characteristics of that biofilm and then designing a therapeutic option particular to that biofilm is currently being defined.
Basic/Clinical Science Advances
Biofilm becomes resistant to therapeutic maneuvers at 48–96 h after formation. By repeatedly attacking it on a regular schedule, one forces biofilm to reattach and reform during which time it is susceptible to antibiotics and host defenses. Identifying the multiple bacteria and fungi that make up a specific biofilm using polymerase chain reaction (PCR) allows directed therapeutic maneuvers such as application of specific topical antibiotics and biocides to increase the effectiveness of the debridement.
Clinical Care Relevance
Most chronic wounds contain biofilm that perpetuate the inflammatory phase of wound healing. Combining debridement with using PCR to identify the bacteria and fungi within the biofilm allows for more targeted therapeutic maneuvers to eliminate a given biofilm.
Conclusion
Therapeutic options in addition to debridement are currently being evaluated to address biofilm. Using PCR to direct adjunctive therapeutic maneuvers may increase the effectiveness of addressing biofilm in a chronic wound.
Christopher Attinger
Background
A chronic wound is a wound that is arrested in the inflammatory phase of wound healing and cannot progress further. The presence of necrotic tissue, foreign material, and/or bacteria impedes the wound's ability to heal by producing or stimulating the production of proinflammatory cytokines, elevated matrix metalloproteases, and excessive neutrophils. In this process, the building blocks (chemotactants, growth factors, mitogens, and so on) necessary for normal wound healing are either rendered inert or destroyed. This hostile environment also allows bacteria to proliferate and further colonize the wound by constructing protected colonies known as biofilm. Over 90% of chronic wounds contain bacteria living within biofilm construct.
Debriding a wound is defined as removing necrotic tissue, foreign material, senescent cells, and bacteria. It, hopefully, removes enough of the inhibitory factors so that the wound can progress beyond the inflammatory stage toward healing. Removing biofilm is one of the difficult things to do, because it is firmly adherent to surrounding tissue, is resistant to and poorly penetrated by antibiotics, is resistant to biocides, and evades the body's local immune response. Strategies to address this hard-to-remove biofilm while keeping the surrounding tissue healthy are evolving.
Target Articles.
1. Wolcott RD, Rumbaugh KP, James G, Schultz G, Phillips P, Yang Q, Watters C, Stewart PS, and Dowd SE: Biofilm maturity studies indicate sharp debridement opens a time dependent therapeutic window. J Wound Care 2010; 19: 320.
2. Wolcott RD, Kennedy JP, and Dowd SE: Regular debridement is the main tool for maintaining a healthy wound bed in most chronic wounds. J Wound Care 2009; 18: 54.
3. Wolcott RD, Kennedy JP, and Dowd SE: Healing and healing rates of chronic wounds in the age of molecular pathogen diagnostics. J Wound Care 2010; 19: 272.
Clinical Problems Addressed
A chronic wound is one that is usually arrested in the inflammatory stage and cannot progress to the proliferative and remodeling phase of healing. Proinflammatory cytokines produced by necrotic tissue, foreign material, and bacteria allow the inflammatory stage to continue. In addition, changes in the cellular DNA synthesis leads to increased formation of metalloproteases that impede the body's attempt to heal by overwhelming the building blocks—chemotactant factors, growth factors, and mitogens—needed for normal wound healing.1 Fibroblasts, an essential cell in the wound healing process, is phenotypically altered in the setting of chronic wounds so that their ability to replicate as well as produce the necessary building blocks for the formation of granulation tissue is altered.2 Further, the keratinocytes at the periphery of the wounds are phenotypically different so that while being able to proliferate, they cannot fully differentiate into migrating keratinocytes.3 This explains the epithelial build up often seen around the edge of the wound.
Evidence suggests that biofilm plays a significant role in the inability of chronic wounds to heal. Biofilm is present in only 6% of acute wounds but over 90% of chronic wounds. In contrast to planktonic bacteria, biofilm represents an aggregation of different bacterial species enclosed within a protective glycocalyx that adheres to the wound surface. The bacteria change their phenotype as they form the biofilm utilizing building blocks from both the host and what they produce. As the biofilm matures, they continue changing their phenotype to living in a lower energy state with separate functions within the colony while sharing their resistance to antibiotics with the community. Every aggregation of bacteria creates a distinct biofilm with differing characteristics4 so that a clinical approach has to be tailored to the specifics of a given biofilm.
Relevant Basic Science Context
The role of debridement in preparing wounds to heal has been well documented most specifically in double-blinded randomized studies looking at the efficacy of becaplermin in healing diabetic foot wounds. In those centers where the wounds were debrided weekly, the wounds healed 83% of the time. In those wounds that were sporadically debrided, only 25% of the wounds went on to heal.5 Piaggesi then showed in a randomized trial that surgical debridement of a foot ulcer when compared with wound care and offloading was more effective in healing the wound by healing it more rapidly and with less complications.6 A retrospective study looking at both 366 venous stasis ulcers and 310 diabetic foot ulcer felt there was enough evidence to suggest that frequent debridement may increase healing rates and time to healing.7
Experimental Model or Material: Advantages and Limitations
Because it is currently accepted that debridement is the key to convert a chronic wound into an acute wound so that it can heal,8 randomized trials that include a nondebridement arm when dealing with chronic wounds are very difficult to design. Current wound healing research evaluates various interventions by their ability to heal wounds within a given time period. To look at debriding techniques with respect to biofilm, one has to assess the type and frequency of debridement as well as the type of topical therapy has an effect. There is some evidence that debridement, hydrotherapy, shockwave therapy, ultrasound, negative pressure with fluid instillation ability, cadexomar iodine, and biofilm-dissolving agents such as lacoferrin all have an effect in removing biofilm. The question is which mode or combination of approaches is the most effective. One then has to assess whether getting rid of biofilm actually speeds up wound healing. These trials have to be randomized and blinded if possible to yield meaningful data.
Discussion of Findings and Relevant Literature
The biofilm matrix provides resistance to biocides,9 host defenses, and antibiotic penetration while bacteria provide resistance to antibiotics via decreased growth/metabolic rate, stress response, and modulation of quorum-sensing pathways.10 Newly formed biofilm is more susceptible to than older mature biofilm to antibiotics, biocides, and host immune mediators. This is because they are in a more active phenotypic stage and the matrix is less developed. This allows a window of opportunity to attack the biofilm before it reaches its mature stage. The first article11 shows that bacteria are much more susceptible to selective antibiotics in the first 48 h of formation than thereafter. This study was carried out in four different laboratories utilizing four different mechanisms of testing and essentially yielded the same results.
Both Staphylococcus aureus and Pseudomonas aeruginosa were exposed to four different tests. The drip flow model showed that bacteria were far less susceptible at 24 and 48 h than at 6 and 12 h to gentamicin. S. aureus took longer (96 h) to reach complete tolerance than P. aerginosa. Hydrodebridement decreases biofilm by 1–3 logs but the remaining biofilm remained tolerant to gentamicin despite regrowth. When both species of planktonic bacteria were placed on porcine explants, they became tolerant to gentamicin at 48 h, although S. aureus took longer to reach complete tolerance. When P. aerginosa biofilm was grown on mice in a chronic wound model, the biofilm at 48 h became more tolerant to both 100% bleach and topical gentamicin. About 39% of the biofilm exposed to bleach and 9% of biofilm exposed to gentamicin was viable at 48 h. When looking at a patient's chronic wound, the biofilm became increasingly resistant to gentamicin after debridement over time such that it reached its predebridement resistance level within 72 h.
The in vitro experiments reported here demonstrate that bacterial biofilms develop antibiotic tolerance over time with less mature biofilms being more susceptible to antibiotic treatment. This occurs relatively quickly with P. aeruginosa biofilms (24 h) and more slowly with S. aureus biofilms (96 h). Partial removal of the biofilm in vitro models exposes more phenotypically inactive bacteria with increased resistance. This suggests that complete removal of biofilm works and that addressing the biofilm in the first 48 h of formation provides the most effective therapeutic window. This is when the biofilm is immature and the bacteria are more active in terms of metabolism and growth.
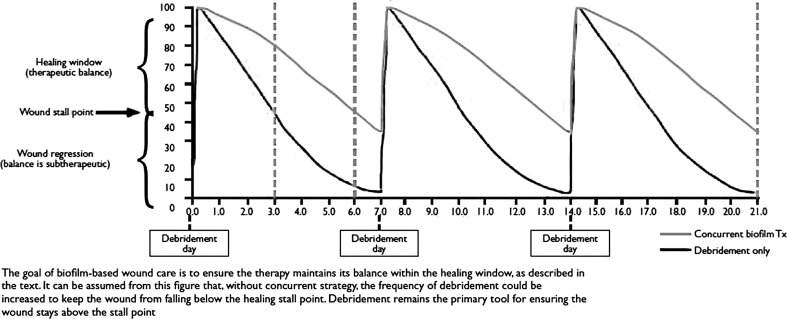
The second article12 summarizes the challenge that biofilm presents to wound healing and emphasizes the importance of repeatedly physically removing biofilm and suppressing its regrowth. Excisional debridement of the wound also helps establish a clean well-vascularized wound base. By repeatedly attacking the biofilm on a regular schedule, one forces it to reattach and reform during which time it is susceptible to antibiotics and host defenses (Fig. 1). Complete removal of the biofilm is unlikely, as it tends to spread perivascularly below the surface of the wound13 and reform very rapidly.14 Because of the rapidity with which biofilm reforms, it is important to quickly identify the type and susceptibility of bacteria involved using polymerase chain reaction.15 Subsequent treatment of the susceptible biofilm with topical biocides as well as systemic antibiotics is critical to limiting regrowth. Debriding the wound every 7 days favors wound healing for 43% of the week while adding appropriate topical biocides and systemic antibiotics increases that time to 86%.
Figure 1.
Graphical illustration of the effects of biofilm-based wound care. The goal of biofilm-based wound care is to ensure the therapy maintains its balance within the healing window, as described in the text. It can be assumed from this figure that, without concurrent strategy, the frequency of debridement could be increased to keep the wound from falling below the healing stall point. Debridement remains the primary tool for ensuring the wound stays above the stall point.12
The diversity of bacteria found within a particular biofilm is such that each biofilm has its own distinct phenotype.16 The third article17 emphasizes the importance of using molecular diagnostics18 in addition to debridement, to rapidly identify the bacteria and yeast in the debrided biofilm for a specific therapeutic intervention leading to more rapid and improved wound healing. The article does that by comparing wound healing the year before and after introducing molecular pathogen diagnostics. Wounds that were treated on the basis of the bioformatic analysis of the biofilm were 22.9% more likely to heal and had a 22% decrease in time to heal when compared with those treated on the basis of single cultures of planktonic bacteria. Bioformatic analysis allowed for more accurate identification of bacteria, both resistant strains and strains hard to culture out. Systemic antibiotic therapy was used more often and specifically in 2009.
Innovation
Sharp debridement has been shown to be critical in the healing of chronic wounds. Its role in the context of dealing with biofilm is currently being better defined as the characteristics and susceptibility of biofilm. The rational for repeated debridement makes more sense when one understands that this keeps biofilm susceptible to outside agents. The use of bioformatics to identify the bacteria within the biofilm helps better guide antibiotic therapy for more effective treatment. The use of alternating biocides and mechanical energy to attack the glycocalyx structure of biofilm weakens it and renders the bacteria within it more vulnerable. This multitiered approach offers a promising way to address biofilm in chronic wound effectively.
Take-Home Message.
Basic science advances
• Biofilm is prevalent in all chronic wounds and rare in acute wounds.
-
• Chronic wounds have many of the same characteristics as other chronic infections (cystic fibrosis, chronic rhinosinusitis, Crohn's) such as:
- Wax and wane with exacerbations.
- Respond incompletely to antibiotics and steroids and reemerge when these are withdrawn.
- Persistent, degrading, and often result in surgical removal of tissue.
• Debridement of biofilm opens a time-dependent window (2–3 days in wounds) where treatment agents are more effective.
• A single treatment will cause some bacteria to drop out of a wound biofilm, but this allows other bacteria to grow up and reconstitute the biofilm.
Clinical science advances
• There is a suggestion that utilizing DNA methods to identify bacteria and fungus is more accurate in identifying infective organisms in chronic wounds.
• There is suggestion that individualizing topical therapy based on the bacteria and/or fungus identified through DNA methods improves clinical outcomes.
-
• Biofilm principles translate from one biofilm disease to another. We are evaluating whether successful treatments from other specialties are applicable to our management of chronic wounds. For example:
- Dentistry utilizes brushing (debridement) with toothpaste (multivalent antibiofilm treating agents) to prevent tooth loss.
- Ear, nose, and throat specialists have utilized topical antibiotics at 1,000 times minimum inhibitory concentration (MIC) to treat otitis externa for over 30 years and this is their gold standard.
- Ophthalmology has used topical antibiotics at 1,000 times MIC to treat biofilm conjunctivitis as a gold standard.
• Frequent debridement disrupts biofilm and this may explain why it is successful in improving wound healing outcomes.
• The single agent antibiotic treatment of planktonic bacteria at best a 50%–50% proposition in chronic wounds. Chronic wounds may benefit from a biofilm strategy of using multiple treating agents simultaneously, which are adjusted as the biofilm changes.
Relevance to clinical care
A key component to chronic wounds is chronic infections, so it is not surprising that wound biofilm may be an important barrier to wound healing. Because biofilm is prevalent in chronic wounds and when appropriately diagnosed and specifically treated utilizing biofilm strategies, level 3 evidence suggests that wound healing outcomes are improved. Biofilm must be targeted by a disruptive strategy including debridement, energy transfer, enzymes, or other methods and then, when disrupted, attacked with multiple treatments to suppress reconstitution. Biofilm treatments may be more effective when directed by diagnostic information identifying the bacteria and fungi it contains. Frequent debridement, use of mechanical energy, application of appropriate biocides, and/or topical antibiotics are all coordinated strategies that may help remove infection as one of the impediments to the healing process in chronic wounds.
Caution, Critical Remarks, and Recommendations
There are no randomized trials showing that the above advocated approach to chronic wounds is effective. There is good research data and level 3 evidence suggesting that this may be a useful tool to heal chronic wounds. The difficulty in addressing biofilm is that not only it is difficult to eradicate on the surface but also it burrows below the surface of the wound by spreading along perivascular channels. Optimizing debridement may require more than surgical debridement and may include also using physical energy such as ultrasound or shockwave therapy. Identifying bacteria in any chronic infection by using pyrosequencing is critical in directing postdebridement therapy. It is more rapid and more accurate. As it becomes more utilized, the price and time to diagnosis will continue to decrease. In addition, such testing will also soon include platforms providing information about inflammatory markers and cell senescence to further guide our treatment. Within 1–2 years, a total evaluation of the wound bed will be available at a cost-effective price.
Future Development of Interest
We are just at the doorway of a major revolution in the management of chronic infections and chronic wounds. As the diagnostics methods evolve to include more host and infection information, treatments will be developed to address this new diagnostic information. One of the more exciting areas is antibiofilm agents. Antibiofilm agents are not antibiotics but affect biofilm by disrupting quorum sensing, degrading extracellular polymeric substance, blocking attachments, and a multitude of other antibiofilm strategies. Antibiofilm agents allow antibiotics to be more effective. Also promising is the use of energy transfer methods such as laser, ultrasound, acoustic, and other methods that can physically disrupt biofilm. These energy transfer methods have the possibility of targeting biofilm cells and sparing the host cells. And finally, electrical treatments should reemerge. A common practice in industry to manage biofilm is to use low electrical currents. Many of the processes and communications of biofilm are electrochemical in nature. As these electrochemical processes are better understood, it should be easier to disrupt by utilizing small electrical currents.
Abbreviations and Acronyms
- MIC
minimum inhibitory concentration
- PCR
polymerase chain reaction
Author Disclosure and Ghostwriting
Christopher Attinger has nothing to disclose. Randy Wolcott has equity interest in PathoGenius, a molecular diagnostic laboratory. No ghostwriter has been used to write this article.
References
- 1.Cornell RS. Meyr AJ. Steinberg JS. Attinger CE. Debridement of the noninfected wound. J Vasc Surg. 2010;52:31S. doi: 10.1016/j.jvs.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Clark RA. Oxidative stress and “senescent” fibroblasts in non-healing wounds as potential therapeutic targets. J Invest Dermatol. 2008;128:2361. doi: 10.1038/jid.2008.257. [DOI] [PubMed] [Google Scholar]
- 3.Morasso MI. Tomic-Canic M. Epidermal stem cells: the cradle of epidermal determination, differentiation, and wound healing. Biol Cell. 2005;97:173. doi: 10.1042/BC20040098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cho KH. Caparon MG. Patterns of virulence gene expression differ between biofilm and tissue communities of Streptococcus pyogenes. Mol Microbiol. 2005;57:6–1545. doi: 10.1111/j.1365-2958.2005.04786.x. [DOI] [PubMed] [Google Scholar]
- 5.Steed DL. Donohoe D. Webster MW. Lindsley L. Effect of extensive debridement and treatment on the healing of diabetic foot ulcers. Diabetic Ulcer Study Group. J Am Coll Surg. 1996;183:61. [PubMed] [Google Scholar]
- 6.Piaggesi A. Schipani E. Campi F. Romanelli M. Baccetti F. Arvia C. Navalesi R. Conservative surgical approach versus non-surgical management for diabetic neuropathic foot ulcers: a randomized trial. Diabet Med. 1998;15:412. doi: 10.1002/(SICI)1096-9136(199805)15:5<412::AID-DIA584>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 7.Cardinal M. Eisenbud DE. Armstrong DG. Zelen C. Driver V. Attinger C. Phillips T. Harding K. Serial surgical debridement: a retrospective study on clinical outcomes in chronic lower extremity wounds. Wound Repair Regen. 2009;17:306. doi: 10.1111/j.1524-475X.2009.00485.x. [DOI] [PubMed] [Google Scholar]
- 8.Kirshen C. Woo K. Ayello EA. Sibbald RG. Debridement: a vital component of wound bed preparation. Adv Skin Wound Care. 2006;19:506. doi: 10.1097/00129334-200611000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Stewart PS. Grab L. Diemer JA. Analysis of biocide transport limitation in an artificial biofilm system. J Appl Microbiol. 1998;85:495. doi: 10.1046/j.1365-2672.1998.853529.x. [DOI] [PubMed] [Google Scholar]
- 10.Rani SA. Pitts B. Beyenal H, et al. Spatial patterns of DNA replication, protein synthesis, and oxygen concentration within bacterial biofilms reveal diverse physiological states. J Bacteriol. 2007;189:4223. doi: 10.1128/JB.00107-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolcott RD. Rumbaugh KP. James G. Schultz G. Phillips P. Yang Q. Watters C. Stewart PS. Dowd SE. Biofilm maturity studies indicate sharp debridement opens a time dependent therapeutic window. J Wound Care. 2010;19:320. doi: 10.12968/jowc.2010.19.8.77709. [DOI] [PubMed] [Google Scholar]
- 12.Wolcott RD. Kennedy JP. Dowd SE. Regular debridement is the main tool for maintaining a healthy wound bed in most chronic wounds. J Wound Care. 2009;18:54. doi: 10.12968/jowc.2009.18.2.38743. [DOI] [PubMed] [Google Scholar]
- 13.Schaber JA. Triffo WJ. Suh SJ, et al. Pseudomonas aeruginosa forms biofilms in acute infection independent of cell-to-cell signaling. Infect Immun. 2007;75:3715. doi: 10.1128/IAI.00586-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis SC. Ricotti C. Cazzaniga A, et al. Microscopic and physiologic evidence for biofilm-associated wound colonization in vivo. Wound Repair Regen. 2008;16:23. doi: 10.1111/j.1524-475X.2007.00303.x. [DOI] [PubMed] [Google Scholar]
- 15.Wolcott RD. Dowd SE. A rapid molecular method for characterising bacterial bioburden in chronic wounds. J Wound Care. 2008;17:513. doi: 10.12968/jowc.2008.17.12.31769. [DOI] [PubMed] [Google Scholar]
- 16.James GA. Swogger E. Wolcott R, et al. Biofilms in chronic wounds. Wound Repair Regen. 2008;16:37. doi: 10.1111/j.1524-475X.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- 17.Wolcott RD. Kennedy JP. Dowd SE. Healing and healing rates of chronic wounds in the age of molecular pathogen diagnostics. J Wound Care. 2010;19:272. [PubMed] [Google Scholar]
- 18.Dowd SE. Sun Y. Secor PR, et al. Survey of bacterial diversity in chronic wounds using Pyrosequencing, DGGE, and full ribosome shotgun sequencing. BMC Microbiol. 2008;6:43. doi: 10.1186/1471-2180-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]