Abstract
Background
Keloids are benign, fibroproliferative lesions that represent abnormal healing resulting in excessive fibrosis. They are composed of mainly type III (early) or type I (late) collagen. Some of the symptoms include pruritus, tenderness, and pain. Often, they are very difficult to treat and prevent from recurrence. In contrast to hypertrophic scars, keloids extend beyond the margin of the wound.
The Problem
There is very limited evidence on the best wound management for minimizing scarring. Multiple available therapeutic modalities have been used for the treatment of keloids; however, high-recurrence rates continue to be reported. Unsuccessful treatment of keloids leads to psychological impact on the patients and increased economic burden.
Basic/Clinical Science Advances
Currently, there are biological and antineoplastic agents that can potentially treat and prevent excessive scar formation. Some of them have been used as "off label" therapies, and others are still in the experimental phase such as interferon alpha (IFN-α), imiquimod, and transforming growth factor beta1 (TGF-β1). The use of IFN-α2b showed 18% recurrence rate when applied to postsurgical excised keloids. Imiquimod 5% can lower recurrence rate on postshaved keloids to 37.5% at 6-month and to 0% at a 12-month follow-up period. TGF-β1 oligonucleotides have shown effective and long-lasting inhibition of TGF-β-mediated scarring in vitro as well as in animal models. Daily injections of neutralizing antibodies against TGF-β1 and -β2 have shown successful reductions in scarring.
Conclusion
Latest discoveries in the use of novel agents suggest therapeutic alternatives for the prevention of recurrences of hypertrophic scars and postexcision keloid lesions.

Brian Berman, MD, PhD
Background
Wound healing is accomplished through different phases: hemostasis, inflammation, proliferation, and remodeling. For a wound to heal effectively, all phases should occur properly and in the right sequence. Scarring is considered abnormal when fibrosis is excessive or suboptimal. Although the precise pathogenesis of keloid formation is unclear, fibroblast proliferation and collagen synthesis are markedly increased. Keloid fibroblasts (KFs) have shown failure to undergo apoptosis, and, therefore, continue to produce connective tissue beyond the period expected for normal scars. Over expression of growth factors, such as transforming growth factor beta (TGF-β), vascular endothelial growth factor, and connective tissue growth factor, may also play a role in keloid formation. TGF-β is a regulator of fibroblast proliferation and collagen synthesis. As supposed to normal scarring, keloids show overproduction and inadequate regulation of TGF-β activity.
Keloids are more frequent in certain ethnic populations with incidence rates as high as 15–20% in the black population. Although they can occur in all skin types, there are no reports of keloid scarring in albinos. Reports have shown various modes of inheritance, thus suggesting that multiple genetic factors influence keloid formation. The location, size, and depth of the lesion; the age of the patient; and the previous response to treatment determine the best strategy to manage this condition.
Target Articles.
1. Berman B, Harrison-Balestra C, Perez OA, Viera M, Villa A, Zell D, and Ramirez C: Treatment of keloid scars post-shave excision with imiquimod 5% cream: a prospective, double-blind, placebo-controlled pilot study. J Drugs Dermatol 2009; 8: 455.
2. Berman B: Biological agents for controlling excessive scarring. Am J Clin Dermatol 2010; 11(Suppl 1): 31.
3. Bran GM, Goessler UR, Schardt C, Hormann K, Riedel F, and Sadick H: Effect of the abrogation of TGF-beta1 by antisense oligonucleotides on the expression of TGF-beta-isoforms and their receptors I and II in isolated fibroblasts from keloid scars. Int J Mol Med 2010; 25: 915.
Clinical Problem Addressed
Keloids are important dermatologic entities that can cause significant physical, esthetic, psychological, and social consequences to patients, and may be associated with substantial emotional and financial costs. In addition to symptoms such as tenderness, pain, and burning, keloids may lead to sleep disturbances, anxiety, depression, and disruption of daily activities. In addition, the cosmetic defect can progress to generate contractures and can moreover produce severe deformities and functional impairment that negatively impact the patient's quality of life.
Hypertrophic scars and keloids have shown some response to therapies such as radiation, cryotherapy, intralesional corticosteroids, 5-fluorouracil, topical silicone, occlusion dressings, and pulsed-dye lazer. Surgical excision frequently results in recurrence unless adjunct therapies are applied. Currently, there is no single therapeutic modality considered entirely safe and effective for the treatment of this condition. The development of successful targeted therapeutic modalities is, therefore, a challenge to the experts in this field. Physicians constantly face with patient's unrealistic expectations. Clinical judgment is required when considering treatment; balancing the potential benefits against the possibility of a poor response or even potential iatrogenic complications. The evidence base for the use of many current treatments is limited, and some may have only placebo benefit.1
Relevant Basic Science Context
Keloids result from derangement of dermal wound repair leading to accumulation of extracellular matrix (ECM) and ultimately to excessive scar formation.2 TGF-β is a cytokine implicated in the pathogenesis of keloids. It is produced and released by platelets, fibroblasts, endothelial, epithelial, and inflammatory cells such as macrophages and lymphocytes after skin injury and participates in the regulatory process of cell proliferation and tissue repair.3 Evidence indicates that TGF-β isoforms 1 and 2 promote collagen synthesis and scar formation. messenger RNA (mRNA) expression of these isoforms is higher in KFs. In contrast, isoform 3 is involved in scar prevention with its mRNA expression being lower in KFs.4–7 TGF-β1 is considered the key mediator of keloid pathogenesis, exerting its biological effect by interacting with the transnmembrane receptors type I and II (TGF-βRI and -βRII).
Keloids have also shown low levels of the inmmune-response modifiers, interferon alpha and gamma (IFN-α, IFN-γ). IFN-γ up regulates the pro-apoptotic gen p53 in epidermal cells, thus reducing collagen type I mRNA and leading toward termination of the repair process.8 IFN-α2b achieves its antifibrotic properties by normalizing collagen, glycosaminoglicans, and collagenase synthesis and activity.9
Imiquimod is an agonist of the toll-like receptors 7 and 8 (TLR7, TLR8) commonly involved in pathogen recognition. Cells activated by imiquimod secrete proinflammatory cytokines, especially IFN-α, interleukin 6 (IL-6), and tumor necrosis factor alpha (TNF-α). When topically applied, this results in antifibrotic and pro-apoptotic effects.
Recent evidence reveal that imiquimod 5% cream increases apoptotic gene expression in nonexcised keloids and reduces keloids recurrence by 30%–70%.10,11
Experimental Model Or Material: Advantages and Limitations
To evaluate the effect of the annulment of TGF-β1 on its isoforms and receptors, samples of keloids were obtained during reconstructive surgery (four after otoplasty and one after tympanoplasty), and control specimens were obtained from adjacent normal skin. After culturing keloid-derived and normal fibroblasts, immunohistochemistry for TGF-β1-β3 and TGF-βRI-βRII was performed. Olidodeoxynucleotides were synthesized, and the antisense was directed against the translation start site of TGF-β1 complementary DNA (cDNA). The isoforms' concentrations were obtained by enzyme-linked immune absorbent assay. RNA was isolated and reversed into cDNA. mRNA from TGF-β isoforms and receptors was measured in all cells by using polymerase chain reaction.
The advantage of this experimental method include convenient sample recollection during an elective surgical removal of keloids without creating a new biopsy site.
Limitations include heterogeneous resections of keloid samples leading to different maturity and clinical stages of the keloids that could have an influence on the reliability of the results.12
In the imiquimod study, 20 shaved keloids of various sizes and from different anatomic areas were administered imiquimod 5% cream versus vehicle and occluded nightly for 2 weeks and then triweekly for 1 month. Limitations include small sample size, self-application of the cream, optional treatment rest periods due to intolerance, the subjective nature of self-assessment of tolerance, the difference in initial size and anatomical locations of the keloids, and the high rate of loss of follow-up. All these aspects may contribute to increased variability of the results.
Discussion of Findings and Relevant Literature
A unique oversensitivity of KFs to TGF-β with an exaggerated response to this cytokine has been demonstrated by researchers.13,14 As mentioned earlier, the expression of TGF-β1 and -β2 (profibrotic) is higher in KFs, whereas TGF-β3 expression (antifibrotic) is significantly lower in KFs. These alterations in expression manifest as uncontrolled fibrotic reaction and keloid formation. In support of this theory, the use of a TGF-β1 and -β2 neutralizing antibody or the exogenous addition of the TGF-β3 peptide has demonstrated reduction of scarring and normalization of the newly formed dermis.15 Keloids have increased TGF-βRI and significantly decreased TGF-βRII mRNA expression.7 These findings suggest two methods to decrease TGF-β transduction and consequent keloid scar formation. The first is to reduce the activity of TGF-β, and the second is to increase the expression of TGF-βR2. Our target article focuses on the first strategy, through the application of antisense oligonucleotides to block the effect of TGF-β1. The results showed significant reduction in the expression of TGF-β1 and β2, increase in TGF-βRII expression, and up-regulation of TGF-β3; these results are consistent with an antifibroproliferative effect. They also support the hypothesis of the possible therapeutic potential of TGF-β-oriented antisense therapy.12
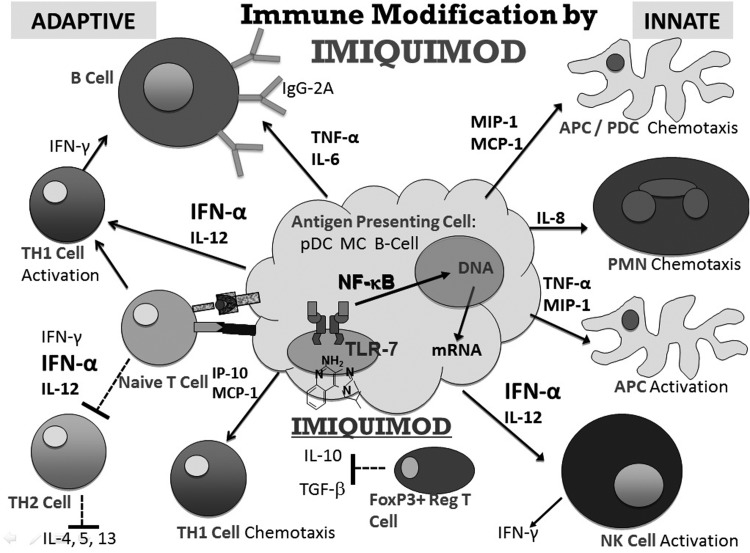
Imiquimod can be used as an adjunctive treatment to surgically removed keloids, thus reducing the recurrence rate, which has been reported to be 45%–100% with excision alone, and 30%–70% with excision plus intralesional steroids (Fig. 1).16,17 In the imiquimod-related target article, at 2 weeks of treatment, tenderness and pain were significantly higher in the imiquimod group versus the control group. However, imiquimod was considered overall well tolerated. Pruritus showed no significant difference. The recurrence rate resulted in 37.5% in the treated group versus 75% in the vehicle group. Although this difference was not statistically significant most likely due to the small sample size, short follow-up period, and poor patient compliance, interestingly the recurrence rate in the treated group was lower than in the group reported with excision alone.12 The efficacy of this therapy is still very controversial. In two similar studies, the use of imiquimod in postsurgical keloids rendered 0% recurrence18 but other case series provided low evidence for efficacy.16,19 Cacao et al.20 treated nine patients with a postsurgical keloid scar with daily application of imiquimod 5% cream for 8 weeks. Keloid recurrence occurred in eight patients (89%). Alternatively, Prado et al. demonstrated that imiquimod therapy improved scar quality and color match after excision21 and in an own series of patients with keloid disease at various locations, long-term application of imiquimod 5% cream for more than a year was able to prevent keloid regrowth. Imiquimod proved to be relatively safe for use.22
Figure 1.
Imiquimod's immune modification: cascade of effects.
The use of TNF-α antagonists for the treatment of keloids is based on the rationale that at low concentrations, TNF-α promotes inflammation and fibrosis and inhibits collagen and glycosaminoglycan breakdown. These effects may be reduced by the use of intralesional etanercept.12 Berman et al. evaluated the tolerability and efficacy of monthly etanercept 25 mg/mL compared with triamcinolone acetonide 20 mg/mL for the intralesional treatment of 20 keloids during 2 months. However, no significant difference between treatments was found, and both were safe and well tolerated.23
As mentioned earlier, keloids are depleted of IFN, which induces apoptosis in keratinocytes and reduces collagen type I mRNA in KFs.8 IFN-α2b has shown to improve scar quality as a result of the suppression of fibroblast function and reduced collagen synthesis.24,25
Other immuneno-modulators include IL-10, which is thought to reduce levels of proinflammatory mediators and promote a favorable environment for scarless healing. Recombinant IL-10 perioperatively administered to 1,400 wounds was found to significantly improve scar appearance at 1 year compared with standard of care.26
Tacrolimus is a potential useful immunomodulator in the treatment of keloids.27 It inhibits glioma-associated oncogene homolog 1 expression in wounded skin.13 In a pilot study, topically applied tacrolimus 0.1% ointment twice daily improved keloids symptoms, although no statistical significance was achieved.28
Innovation
Recent studies indicate that keloids treated with TGF-β1 show up-regulation of transcription factor SMAD and reduction of SMAD interacting protein 1 (SIP1). The SIP1 level inversely correlates with type I collagen and directly correlates with matrix metalloproteinase 1 (MMP1) level. Over expression of SIP1 in keloids represses collagen and induces MMP1 expression. These findings suggest that SIP1 may be a regulator of skin fibrosis.29
Integrins (proteins controlling cellular matrix communication) were found highly expressed in KF. However, after incubation with TGF-β1-antisense, these proteins were reduced, concluding that integrin expression is directly modulated by TGF-β1.30
Antineoplastic agents such as IFN-α2b, IFN-γ, mitomycin-C, bleomycin, and 5-fluorouracil appear to markedly reduce pathologic scars and improve rates of recurrence and patient satisfaction. There is mounting evidence that these drugs used alone or in combination can be successful in treating hypertrophic scars and keloids.31
More recently, researchers have discovered that treatment with IFN-α2b may be associated with a decreased number of fibrocytes and angiogenesis.24
Fetal dermal wound healing is characterized by minimal inflammationand scarless repair and associated to decreased IL-6 and IL-8. IL-10 is an anti-inflammatory cytokine that decreases production of IL-6 and IL-8. Therefore, it is hypothesized that IL-10 seems to be a promising treatment to achieve scarless wound repair.32
Caution, Critical Remarks, and Recommendations
Prevention is the best way to avoid the development of cosmetically unacceptable scars. Adequate preoperatory planning is essential. Techniques such as hiding incisions in natural orifices, behind anatomical prominences, or in the hairline; placing incisions between two facial esthetic units, or in the relaxed skin tension lines (RSTLs) are very useful alternatives. Incisions following the plane of the RSTLs tend to heal better than perpendicular incisions to the RSTLs. Clean wounds tend to heal better than infected wounds or under immunocompromised states.16,18–20
The key in keloid therapy is to avoid performing elective or cosmetic surgeries in patients with a previous history of keloids. However, the overall risk is lower among patients of light skin color and on those who have solitary earlobe lesions. The best surgical outcomes are seen with excellent wound edge closure, combining minimal tension with maximal eversion, ensuring incisions are made along skin creases and/or RSTLs, and avoiding crossing of joint spaces. Mid-chest incisions should be avoided, as they are one of the body areas under more stretching forces. Surgical wounds should be dressed with minimal tension.
Future Development of Interest
Comprehension of the molecular mechanisms behind keloid disease has led to the development of new promising therapies such as recombinant TGF-β3, which has shown improvement in subsequent scar appearance in rat wounds.15 Imatinib mesylate is a tyrosine kinase inhibitor that blocks non-SMAD signal transduction downstream of TGF-β.34 Distler et al. showed that imatinib mesylate reduced collagen synthesis in systemic sclerosis and inhibited the induction of ECM proteins after stimulation with TGF-β. The results suggest that imatinib mesylate has potent antifibrotic effects and is a potential candidate for the treatment of fibrotic diseases such as keloids.35
The absence of IL-10 leads to enhancement of inflammatory cytokine cascade, stimulation of fibroblasts, and abnormal collagen deposition, thus resulting in scar formation. Human recombinant IL-10 may be a promising treatment for keloids.
Take-Home Message.
Basic science advances
TGF-β1 is a key mediator of keloid pathogenesis, exerting its biological effect by interaction with its receptors TGF-βRI and -βRII. TGF-β1 and -β2 are increased in KFs and have pro-fibrotic activities that promote collagen synthesis and scar formation. Conversely, isoform 3 is decreased in KFs and is involved in scar prevention. Keloids show increased expression of receptor I and significantly decreased expression of receptor II. Blockage of TGF-β1 results in significant reduction in TGF-β1 and -β2 and increase in TGF-βRII, which is consistent with an antifibroproliferative effect and correlates with prevention of keloid formation.
Keloids also show depletion of IFN-γ, which up regulates the pro-apoptotic gen p53 in epidermal cells, thus reducing collagen type I mRNA and leading toward termination of the repair process; therefore, low levels of IFN-γ lead to abnormal scarring. IFN-α2b has antifibrotic effects by normalizing collagen and glycosaminoglicans synthesis, and collagenase activity.9
Imiquimod activates immune cells through TLRs to secrete proinflammatory cytokines (IFN-α, IL-6, and TNF-α), thus resulting in antifibrotic and pro-apoptotic effects.33
Clinical science advances
Treatment of keloids has transitioned from invasive methods including gross excision and radiation to intralesional and topical therapies that act at a cellular level. Keloids are challenging to treat and especially to prevent from recurring. Some biological agents potentially prevent formation and recurrence of keloid formation, such as IFN-α, imiquimod, and TGF-β. Other treatment modalities have been reported to obtain mixed results including tacrolimus, IL-10, mitomycin-C, bleomycin, 5-fluorouracil, and etanercept among other agents. These drugs, used alone or in combination therapy, have the potential to play an important role on the treatment of hypertrophic scars and keloids.
Relevance to clinical care
Targeting therapy at aberrant collagen proliferation through the understanding of the cytokines' function, the discovery of the therapeutic potential of imiquimod, other biological agents, and TGF-β-oriented antisense therapy could lead to development of clinically useful therapeutic modalities for treatment of keloid disease.
Understanding the TGF-β pathway, how to decrease both profibrotic isoforms, and increase the antifibrotic isoform could be crucial in accomplishing control of the fibrotic process underlying keloids. Moreover, reducing keloid activity and its concomitant symptoms will significantly reduce the concurrent psychological effects that usually accompany this condition and increase quality of life. Nevertheless, new strategies need to be identified and developed to successfully treat and prevent the formation of keloids as well as decrease the recurrence rate.12
Intradermal tacrolimus has demonstrated to be a potential, efficient, and well-tolerated treatment to prevent scar hypertrophy in a preclinical model. Further investigations are required to propose this new treatment for the management of keloids.36
Abbreviations and Acronyms
- cDNA
complementary DNA
- ECM
extracellular matrix
- IFN
interferon
- IL
interleukin
- KFs
keloid fibroblasts
- MMP1
matrix metalloproteinase 1
- mRNA
messenger RNA
- RSTLs
relaxed skin tension lines
- SIP1
SMAD interacting protein 1
- TGF-β
transforming growth factor beta
- TGF-βRI-βRII
transnmembrane receptor type I and 2
- TLR7 and TLR8
toll-like receptors 7 and 8
- TNF-α
tumor necrosis factor alpha
Acknowledgments and Funding Sources
The authors have not received funding for this work.
Author Disclosure and Ghostwriting
B.B. is a consultant for Graceway Pharmaceuticals, PharaDerm, and LEO. M.H.V. and A.C.V. have no conflicts of interest to disclose. The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article.
References
- 1.Bayat A. McGrouther DA. Ferguson MWJ. Skin scarring. BMJ. 2003;326:88. doi: 10.1136/bmj.326.7380.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alster TS. Tanzi EL. Hypertrophic scars and keloids: etiology and management. Am J Clin Dermatol. 2003;4:235. doi: 10.2165/00128071-200304040-00003. [DOI] [PubMed] [Google Scholar]
- 3.Viera MH. Amini S. Valins W. Berman B. Innovative therapies in treatment of keloids and hypertrophic scars. J Clin Aesthetic Dermatol. 2010;3:20. [PMC free article] [PubMed] [Google Scholar]
- 4.Bock O. Yu H. Zitron S. Bayat A. Ferguson MW. Mrowietz U. Studies of transforming growth factors beta 1–3 and their receptors I and II in fibroblast of keloids and hypertrophic scars. Acta Derm Venereol. 2005;85:216. doi: 10.1080/00015550410025453. [DOI] [PubMed] [Google Scholar]
- 5.Al-Attar A. Mess S. Thomassen JM. Kauffman CL. Davison SP. Keloid pathogenesis and treatment. Plast Reconstr Surg. 2006;117:286. doi: 10.1097/01.prs.0000195073.73580.46. [DOI] [PubMed] [Google Scholar]
- 6.Assoian RK. Komoriya A. Meyers CA. Miller DM. Sporn MB. Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J Biol Chem. 1983;258:7155. [PubMed] [Google Scholar]
- 7.Viera MH. Amini S. Konda S. Berman B. Do postsurgical interventions optimize ultimate scar cosmesis. G Ital Dermatol Venereol. 2009;144:243. [PubMed] [Google Scholar]
- 8.Berman B. Biological agents for controlling excessive scarring. Am J Cin Dermatol. 2010;11(Supp 1):31. doi: 10.2165/1153419-S0-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 9.Berman B. Harrison-Balestra C. Perez OA. Viera M. Villa A. Zell D. Ramirez C. Treatment of keloid scars post-shave excision with imiquimod 5% cream: a prospective, double-blind, placebo-controlled pilot study. J Drugs Dermatol. 2009;8:455. [PubMed] [Google Scholar]
- 10.Jacob SE. Berman B. Nassiri M. Vincek V. Topical application of imiquimod 5% cream to keloids alters expression genes associated with apoptosis. Br J Dermatol. 2003;149(Suppl 66):62. doi: 10.1046/j.0366-077x.2003.05636.x. [DOI] [PubMed] [Google Scholar]
- 11.Meyer T. Nindl I. Schmook T. Ulrich C. Sterry W. Stockfleth E. Induction of apoptosis by toll-like receptor-7 agonist in tissue cultures. Br J Dermatol. 2003;149(Suppl 66):9. doi: 10.1046/j.0366-077x.2003.05632.x. [DOI] [PubMed] [Google Scholar]
- 12.Bran GM. Goessler UR. Schardt C. Hormann K. Riedel F. Sadick H. Effect of the abrogation of TGF-β1 by antisense oligunucleotides on the expression of TGF-β-isoforms and their recectors I and II in isolated fibriblasts from keloid scars. Int J Mol Med. 2010;25:915. doi: 10.3892/ijmm_00000422. [DOI] [PubMed] [Google Scholar]
- 13.Younai S. Nichter LS. Wellisz T. Reinisch J. Nimni ME. Tuan TL. Modulation of collagen synthesis by transforming growth factor beta in keloid and hyperttophic scar fibroblasts. Ann Plast Surg. 1994;33:148. doi: 10.1097/00000637-199408000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Bettinger DA. Yager DR. Diegelmann RF. Cohen IK. The effect of TGF-beta on keloid fibroblast proliferation and collagen synthesis. Plast Reconstr Surg. 1996;98:827. doi: 10.1097/00006534-199610000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Shah M. Foreman DM. Ferguson MW. Neutralisation of TGF-beta1 and TGF-beta2 or exogenous addition of TGF-beta3 to cutaneous wounds reduce scarring. J Cell Sci. 1995;108(Pt 3):985. doi: 10.1242/jcs.108.3.985. [DOI] [PubMed] [Google Scholar]
- 16.Berman B. Kaufman J. Pilot study of the effect of postoperative imiquimod 5% cream on the recurrence rate of excised keloids. J Am Acad Dermatol. 2002;47(Suppl 4):S209. doi: 10.1067/mjd.2002.126585. [DOI] [PubMed] [Google Scholar]
- 17.Stashower ME. Successful treatment of earlobe keloids with imiquimod after tangential shave excision. Dermatol Surg. 2006;32:380. doi: 10.1111/j.1524-4725.2006.32077.x. [DOI] [PubMed] [Google Scholar]
- 18.Martin-Garcia RF. Busquets AC. Postsurgical use of imiquimod 5% cream in the prevention of earlobe keloid recurrences: results of an open-label, pilot study. Dermatol Surg. 2005;31:1394. doi: 10.2310/6350.2005.31203. [DOI] [PubMed] [Google Scholar]
- 19.Patel PJ. Skinner RB., Jr. Experience with keloids after excision and application of 5% imiquimod cream. Dermatol Surg. 2006;32:462. doi: 10.1111/j.1524-4725.2006.32094.x. [DOI] [PubMed] [Google Scholar]
- 20.Cacao FM. Tanaka V. Messina MC. Failure of imiquimod 5% cream to prevent recurrence of surgically excised trunk keloids. Dermatol Surg. 2009;35:629. doi: 10.1111/j.1524-4725.2009.01101.x. [DOI] [PubMed] [Google Scholar]
- 21.Prado A. Andrades A. Benitez S. Umana M. Scar management after breast surgery: preliminary results of a prospective, randomized and double-blind clinical study with aldara cream 5% (imiquimod) Plast Reconstr Surg. 2005;115:966. doi: 10.1097/01.prs.0000153823.52784.7e. [DOI] [PubMed] [Google Scholar]
- 22.Mrowietz U. Seifert O. Keloid scarring:new treatments ahead. Actas Dermosifiliogr. 2009;100(Supp 2):75. doi: 10.1016/s0001-7310(09)73382-4. [DOI] [PubMed] [Google Scholar]
- 23.Berman B. Patel JK. Perez OA. Viera MH. Amini S. Block S. Zell D. Tadicherla S. Villa A. Ramirez C. De Araujo T. Evaluating the tolerability and efficacy of etanercept compared to triamcinoloneacetonide for the intralesional treatment of keloids. J Drugs Dermatol. 2008;6:28. [PubMed] [Google Scholar]
- 24.Wang J. Chen H. Shankowsky HA. Scott PG. Tredget EE. Improved scar in postburn patients following interferon-alpha2b treatment is associated with decreased angiogenesis mediated by vascular endothelial cell growth factor. J Interferon Cytokine Res. 2008;28:423. doi: 10.1089/jir.2007.0104. [DOI] [PubMed] [Google Scholar]
- 25.Tredget EE. Shen YJ. Liu G. Forsyth N. Smith C. Robertson Harrop A. Scott PG. Ghahary A. Regulation of collagen synthesis and messenger RNA levels in normal and hypertrophic scar fibroblasts in vitro by interferon alfa-2b. Wound Repair Regen. 1993;1:156. doi: 10.1046/j.1524-475X.1993.10305.x. [DOI] [PubMed] [Google Scholar]
- 26.Renovo phase II results. Press release: renovo announces positive phase II results for prevascar. Renovo, United Kingdom: FierceBiotech; Renovo announces positive phase II results for prevascar. [Google Scholar]
- 27.Gisquet H. Liu H. Blondel WC. Leroux A. Latarche C. Merlin JL. Chassagne JF. Peiffert D. Guillemin F. Intradermaltacrolimus prevent scar hypertrophy in a rabbit ear model: a clinical, histological and spectroscopical analysis. Skin Res Technol. 2011;17:160. doi: 10.1111/j.1600-0846.2010.00479.x. [DOI] [PubMed] [Google Scholar]
- 28.Berman B. Poochareon V. Villa AM. An open-label pilot study to evaluate the safety and tolerability of tacrolimus ointment 0.1% for the treatment of keloids. Cosm Dermatol. 2005;18:399. [Google Scholar]
- 29.Zhang ZF. Zhang YG. Hu DH. Shi JH. Liu JQ. Zhao ZT. Wang HT. Bai XZ. Cai WX. Zhu HY. Tang CW. Smad interacting protein 1 as a regulator of skin fibrosis in pathological scars. Burns. 2011;37:665. doi: 10.1016/j.burns.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 30.Bran G. Sommer U. Meinzer F. Goessler UR. Hörmann K. Riedel F. Sadick H. Impact of TGF-beta1 antisense on collagen-binding integrins in keloid. HNO. 2010;58:605–610. doi: 10.1007/s00106-010-2124-8. [DOI] [PubMed] [Google Scholar]
- 31.Shridharani SM. Magarakis M. Manson PN. Singh NK. Basdag B. Rosson GD. The emerging role of antineoplastic agents in the treatment of keloids and hypertrophic scars: a review. Ann Plast Surg. 2010;64:355. doi: 10.1097/SAP.0b013e3181afaab0. [DOI] [PubMed] [Google Scholar]
- 32.Liechty KW. Kim HB. Adzick NS. Crombleholme TM. Fetal wound repair results in scar formation in interleukin-10-deficient mice in a syngeneicmurine model of scarless fetal wound repair. J Pediatr Surg. 2000;35:866. doi: 10.1053/jpsu.2000.6868. [DOI] [PubMed] [Google Scholar]
- 33.Hemmi H. Kaisho T. Takeuchi O. Sato S. Sanjo H. Hoshino K. Horiuchi T. Tomizawa H. Takeda K. Akira S. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- 34.Krause DS. Van Etten RA. Tyrosine kinases as targets for cancer therapy. N Engl J Med. 2005;353:172. doi: 10.1056/NEJMra044389. [DOI] [PubMed] [Google Scholar]
- 35.Distler JH. Jungel A. Huber LC. Schulze-Horsel U. Zwerina J. Gay RE, et al. Imatinibmesylate reduces production of extracellular matrix and prevents development of experimental dermal fibrosis. Arthritis Rheum. 2007;56:311. doi: 10.1002/art.22314. [DOI] [PubMed] [Google Scholar]
- 36.Gisquet H. Liu H. Blondel WC. Leroux A. Latarche C. Merlin JL. Chassagne JF. Peiffert D. Guillemin F. Intradermaltacrolimus prevent scar hypertrophy in a rabbit ear model: a clinical, histological, spectroscopical analysis. 2011. p. 160. [DOI] [PubMed]