Abstract
Problem
Lower extremity ulcers (venous, diabetic) are often unresponsive to standard treatment. Various systemic and local cellular, vascular, and anatomical factors can result in nonhealing wounds that are refractory to normal healing processes and standard care.
Solution
Several published wound care guidelines strongly suggest that if an ulcer does not respond to standard good wound care within 4 weeks, then advanced wound therapies should be considered. These advanced therapies include wound bed preparation agents (negative wound pressure therapy, hyperbaric oxygen), recombinant growth factors, or bioengineered cell therapies.
New Technology
The Cascade® system produces platelet-rich fibrin matrix (PRFM), a novel autologous sterile biologic, produced at the bedside from a small volume (18 mL) of the patient's own blood by using Vacutainer® separation technology optimized for fibrin and platelet isolation. Prepared as an easy to apply, suturable membrane, without the use of exogenous thrombin, PRFM consists of a dense cross-linked fibrin lattice containing intact, viable platelets with their full complement of platelet-derived growth factors.
Indications for Use
From the FDA 510(k) clearance: The Cascade system “is designed to be used for the safe and rapid preparation of autologous platelet-rich plasma from a small sample of blood at the patient point of care.” PRFM has been used to successfully treat severe venous leg ulcer (VLU), neuropathic diabetic foot ulcer (DFU), mixed arterial and Charcot-deformity associated foot ulcers.
Cautions
When treating venous or DFUs, the Cascade system should be used together with standard wound care practice (therapeutic compression for VLU and weight off-loading, debridement, and infection control for DFU) in patients with an adequate blood supply to the lower limb.

Sean M. O'Connell
Unmet Need
Normal healing follows a series of complex but orderly physiologic and molecular processes, which include coagulation, inflammation, cell recruitment, migration, proliferation, and connective tissue production followed by tissue remodeling and maturation.1 In contrast, chronic wounds are characterized as having stalled somewhere in this progression to healing due to a variety of systemic and local factors. Detrimental factors include poor perfusion and low oxygen tension, excess microbial burden, necrotic tissue, chronic venous insufficiency, diabetes mellitus, wound bed cells that are unresponsive to normal cell signaling,2 and decreased growth factor (GF) production. Even with optimal wound bed preparation to minimize these factors, significant healing is still not achieved in 30% or more of ulcers seen. Published guidlines recommend considering advanced therapies after 4 weeks of non-response to standard care.3
Product Technology
Conventional platelet-rich plasma (PRP) is plasma with a platelet concentration above the “normal” physiologic levels found in blood.4 Increased concentration of platelets also yields an increase in the concentration of GFs stored in the α-granules of platelets.4 Currently, several methods are available for PRP preparation, most producing a liquid end product. PRP is clinically used in liquid or gel form to promote tissue repair. Due to poor mechanical properties, conventional PRP is often difficult to handle in clinical settings that require secure implantation in a specific site or where released GFs could be washed out during an operation. The physical properties of PRP can be changed if plasma and platelets are stimulated, usually by the addition of CaCl2 and thrombin, to produce a fibrin network. However, this method leads to a clot with high concentrations of red and white blood cells and is associated with an almost total platelet activation, degranulation, and GF release.5,6
Innovation
The Cascade® system produces a platelet-rich fibrin matrix (PRFM), prepared as an easy to apply membrane without the use of exogenous thrombin (Fig. 1A).7,8 Instead of producing a platelet concentrate and platelet-poor plasma, as in conventional PRP systems, a thixotropic separator gel is employed in a low-speed (1,100 g) radial (swing-bucket) centrifugation to rapidly isolate both the platelets and fibrinogen-containing plasma from the packed red and white cell fraction. In vitro studies demonstrated that platelets isolated from blood using the Cascade system are essentially intact and unactivated (cluster designation 62p, a membrane-associated glycoprotein expressed by activated platelets expression <5%). In contrast, 5 min exposure to 100 U/mL bovine thrombin resulted in >95% activation and >65% degranulation exposed platelets.5 After the first separation centrifugation, the platelet-containing plasma is sterilely transferred to a second container, preloaded with CaCl2, and centrifuged for a second time at high speed (>3,500 g) for 25 min to produce the PRFM membrane.
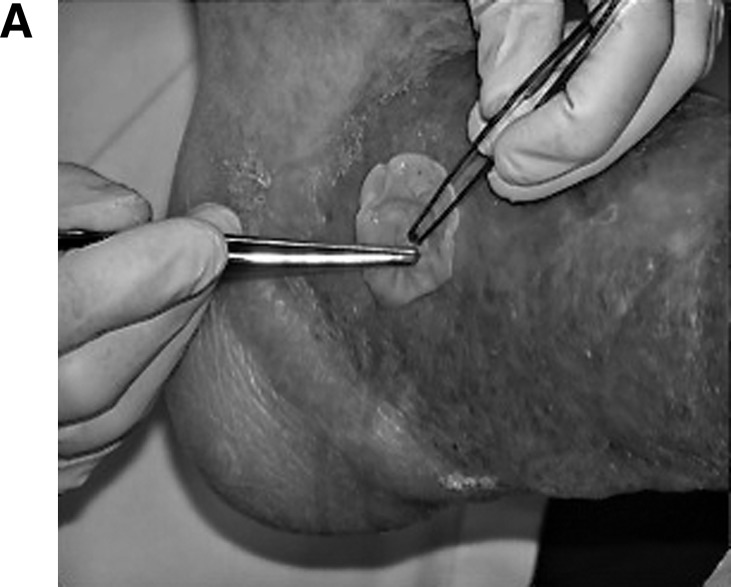
Figure 1A.
Cascade® system PRFM membrane produced by separation and concentration of fibrinogen and platelets from whole blood. Application of PRFM membrane to a prepared lower-extremity (venous) ulcer.
Peer-Reviewed Data
In 2008, the authors' group reported the results of a prospective pilot trial (n = 21).7 Twelve patients were enrolled with 17 venous leg ulcers (VLUs) and nine bearing 13 nonvenous lower-extremity ulcers. Enrolled patients had failed to respond to a variety of standard treatments (median 1 year) with an initial mean ulcer size of 11.2 cm2. Each patient who had been treated for PRFM received up to three applications of the fenestrated membrane. The primary endpoints were percent and rate of complete closure. Patients were followed weekly for 12 weeks with a follow-up visit at 16 weeks. Patients with <50% closure received repeated applications (up to 3). Complete closure was achieved in 66.7% of the patients with VLU in 7.1 weeks (median, 6 weeks) with an average of two applications per patient (Fig. 2). Forty-four percent complete closure was seen in patients without VLU (31% of treated ulcers).
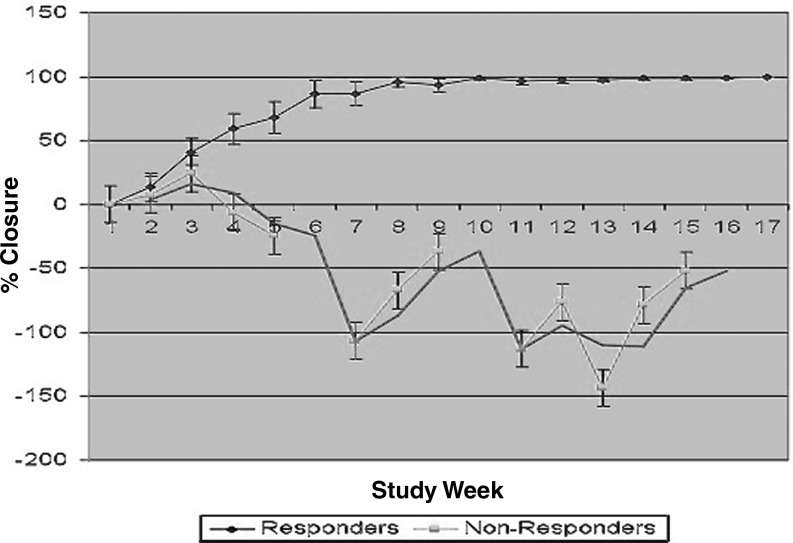
Figure 2.
Cumulative closure for venous ulcers treated with PRFM membrane.7 Graph depicts wounds responding to PRFM treatment (n = 13; >75% closure by week 16) and nonresponding (n = 4; <75% closure by week 16). Percent closure was calculated from ulcer area reduction normalized to initial baseline area. Values are given as mean percent closure ± standard error of the mean for each visit.
It is interesting to note that in the VLU-treated group, responders (those whose wounds achieved 75% closure or more during the study) diverged rapidly (by 3 weeks from the start or 1 week after the second application) from nonresponders (patients whose wounds did not reach 75% closure by week 16).
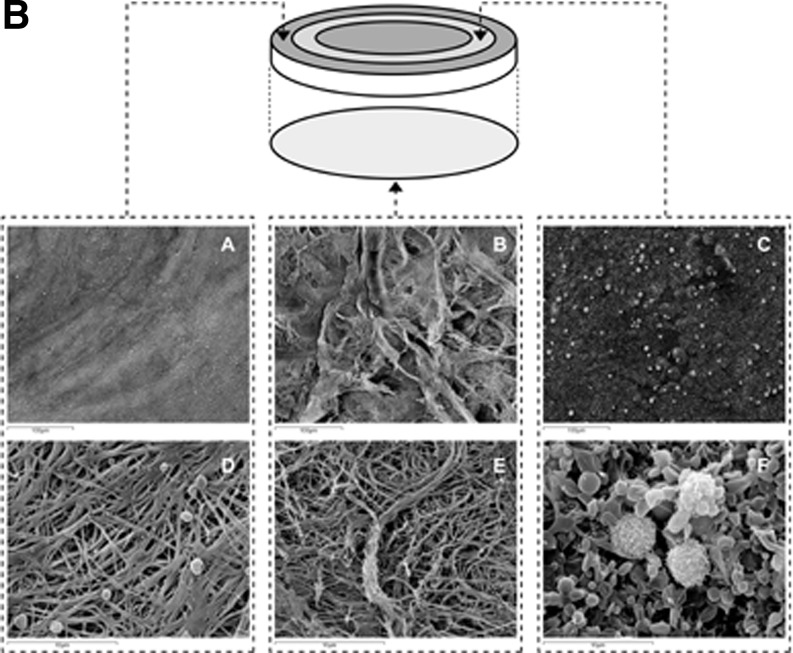
Lucarelli et al. studied the ultra structural and physical characteristics of the PRFM membrane8 describing it “. as a translucent yellow-white disk of 0.105 ± 0.021 mm thickness and 33 mm in diameter,” that is “easy to handle and does not tear when manipulated with forceps” (Fig. 1A). PRFM membrane showed significant tensile properties with elasticity, peak breaking strength and elongation to break equivalent to intact aorta and far greater than conventional thrombin-generated PRP clots. Confocal scanning fluorescence microscopy and SEM revealed a dense highly cross-linked fibrin matrix with high concentrations (>100×) of intact platelets localized on one side of the membrane (Fig. 1B). Using a “washed–out” protocol, where the media is replaced after each time point, the authors demonstrated that the conditioned media from in vitro PRFM cultures (37°C) produced high levels of platelet-derived growth factors (PDGFs) with maximal release in the first 24 hrs (PDGF 28 ng/mL, vascular endothelial growth factor 240pg/mL, and transforming growth factor beta 10 ng/mL) and decreasing levels out to day 7. Further, the authors showed that media supplemented with conditioned media from the PRFM cultures (20% v/v) were able to support proliferation of human mesenchymal stem cells to a level significantly greater than that achieved with 20% fetal bovine serum supplementation.
Figure 1B.
The reverse side of the membrane in which no platelets or cells are visible, but a coarse surface of fibrin strands and bundles is visible. (C) Representative picture of the platelet/cell-rich area. (D) The same field as in A at higher magnification shows platelets on the surface of the fibrin network. (E) A higher magnification of the same field as in B of the reverse side of the membrane in which no platelets or cells are visible, but a coarse surface of fibrin strands and bundles is visible. (F) A higher magnification of the same field as in C shows a thick layer of unactivated platelets in which a few nucleated cells are visible. PRFM, platelet-rich fibrin matrix.
Non-Peer-Review Observation
Gosch at al. evaluated PRFM membrane for the treatment of neuropathic, diabetic foot ulcers (DFUs) refractory to standard care.9 In this small-scale, 12-week pilot study, 100% of patients treated with PRFM reached 75% closure, with 75% achieving complete closure in 9 weeks. In a similar case-study series, Kimmel and co-workers showed that PRFM membrane has potential utility in closing DFUs associated with concomitant Charcot deformity that had failed a variety of treatments.10
The results of these pilot studies indicate that PRFM shows significant potential for closing of chronic leg ulcers.
Caution, Critical Remarks, and Recommendations
The clinical studies cited in this article are small-scale pilot trials involving relatively few patients. The results of the studies should be confirmed by using larger randomized controlled trials. The Cascade system is cleared by the U.S. Food and Drug administration as a class II device and designed to be used for the safe and rapid preparation of autologous PRP from a small sample of blood at the patient point of care.
Abbreviations and Acronyms
- α-Granules
organelles within the platelet that contain growth factors and other proteins which are released on platelet activation
- CaCl2
calcium chloride
- DFU
diabetic foot ulcer
- FDA
United States Food and Drug Administration
- g
force of gravity, a measure of centrifugal force
- GF
growth factor
- n
number of patients or ulcers examined in a clinical study
- ng
nanogram (10−9 grams)
- nonresponders
ulcers achieving <75% reduction in ulcer are by week 16 of the study
- PDGF
platelet-derived growth factor
- pg
picogram (10−12 grams)
- PRFM
platelet-rich fibrin matrix
- PRP
platelet-rich plasma
- responders
ulcers which have reach 75% or greater reduction in area (including complete closure) by week 16 of the study
- SEM
scanning electron microscopy
- U
international thrombin unit
- v/v
volume to volume, the ratio of the one liquid to another in a mixture
- VLU
venous leg ulcer
- washed-out
method for assessing growth factor release over time in vitro culture. Conditioned media are removed from culture, rinsed, and replaced with fresh media (diluent) at each time point.
Acknowledgement and Funding Source
The author would like to acknowledge Toni Ricciardi for chapter review and technical assistance.
Author Disclosure and Ghostwriting
Dr. O'Connell and Dr. Dardik are consultants and shareholders in Cascade Medical Enterprises. No ghostwriters were used to write this article.
References
- 1.Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:736. doi: 10.1016/S0140-6736(05)67700-8. [DOI] [PubMed] [Google Scholar]
- 2.Woo K. Ayello EA. Sibbald RG. The edge effect: current therapeutic options to advance the wound edge. Adv Skin Wound Care. 2007;20:99–117. doi: 10.1097/00129334-200702000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Margolis DJ. Allen-Taylor L. Hoffstad O. Berlin JA. The accuracy of venous leg ulcer prognostic models in a wound care system. Wound Repair Regen. 2004;12:163. doi: 10.1111/j.1067-1927.2004.012207.x. [DOI] [PubMed] [Google Scholar]
- 4.Marx RE. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10:225. doi: 10.1097/00008505-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 5.O'Connell S. Carroll R. Beavis A, et al. Flow Cytometric Characterization of Cascade Platelet-Rich Fibrin Matrix (PRFM); the Impact of Exogenous Thrombin on Platelet Concentrates (PC) Edison, NJ: Musculoskeletal Transplant Foundation; 2008. [Google Scholar]
- 6.Castillo TN. Pouliot MA. Kim HJ. Dragoo JL. Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. Am J Sports Med. 2011;39:266. doi: 10.1177/0363546510387517. [DOI] [PubMed] [Google Scholar]
- 7.O'Connell S. Impeduglia T. Hessler K. Wang X-J. Carroll RJ. Dardik H. Autologous platelet-rich fibrin matrix as cell therapy in the healing of chronic lower extremity ulcers. Wound Repair Regen. 2008;16:749. doi: 10.1111/j.1524-475X.2008.00426.x. [DOI] [PubMed] [Google Scholar]
- 8.Lucarelli E. Beretta R. Dozza B. Tazzari PL. O'Connell SM. Ricci F. Pierini M. Squarzoni S. Pagliaro PP. Oprita EI. Donati D. A recently developed bifacial platelet-rich fibrin matrix. Eur J Cell Mater. 2010;20:13. doi: 10.22203/ecm.v020a02. [DOI] [PubMed] [Google Scholar]
- 9.Gosch C. Zeichner A. Carroll R. Bois J. Evaluation of an autologous platelet rich fibrin matrix technology for diabetic foot ulcer treatment. Wound Rep Regen. 2007;15:A38.90. Abstract. [Google Scholar]
- 10.Kimmel HM. Johnson M. Valentine C. The use of Cascade platelet rich plasma membrane in healing chronic diabetic ulcers. J Wound Technol. 2010;10:10. [Google Scholar]