Abstract
Background
Although significant resources are invested in wound care and healing annually, chronic wounds remain a major medical problem as they often present a more difficult challenge than the underlying disease. Current treatment options include a multitude of dressing materials, topical agents including antibiotics, enzymatic debriders, and growth factors, mechanical debridement, and optimization of medical comorbidities.
The Problem
Even under optimal circumstances, the healing process leads to some form of fibrosis and scarring.
Basic/Clinical Science Advances
Studies suggest that mesenchymal stem/stromal cells (MSCs) isolated from these diverse tissues possess similar biological characteristics, differentiation potential, and immunological properties. Enthusiasm about MSCs for use in reconstruction and regenerative medicine has been fueled by evidence that these cells possess the ability to participate in the tissue repair process through a variety of paracrine mechanisms affecting tissue regeneration and inflammation.
Clinical Care Relevance
Recent advances in stem cell immunobiology have led to increased interest in MSCs as a new therapeutic modality to address chronic wounds and other inflammatory pathology.
Conclusion
A thorough understanding of the immunobiology of MSCs is necessary to realize the complement of pathological processes that could be affected by MSC-based therapy. The novel methods reviewed here are highly promising, with the collective goal of identifying new therapeutic approaches to wound healing that are broadly applicable to many chronic diseases, and can safely accelerate the transition of basic research findings into clinical advances in many areas of regenerative medicine and reconstructive surgery.

Summer E. Hanson
Background
New treatment strategies in wound healing and reconstruction, such as bioengineered scaffolds and cellular applications, aim to replace senescent resident cells and reestablish their normal life cycle.1 Recent advances in bioengineering have led to hundreds of new products with Food and Drug Administration approval, including a few that contain living cells. Mesenchymal stem/stromal cells (MSCs) are fibroblast-like cells first isolated in the bone marrow (BM) and their primary function has been assumed to be providing the microenvironmental support for hematopoietic cells. These cells are characterized by combinations of cell surface markers and functional characteristics such as the potential to differentiate along multiple lineages including bone, fat, cartilage, neural cells, and endothelial cells, among others.2 It is now evident that MSCs reside within most adult connective tissues and organs.3 During the last decade, adult tissue-derived MSCs have rapidly moved from in vitro and animal studies into human trials as an investigational therapeutic modality for a diverse group of clinical applications.4
Target Articles.
1. Hanson SE, Bentz ML and Hematti P: Mesenchymal stem cell therapy for nonhealing cutaneous wounds. Plast Reconstr Surg 2010; 125: 510.
2. Hanson SE, Gutowski KA and Hematti P: Clinical applications of mesenchymal stem cells in soft tissue augmentation. Aesthet Surg J 2010; 30: 838.
Clinical Problem Addressed
Normal wound healing is a complex, coordinated sequence of events proceeding from hemostasis through inflammation, to organized tissue regeneration. Tissue injury initiates the healing process characterized by an infiltration of proinflammatory cytokine-producing cells such as neutrophils and macrophages, followed by extracellular matrix (ECM) depositing fibroblasts, leading to new tissue formation. However, even under the best circumstances, this process typically results in fibrosis or scarring, and given the complex nature of wound healing, there are a number of steps along the way that can influence clinical decision making and outcome.
Millions of reconstructive procedures are performed each year to address a variety of defects. Within this armamentarium, there are techniques ranging from primary closure and secondary intention or granulation, to complex, composite tissue transplantation moving skin, muscle, soft tissue, and bone from one part of the body to another based on microsurgical anastomosis and revascularization of the blood supply of the transferred tissues. Adjunctive treatment modalities in clinical practice include debridement, pressure offloading, dressing regimens, hyperbaric oxygen, antibiotics, and topical growth factors. However, even with most current therapies, greater than 50% of chronic wounds remain refractory to treatment.5 Chronic wounds remain a major challenge in modern medicine and represent a significant burden, affecting not only physical and mental health but also productivity, long-term morbidity, and healthcare expenditure.
Relevant Basic Science Context
MSCs have been shown to possess a variety of functional characteristics, which make them a desirable cell type for reconstructive applications. Of particular interest in wound healing is their ability to migrate to the site of injury or inflammation, participate in regeneration of damaged tissues, stimulate proliferation and differentiation of resident progenitor and stem cells, promote recovery of injured cells through growth factor secretion and matrix remodeling, and exert unique immunomodulatory and anti-inflammatory effects.6–8 In fact, one of the most intriguing properties of ex vivo expanded MSCs is their ability to affect the immune response through interaction with a broad range of immune and inflammatory cells, including T-lymphocytes, B-lymphocytes, natural killer cells, dendritic cells, monocytes, and macrophages.9 Hypoxia, inflammation, repetitive ischemia-reperfusion injury, and aging have all been suggested as factors contributing to impaired healing and are all potential pathways for MSCs to take effect.10 Thus, in contrast to most pharmacological agents targeting single pathophysiological pathways, MSCs could affect tissue healing and regeneration through many different routes.
Experimental Model or Material: Advantages and Limitations
Although there is increasing interest in using MSCs as a therapeutic option for a variety of clinical applications, there are few well-characterized preclinical models, particularly of impaired wound healing. Among the common animal models, the pig has an integumentary architecture similar to humans except for its lack of sweat glands. Our group is investigating a mini-pig model for use of local delivery of MSCs. Porcine skin, in terms of dermal thickness, papillary structure, collagen composition, and distribution of dermal blood vessels, is the most comparable to humans among animal models currently used to study wound healing, including rodents, rabbits, and mini and large pigs.11 Clinically, BM-MSCs have been shown to be safe and potentially efficacious in several phase I, II, and III clinical trials for a variety of diseases including myocardial infarction, chronic obstructive pulmonary disease, Crohn's disease, diabetes mellitus type 1, and stroke.12 This wealth of clinical data on the safety of BM-derived MSCs warrants considering the use of these cells, as well as adipose-derived MSCs, in a wider range of applications, including wound repair.
Discussion of Findings and Relevant Literature
The few clinical series reported in the literature that have focused on the application of MSCs for the treatment of human wounds have shown potentially promising results. When evaluating the literature, particular attention should be paid to nomenclature and methodology. Published reports attributed to the use of MSCs in the clinical setting range from unmanipulated BM or liposuction aspirate, as these tissues harbor MSCs in their natural form, to ex vivo culture expanded MSCs applied alone or with a skin graft or other dressing material. All of these factors should be considered when comparing results of different clinical trials. An early proof-of-principle report of this nature in cutaneous wounds included the direct application of autologous BM aspirate to wounds present for more than a year that were recalcitrant to standard therapeutics.13 Remaining aspirate was cultured and cells were subsequently administered. Ultimately, the authors report healing of all of the wounds (n = 3) within three consecutive treatments. However, it is not clear whether the cells cultured by these investigators were MSCs or other types of cells present in the BM, as there was no information on the identity of the cells cultured.
A variety of chronic nonhealing wounds (i.e., burns, lower extremity ulcers, and decubiti) were treated with autologous BM-MSCs expanded in culture, in conjunction with a dermal replacement (Pelnac), and autologous skin graft by Yoshikawa and colleagues.14 The authors report that 18 of the 20 wounds appeared to be completely healed with the cell–composite graft transfer and the addition of MSCs facilitated regeneration of the native tissue by histologic examination. However, these authors only used a low concentration of cells that were available at the end of passage 0 and did not report on the characterization of cultured cells. This is especially important, because passage 0, when the culture flasks are confluent after initial plating, potentially contains many other types of cells, including macrophages, which would affect wound healing as well.
The largest series thus far using BM-derived MSCs included 24 patients with nonhealing lower extremity ulcers (18 with Buerger disease and 6 were diabetic foot ulcers) randomized to receive standard wound dressings with or without autologous BM-MSCs.15 For the MSC-treated groups, cells were expanded in culture for several days and injected both into the ulcer edges directly as well as throughout the lower extremity intramuscularly. The authors found a significant difference in overall ulcer size, total reduction in ulcer size, and pain-free walking distance between the two groups, with more favorable outcomes seen in the MSC-treated patients. Further, there was no difference in biochemical parameters studied including liver function tests, fasting blood glucose, or renal function in patients locally treated with MSCs compared with control subjects.
Although the majority of wounds clinically treated with cell-based therapies have been chronic in nature, there are reports of severe radiation burn injury successfully treated with a combination of serial debridement, split thickness skin graft, and cell injection.16 The cells were cultured from autologous BM aspirate and directly injected into the wound after a two-step expansion process. The cells administered were positive for surface markers characteristic of MSCs, and pluritpotency was confirmed with differentiation assays. Complete healing was observed within 6 months with no functional impairments noted. In a separate study, purified lipoaspirate (a tissue rich in MSCs) was centrifuged, the oil/liquid layer was discarded, and the remaining cell-augmented adipose tissue was injected into wounded tissue of 20 patients resulting from radiation therapy to the chest wall or supraclavicular region.17 Patients received from one to six injections, based on the severity of their wound. Outcomes measured included clinical healing, symptom improvement, and recurrence. In only one case there was no sign of improvement. An additional case of severe radiation burn causing a desquamating wound along the entire posterior surface of the arm, from shoulder to elbow, with limited range of motion was salvaged with reconstructive surgery and culture-expanded autologous BM-MSCs in a series of applications.18 After a regimen of serial debridement, complex reconstructions including latissimus dorsi and radial forearm rotational flaps and several local injections of autologous BM MSCs were carried out (five injections total, each >100 × 106 cells). The authors report limitation in motion at the shoulder and elbow, but good soft tissue coverage and resolution of pain, in an injury that would more commonly have led to amputation at the shoulder.
Taken together, this emerging literature shows that the addition of MSCs to nonhealing wounds is associated with dermal rebuilding (in addition to remodeling), an increase in wound vascularity, and reduced fibrosis or scarring. Tissue hypoxia, inflammation, repetitive ischemia-reperfusion injury, and aging or cellular senescence are factors leading to dysfunctional wound healing5 and are all potential pathways for MSCs to exert effect. In particular, MSCs have been shown in vitro to secrete increased amounts of vascular endothelial growth factor-a, insulin like growth factor, epidermal growth factor, keratinocyte growth factor, angiopoietin-1, stromal-derived growth factor-1, macrophage inflammatory proteins 1a and 1b, and erythropoietin compared with dermal fibroblasts in vitro under hypoxic conditions meant to mimic wounded tissue.19 The paracrine mechanism of action of MSCs is further supported by evidence that BM-MSC–conditioned media has a mitogenic effect on CD14+ monocytes, endothelial cells, and keratinocytes in vitro compared with dermal fibroblast-conditioned media.19 Although these reports demonstrate the heterogeneity of the type of wounds treated with MSCs, they also illustrate the variations in culture and application techniques that should be considered when critically evaluating the current body of evidence in support of MSC therapy.
Innovation
Too often, reconstructive surgeons are faced with the additional challenge of complex tissue composition and unique functional requirements often associated with large defects. Tissue engineering strategies have been more recently explored in select clinical scenarios ranging from using fibrin glue in conjunction with MSCs in the setting of both acute and chronic wounds to a composite tissue graft for airway replacement consisting of decellularized trachea seeded with MSCs. Tissue-engineered constructs offer a unique delivery system to maintain MSCs in the acute wound bed and, at the same time, allow for migration or differentiation as healing progresses. An equally important matter in the development of tissue-engineered constructs is their interaction with the immune system, especially considering the potential effect of MSCs on inflammatory processes. Our group has shown a potential role for MSC–hyaluronic acid constructs in the immunomodulation of macrophages, which are key players in the healing process (unpublished data). One can anticipate the impact of such novel multimodality therapy to mediate both the inflammatory resolution and tissue reparative phases of wound healing.
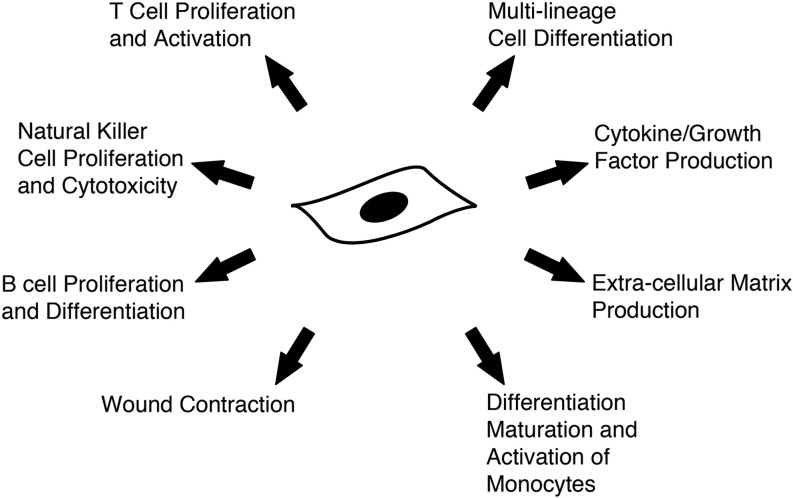
Summary Illustration
Functional mechanisms of mesenchymal stem cells involved in wound healing are shown in the above figure. There are several functional mechanisms identified with culture-expanded mesenchymal stem cells that are favorable when developing cell-based therapies for wound healing and regenerative medicine. These affect both the inflammatory and reparative pathways of the healing process, potentially offering a multimodal cell-based therapy.
Take-Home Message.
Basic science advances
• Studies suggest that MSCs isolated from diverse tissues possess similar biological characteristics, differentiation potential, and immunological properties.
• Enthusiasm about MSCs for use in reconstruction and regenerative medicine has been fueled by evidence that these cells possess the ability to participate in the tissue repair process through a variety of cytokine-mediated paracrine mechanisms affecting tissue regeneration and inflammation.
Clinical science advances
• The majority of literature regarding the therapeutic use of MSCs in wound healing is based on small, nonrandomized clinical trials or case reports.
• Tissue hypoxia, inflammation, repetitive ischemia-reperfusion injury, and aging or cellular senescence are factors leading to dysfunctional wound healing and are all potential pathways for MSCs to take effect.
• Although the outcomes were varied among the clinical applications, these reports are promising, offering novel solutions for challenging clinical disorders and ushering in a new era in regenerative medicine.
Relevance to clinical care
• Recent advances in stem cell immunobiology can offer insight to the multiple mechanisms through which MSCs could affect underlying pathophysiologic processes associated with nonhealing wounds.
• Critical evaluation of the current literature is necessary in understanding how MSCs could potentially revolutionize our approach to skin and soft tissue defects and designing clinical trials to address their role in wound repair and regeneration.
Caution, Critical Remarks, and Recommendations
There are many questions that have arisen in the development of novel MSC-based therapies in clinical applications. Not all reports are optimistic. In particular, it is important to consider MSC engraftment, migration, and tumorogenicity. One major confounding factor in these trials is the lack of a standardized procedure for isolation and characterization of the cells used. Some studies have used unmanipulated BM or fat aspirates, with the assumption that these tissues are rich in MSCs, whereas others have used highly enriched populations of MSCs through culture expansion. The exact fate of locally or systemically delivered MSCs has not been clearly established. Many preclinical and clinical studies show variations in engraftment of MSCs in a variety of tissues potentially affecting their anti-inflammatory or regenerative effects. Tissue injury, and sometimes pathology, is thought to elicit enhanced MSC migration through a variety of inflammatory markers.20 Although this is advantageous in a wound healing model, similar inflammatory markers could promote MSCs to a tumor bed. Moreover, it has been speculated that BM-derived MSCs are a source of tumor-associated stromal cells and, as such, can enhance tumor growth, at least in an animal model.20 Further understanding of not only how MSCs can affect the complex wound healing process, but also how these progenitor cells in turn are affected by the wounded or diseased tissues of interest are needed to clarify the clinical utility of MSCs in wound repair and reconstruction.
Future Development of Interest
There is no doubt future developments in the area of tissue engineering will lead to new treatments to address wound healing and soft tissue regeneration. Bioengineered scaffolds could provide a microenvironment that allows for nutrient diffusion as well as biochemical, physical, and cellular stimuli guiding proliferation, differentiation, and migration of implanted or tissue-resident cells. Although many of these scaffolds are clinically used without cells for soft tissue augmentation or repair, such as collagen or hyaluronic acid, there is increasing interest in combining these ECM-based scaffold materials with cells for in vivo tissue engineering strategies. Biomaterials, such as MSC–hydrogel constructs, hold great promise in tissue engineering, but before the initiation of clinical trials, further preclinical studies are needed to investigate their potential interactions with inflammatory mediators.
Abbreviations and Acronyms
- BM
bone marrow
- ECM
extracellular matrix
- MSCs
mesenchymal stem/stromal cells
Acknowledgments and Funding Sources
The author thanks Peiman Hematti, M.D., and Michael L. Bentz, M.D., for their support and review of this work. The author is also grateful to Christopher Hanson for his technical contribution to the illustration.
Author Disclosure and Ghostwriting
The author has no competing disclosures or conflicts of interest. There were no ghostwriters in the preparation of this article.
References
- 1.Panuncialman J. Falanga V. The science of wound bed preparation. Clin Plast Surg. 2007;34:621. doi: 10.1016/j.cps.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Dominici M. Le Blanc K. Mueller I. Slaper-Cortenbach I. Marini F. Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006;8:315. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 3.da Silva Meirelles L. Chagastelles PC. Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119(Pt 11):2204. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 4.Giordano A. Galderisi U. Marino IR. From the laboratory bench to the patient's bedside: an update on clinical trials with mesenchymal stem cells. J Cell Physiol. 2007;211:27. doi: 10.1002/jcp.20959. [DOI] [PubMed] [Google Scholar]
- 5.Mustoe TA. O'Shaughnessy K. Kloeters O. Chronic wound pathogenesis and current treatment strategies: a unifying hypothesis. Plast Reconstr Surg. 2006;117(7 Suppl):35S. doi: 10.1097/01.prs.0000225431.63010.1b. [DOI] [PubMed] [Google Scholar]
- 6.Phinney DG. Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair—current views. Stem Cells. 2007;25:2896. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- 7.Hanson SE. Bentz ML. Hematti P. Mesenchymal stem cell therapy for nonhealing cutaneous wounds. Plast Reconstr Surg. 2010;125:510. doi: 10.1097/PRS.0b013e3181c722bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanson SE. Gutowski KA. Hematti P. Clinical applications of mesenchymal stem cells in soft tissue augmentation. Aesthet Surg J. 2010;30:838. doi: 10.1177/1090820X10386364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nauta AJ. Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 10.Chamberlain G. Fox J. Ashton B. Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 11.Zhu KQ. Carrougher GJ. Gibran NS. Isik FF. Engrav LH. Review of the female Duroc/Yorkshire pig model of human fibroproliferative scarring. Wound Repair Regen. 2007;15(Suppl 1):S32. doi: 10.1111/j.1524-475X.2007.00223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen SL. Fang WW. Qian J. Ye F. Liu YH. Shan SJ, et al. Improvement of cardiac function after transplantation of autologous bone marrow mesenchymal stem cells in patients with acute myocardial infarction. Chin Med J (Engl) 2004;117:1443. [PubMed] [Google Scholar]
- 13.Badiavas EV. Abedi M. Butmarc J. Falanga V. Quesenberry P. Participation of bone marrow derived cells in cutaneous wound healing. J Cell Physiol. 2003;196:245. doi: 10.1002/jcp.10260. [DOI] [PubMed] [Google Scholar]
- 14.Yoshikawa T. Mitsuno H. Nonaka I. Sen Y. Kawanishi K. Inada Y, et al. Wound therapy by marrow mesenchymal cell transplantation. Plast Reconstr Surg. 2008;121:860. doi: 10.1097/01.prs.0000299922.96006.24. [DOI] [PubMed] [Google Scholar]
- 15.Dash NR. Dash SN. Routray P. Mohapatra S. Mohapatra PC. Targeting nonhealing ulcers of lower extremity in human through autologous bone marrow-derived mesenchymal stem cells. Rejuvenation Res. 2009;12:359. doi: 10.1089/rej.2009.0872. [DOI] [PubMed] [Google Scholar]
- 16.Lataillade JJ. Doucet C. Bey E. Carsin H. Huet C. Clairand I, et al. New approach to radiation burn treatment by dosimetry-guided surgery combined with autologous mesenchymal stem cell therapy. Regen Med. 2007;2:785. doi: 10.2217/17460751.2.5.785. [DOI] [PubMed] [Google Scholar]
- 17.Rigotti G. Marchi A. Galie M. Baroni G. Benati D. Krampera M, et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg. 2007;119:1409–1422. doi: 10.1097/01.prs.0000256047.47909.71. discussion 1423, 1424. [DOI] [PubMed] [Google Scholar]
- 18.Bey E. Prat M. Duhamel P. Benderitter M. Brachet M. Trompier F, et al. Emerging therapy for improving wound repair of severe radiation burns using local bone marrow-derived stem cell administrations. Wound Repair Regen. 2010;18:50. doi: 10.1111/j.1524-475X.2009.00562.x. [DOI] [PubMed] [Google Scholar]
- 19.Chen L. Tredget EE. Wu PY. Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008;3:e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kidd S. Spaeth E. Klopp A. Andreeff M. Hall B. Marini FC. The (in) auspicious role of mesenchymal stromal cells in cancer: be it friend or foe. Cytotherapy. 2008;10:657. doi: 10.1080/14653240802486517. [DOI] [PubMed] [Google Scholar]