Abstract
OBJECTIVES:
Pancreatic duct (PD) dilation proximal to a solid focal pancreatic lesion on computed tomography (CT) scan is considered highly suggestive of pancreatic adenocarcinoma. There is, however, no published data on the differential diagnosis of focal non-cystic pancreatic lesions with and without PD dilation. We assessed the diagnostic utility of this radiologic finding.
METHODS:
This is a retrospective analysis of a prospectively maintained database of university-based clinical practice. A total of 445 non-jaundiced patients who underwent endoscopic ultrasound (EUS) (2002–2010) for evaluation of solid pancreatic lesions noted on CT scan were included. Final diagnosis was based on surgical pathology or definitive cytology with supporting clinical follow-up of ≥12 months. Main outcome measurements included (1) differential diagnoses and (2) performance characteristics of EUS-fine needle aspiration (FNA) for diagnosing neoplasm in patients with non-cystic pancreatic lesions with and without PD dilation.
RESULTS:
A neoplasm was finally diagnosed in 152 of 187 patients with and 87 of 258 patients without PD dilation on CT scan. Chronic pancreatitis (diffuse and focal) was the predominant non-malignant diagnosis in patients with PD dilation. In patients without PD dilation, malignant lesions included neuroendocrine tumor, adenocarcinoma, metastasis, PEComa (perivascular epitheloid cell tumor), and lymphoma; and the non-neoplastic diagnosis included chronic pancreatitis, intrapancreatic lymph nodes, and infected pancreatic fluid collection. EUS-FNA had 97.6% accuracy for diagnosing a neoplasm in these patients.
CONCLUSIONS:
Dilation PD proximal to a focal solid pancreatic lesion increases the likelihood of malignancy but the performance characteristics of this radiologic finding are probably inadequate to guide clinical management. Neoplasms without dilated PD often require immunostaining for a definitive diagnosis.
INTRODUCTION
Dilation of the pancreatic duct (PD) beyond a focal non-cystic lesion in the pancreas on computed tomography (CT) scan is considered to be highly suggestive of pancreatic adenocarcinoma.1, 2 This radiologic finding is often used to guide further management although there is insufficient published data on its predictive value for malignant etiology of pancreatic lesion. Information on the differential diagnosis of non-cystic focal pancreatic lesions with and without proximal dilation, PD dilation would help in devising appropriate strategies for further diagnostic evaluation. Endoscopic ultrasound (EUS) is increasingly being used for a definitive diagnosis in these patients. EUS expertise is not always readily available and absence of proximal PD dilation in an otherwise asymptomatic patient with a focal non-cystic pancreatic lesion is considered to be reassuring by several practitioners often leading to a conservative management approach with follow-up imaging in 6–12 weeks.
In this article, we evaluated the differential diagnosis of the solid focal pancreatic lesions associated with or without proximal dilation of PD noted on CT scan. We also evaluated the performance characteristics of EUS in identifying neoplastic lesions in this subgroup of patients.
METHODS
This is a retrospective analysis from our database of patients who underwent EUS/EUS-fine needle aspiration (FNA) at Saint Louis University and Missouri Baptist Medical center. This database was started in March 2002 and has been maintained prospectively since. In our database, follow-up information is rigorously collected for quality assurance in our clinical practice. It comprises of periodic phone calls to patients, correspondence from the referring and other physicians involved in the patient's clinical care, laboratory data, imaging, operative notes, and surgical pathology reports. Patients without evidence of cancer based on EUS/EUS-FNA undergo repeat imaging by EUS or CT/magnetic resonance imaging in 3, 6, and/or 12 months (as deemed clinically appropriate).
We identified all patients in our database that presented without obstructive jaundice and had non-cystic pancreatic mass lesions on CT scans. PD dilation on EUS was defined as PD diameter ≥3 mm in the head, ≥2 mm in the body, and ≥1 mm in the tail. Patients in whom the focal pancreatic lesion was not identified to be cystic on CT scans but was found to be cystic by EUS/EUS-FNA were not excluded. Patients with recent acute pancreatitis (within 6 weeks) were also excluded. A total of 445 patients were finally included for analysis. Medical records including radiology, EUS, cytology, and surgical pathology and operative notes were reviewed.
This study was approved by the Institutional Review Board of Saint Louis University School of Medicine.
EUS examination
EUS examinations were performed initially using radial echoendoscope (EUM-130 and EUM-160 Olympus, Melville, NY). Whenever a suspicious ‘mass' lesion was identified during the radial EUS exam, FNA was performed using a linear echoendoscope (FG-32A or FG-36A Pentax, Orangeburg, NY). Multiple FNA passes (up to seven) were made using the 22G or 25G Echo-tip EUS-FNA needle (Wilson Cook, Winston-Salem, NC) until the cytologist was able to make a preliminary diagnosis. The cytology specimens were stained by the Diff-Quik and Papanicoulou method (Pap smear) and assessed immediately by an attending cytologist. A sample was also collected for cell blocks. The final diagnosis was based on examination of the Pap smears and the cell blocks using standard cytological criteria.3 Special stains were used as indicated for identifying neuroendocrine tumors and lymphoid tissue. Flow cytometry was used to look for lymphoma if the pancreatic lesion was found to be lymphoid. The cystic lesions were aspirated as completely as possible. Cyst aspirates were submitted for cytology and biochemical analysis, including carcinoembryonic antigen, amylase, and lipase concentrations.4 Chronic pancreatitis was diagnosed based on the presence of ≥5 criteria.5
Radiologic imaging
To best address this frequently encountered clinical dilemma, the study was based on data actually used in patient management. The CT findings were based upon imaging that was performed before the EUS exam and included data that prompted the referral for EUS±FNA examination. The CT scans were performed using helical scanners with contrast. The CT scans were not reviewed by a designated radiologist(s) for purposes of this study. Radiologic findings of a dilated PD and the presence of mass lesion were considered present if they had been noted in the clinical CT report.
Follow-up and final diagnosis
Final diagnosis was based on surgical pathology or definitive cytology with supporting clinical follow-up of ≥12 months. The median follow-up was 17.6 months (range 12–74), and was 18.4 months (range 14–74) for patients with benign diagnosis and 13.9 months (range 12–48) in patients found to have a neoplastic lesion by EUS-FNA. Cytological specimens that were considered ‘atypical' (n=8) were counted as negative for malignancy for purposes of this study. Diagnosis of neuroendocrine tumor, PEComa (perivascular epitheloid cell tumor), spindle cell, and giant cell neoplasm was based on immunostaining in conjunction with suggestive cytology. For the purposes of calculating the performance characteristics of EUS/EUS-FNA, all these neoplasms were counted as malignant. Embedded lymph nodes in the pancreas were diagnosed only after immunostaining the EUS-FNA specimens with lymphoid markers when the neuroendocrine markers were negative. Lymphoma was diagnosed with flow cytometry. Immunostaining was also used to determine or confirm the site of primary in patients with metastasis in the pancreas, the choice of immunomarkers being based on patient's clinical history including that of tumors in the past.
Statistical analysis
Statistical analysis was conducted using a statistical software package (SPSS 17.0, Chicago, IL). All analysis were two-tailed and statistical significance was accepted at P<0.05. For descriptive analysis, continuous variables were reported as mean±s.d., range, and median. A Student's t-test or analysis of variance was used to assess statistical differences between continuous variables and χ2 tests were used to evaluate categorical variables.
RESULTS
Patient characteristics
The clinical characteristics of study patients are summarized in Table 1. The mean age of 445 study patients (214 men) was 63.6±13.3 years. A history of abdominal pain was present in 258 patients (58%, 95% confidence interval (CI) 53.3, 62.4) and a history of weight loss in the preceding 3 months was present in 162 patients (36.4%, 95% CI 32.0, 40.9). In 136 patients (30.5%, 95% CI 26.4, 35.0), the focal pancreatic lesion was noted incidentally on CT scan performed for unrelated symptoms. The mean size of pancreatic lesion was 26.5±13.2 mm. The median time between CT exam and EUS was 8 weeks (range 1–18 weeks). A final diagnosis of malignancy was made in 239 patients (53.7%, 95% CI 49.0, 58.2). Pancreatic lesions with proximal dilation of the PD had a significantly higher likelihood of malignant etiology (odds ratio 8.5, 95% CI 5.4, 13.3; P<0.001).
Table 1. Patient characteristics.
| Patients with proximal PD dilation, N=187 (%) | Patients without proximal PD dilation, N=258 (%) | Overall, N=445 (%) | P value | |
|---|---|---|---|---|
| Age (years) | 67.3±12.4 | 60.9±13.3 | 63.6±13.3 | <0.0001 |
| Gender | ||||
| Male | 96 (51.3) | 118 (45.7) | 214 (48.1) | 0.25 |
| Female | 91 (48.6) | 140 (54.2) | 231 (51.9) | 0.25 |
| Associated symptoms | ||||
| Abdominal pain | 113 (60.4) | 145 (56.2) | 258 (58.0) | 0.38 |
| Weight loss | ||||
| ≥10 lbs | 70 (37.4) | 35 (13.5) | 105 (23.6) | <0.0001 |
| <10 lbs | 25 (13.3) | 32 (12.4) | 57 (12.8) | 0.78 |
| No weight loss | 92 (49.1) | 191 (74.0) | 283 (63.6) | <0.0001 |
| Incidental mass on CT scan | 45 (24.0) | 91 (35.2) | 136 (30.6) | 0.0124 |
| Benign/malignant | ||||
| Malignant | 152 (81.2) | 87 (33.7) | 239 (53.7) | <0.0001 |
| Benign | 35 (18.7) | 171 (66.2) | 206 (46.3) | <0.0001 |
CT, computed tomography; PD, pancreatic duct.
Final diagnosis of pancreatic lesion in patients with PD dilation
The mean size of pancreatic tumor in patients with PD dilation was 29.8±15.8 mm, ranged from 4 to 80 mm, the median size being 25 mm. Table 2 summarizes the final diagnoses based on the location of the pancreatic lesion, in patients with proximal dilation of the PD. In 152 patients (81.2%, 95% CI 75.0, 86.2), the lesion was finally diagnosed to be malignant, the most common etiology being pancreatic adenocarcinoma. However, the focal lesions with pancreatic ductal dilation also included neuroendocrine tumors, giant cell neoplasm, metastatic non-small cell carcinoma to the pancreas, and spindle cell carcinoma. Diffuse chronic pancreatitis was the most common benign etiology along with benign cysts (serous cystadenoma and mixed type intraductal papillary mucinous neoplasm). Three patients with focal chronic pancreatitis (confirmed on surgical pathology) also had proximal pancreatic ductal dilation.
Table 2. Final diagnosis of focal pancreatic lesion with PD dilation.
|
Location of the pancreatic mass on CT scan |
|||||
|---|---|---|---|---|---|
| Uncinate | Head/neck | Body | Tail | N=187 (%) | |
| Malignant lesions=152 (81.2%) | |||||
| Adenocarcinoma of pancreas | 2 | 62 | 59 | 11 | 134 (71.6) |
| Neuroendocrine tumor | 1 | 7 | 5 | 1 | 14 (7.4) |
| Giant cell neoplasm | — | 1 | — | — | 1 (0.5) |
| Non-small cell carcinoma | — | 2 | — | — | 2 (1.0) |
| Spindle cell carcinoma | — | — | — | 1 | 1 (0.5) |
| Benign lesions=35 (18.7%) | |||||
| Diffuse chronic pancreatitis | 2 | 10 | 4 | — | 16 (8.5) |
| Focal chronic pancreatitis | 0 | 2 | 0 | 1 | 3 (1.6) |
| IPMN | — | 7 | 1 | — | 8 (4.2) |
| Serous cystadenoma | — | 5 | 1 | 1 | 7 (3.7) |
| Normal pancreas | — | 1 | — | — | 1 (0.5) |
| Total | 5 | 97 | 70 | 15 | 187 |
CT, computed tomography; IPMN, intraductal papillary mucinous neoplasm; PD, pancreatic duct.
Final diagnosis of the pancreatic lesion in patients without proximal dilation of PD
The mean size of pancreatic tumor in patients with PD dilation was 18.9±9.9 mm ranged from 4 to 60 mm, the median size being 19 mm. Table 3 summarizes the final diagnoses in patients without proximal dilation of PD. The focal pancreatic lesion was finally diagnosed to be neoplastic in 87 patients (33.7%, 95% CI 28.2, 39.7) and benign in 171 patients (66.2%, 95% CI 60.3, 71.7). Among patients with neoplastic lesions, 38 had neuroendocrine tumor, 32 had pancreatic adenocarcinoma (uncinate process n=17, body n=2, and tail n=13), 15 had metastatic tumor to pancreas, 1 had PEComa, and 1 had lymphoma. Of the remaining 171 patients, 25 patients had focal chronic pancreatitis, 16 had lymph node embedded in pancreas, and 2 had infected pancreatic fluid collection. In 23 patients with an otherwise normal EUS exam of pancreas, a focal lesion was noted by EUS and the FNA from which revealed benign pancreatic tissue with inflammatory changes and all these were confirmed to be benign based on follow-up. No identifiable focal lesion was noted by EUS in 27 patients who had EUS findings suggestive of diffuse chronic pancreatitis and 42 patients with normal appearing pancreas. In three patients, the abnormal CT appearance of a pancreatic mass lesion was due to a periampullary diverticulum.
Table 3. Final diagnosis of focal pancreatic lesion without PD dilation.
|
Location of the pancreatic mass on CT scan |
|||||
|---|---|---|---|---|---|
| Uncinate | Head/neck | Body | Tail | N=258 (%) | |
| Malignant lesions=87 (33.7%) | |||||
| Neuroendocrine tumor | — | 21 | 7 | 10 | 38 (14.7) |
| Adenocarcinoma of pancreas | 17 | — | — | 13 | 30 (11.6) |
| Cystadenocarcinoma of pancreas | — | — | 2 | 0 | 2 (0.7) |
| Metastasis to pancreas | — | 7 | 6 | 2 | 15 (5.8) |
| PEComa | — | 1 | — | — | 1 (0.3) |
| Lymphoma | — | 1 | — | — | 1 (0.3) |
| Benign lesions=171 (66.2%) | |||||
| Focal chronic pancreatitis | 3 | 17 | 4 | 1 | 25 (9.6) |
| Diffuse chronic pancreatitis | 2 | 16 | 4 | 5 | 27 (10.4) |
| Normal pancreas | 3 | 10 | 6 | 4 | 23 (8.9) |
| Normal pancreas without mass on EUS | — | 38 | 2 | 2 | 42 (16.2) |
| Benign cysts | 0 | 14 | 15 | 4 | 33 (12.7) |
| LN embedded in pancreas | 1 | 5 | 2 | 8 | 16 (6.2) |
| Infected pancreatic fluid collection | — | 1 | — | 1 | 2 (0.7) |
| Periampullary diverticulum | — | 3 | — | — | 3 (1.1) |
| Total | 26 | 134 | 48 | 50 | 258 |
CT, computed tomography; EUS, endoscopic ultrasound; LN, lymph node; PD, pancreatic duct; PEComa, perivascular epitheloid cell tumor.
Performance characteristics of EUS/EUS-FNA
The performance characteristics of EUS/EUS-FNA for diagnosing neoplasm in study patients are summarized in Table 4. EUS-FNA had an accuracy of 97.3% with 95.3% sensitivity, 99.5% specificity, and 94.9% negative predictive value. Eleven patients in our study cohort had false-negative diagnoses for malignancy based on EUS-FNA (pancreatic adenocarcinoma (n=6), neuroendocrine tumor (n=3), and metastasis to pancreas (n=2)). Four patients with false-negative diagnosis (pancreatic adenocarcinoma (n=3) and neuroendocrine tumor (n=1)) had chronic pancreatitis. Both the patients with metastasis to pancreas had a known history of carcinoma (esophageal carcinoma (n=1) and renal cell carcinoma (n=1)) and were subsequently diagnosed malignant on the follow-up repeat EUS-FNA.
Table 4. Performance characteristics of EUS/EUS-FNA in diagnosing malignancy in study patients.
| Patients with PD dilation (group A), N=187 | Patients without PD dilation (group B), N=258 | Overall, N=445 | |
|---|---|---|---|
| True positive | 146 | 82 | 228 |
| True negative | 35 | 170 | 205 |
| False negative | 6 | 5 | 11 |
| False positive | 0 | 1 | 1 |
| Sensitivity (95% CI) | 146/152 96.0% (91.2, 98.3) | 82/87 94.2% (86.4, 97.8) | 228/239 95.3% (91.6, 97.5) |
| Specificity | 35/35 100% | 170/171 99.4% (96.2, 99.9) | 205/206 99.5% (96.9, 99.9) |
| Positive predictive value | 146/146 100% | 82/83 98.7% (92.5, 99.9) | 228/229 99.5% (97.2, 99.9) |
| Negative predictive value | 35/41 85.3% (70.1, 93.9) | 170/175 97.1% (93.1, 98.9) | 205/216 94.9% (90.8, 97.3) |
| Accuracy | 181/187 96.7% (93.1, 98.8) | 252/258 97.6% (95.0, 99.1) | 433/445 97.3% (95.2, 98.5) |
CI, confidence interval; EUS, endoscopic ultrasound; FNA, fine needle aspiration; PD, pancreatic duct.
The complication rate of EUS-FNA was 0.8%, with acute pancreatitis in three patients (0.6%, 95% CI 0.1, 2.0) and aspiration pneumonia requiring hospitalization in one patient (0.2%, 95% CI 0.001, 1.3).
DISCUSSION
In patients found to have a focal non-cystic lesion in the pancreas on imaging with CT/magnetic resonance imaging, dilation of the PD beyond the lesion is considered highly suggestive of an underlying malignancy and is routinely looked for by the radiologists. There is, however, scant literature on the significance of this radiologic finding, especially the prevalence of malignancy and the differential diagnosis in lesions with and without PD dilation. As is generally believed, we also observed that the likelihood of a malignant etiology in a focal non-cystic pancreatic lesion noted on CT scan in a non-jaundiced patient is significantly higher in patients with proximal dilation of the PD (odds ratio 8.5, 95% CI 5.4, 13.3). In patients with proximal dilation of the PD, the pancreatic lesion was finally diagnosed to be malignant in 81.2% of patients and pancreatic adenocarcinoma was the most common etiology comprising 71.6% of the lesions. In patients with normal sized PD, the pancreatic lesion was neoplastic in 33.7% of patients and neuroendocrine tumors and pancreatic adenocarcinoma being the two most common neoplasms.
In a prospective study to identify endosonographic features suggestive of malignancy in 73 patients with suspected pancreatic cancer, Rodriguez and Faigel6 noted that 5/30 patients with malignant neoplasms (17%) did not have PD dilation. In the present cohort, 30 of 164 patients (18.2%, 95% CI 12.4, 24.2) with pancreatic adenocarcinoma had normal sized PD. Thus, absence of PD dilation does not reliably exclude a pancreatic adenocarcinoma. Does the location of the pancreatic lesion determine the significance of PD dilation in predicting the etiology of the lesion? In our cohort, the tumors in patients with pancreatic adenocarcinoma without PD dilation were located in the uncinate process and the tail of pancreas. Padilla-Thornton et al.7 have also reported that the pancreatic adenocarcinomas arising in the uncinate process are significantly less likely to have dilation of PD. The two primary pancreatic adenocarcinomas located in the body of pancreas without proximal PD dilation in our cohort were mucinous cystadenocarcinoma and had not been identified to be cystic on CT scan. Absence of pancreatic ductal dilation argued against a diagnosis of pancreatic adenocarcinoma when the lesion is situated in the pancreatic head, neck, or body. For focal lesions without a dilated PD situated in the uncinate process and the tail of pancreas, pancreatic adenocarcinoma was still the most common neoplastic etiology.
In absence of proximal PD dilation, most focal pancreatic lesions in head, neck, and body of pancreas were diagnosed to be benign (137 of 182 in present cohort) although the remaining 45 were neoplastic. Chronic pancreatitis (focal or diffuse), benign cysts, and intrapancreatic lymph nodes were the most common benign etiologies in these patients. Neuroendocrine tumor or metastases to pancreas were the two most common neoplastic diagnoses in these patients. Immunostaining of the FNA specimens is usually needed to diagnose most of these neoplasms and the cytologists should probably be alerted to the differential diagnosis so that they can process the specimens accordingly. We use immunostaining with lymphoid markers to diagnose an intrapancreatic lymph node if the neuroendocrine markers are negative. We also use immunostaining to identify the source of primary tumor if the FNA from a pancreatic lesion without proximal PD dilation reveals an adenocarcinoma.
Although all patients with pancreatic adenocarcinoma located in the head, neck, and body had PD dilation, not all focal lesions with dilated PD were adenocarcinoma or were malignant. PD dilation was also noted in several patients with neuroendocrine tumors, rarely with tumor metastasis and some rare tumors besides in patients with benign diagnosis such as diffuse or focal chronic pancreatitis and benign cysts (serous cystadenoma and mixed type intraductal papillary mucinous neoplasm). Thus, the presence of PD dilation on a focal lesion noted on CT scan is not sufficient to assume the lesion to be an adenocarcinoma or be malignant.
Presence or absence of PD dilation proximal to a focal pancreatic lesion is often used to guide further management decisions, especially in if the pancreatic lesion is small or is poorly defined lesion on CT images. Asymptomatic patients with poorly defined lesions on CT scan without PD dilation are often managed using follow-up imaging in 6–12 weeks. However, in patients with pancreatic adenocarcinoma, this delay in diagnosis and consequently initiation of treatment, can lead to worsening outcomes. The sensitivity and positive predictive value of this radiologic finding of PD dilation proximally for diagnosing a neoplasm was 63.5% and 81.2%, respectively. Absence of PD dilation had 66.2% negative predictive value to exclude a neoplastic etiology. EUS/EUS-FNA had 95.3% sensitivity with 99.5% positive predictive value and 94.9% negative predictive value for diagnosing malignancy in same cohort. Thus, although the radiologic finding of proximal pancreatic ductal dilation in patients with focal pancreatic non-cystic lesion is a useful rule of thumb, the performance characteristics of this sign are probably not adequate to make decisions regarding further clinical management and can be improved upon by the use of EUS/EUS-FNA.
In 51 patients, the pancreatic lesion that appeared non-cystic on CT was identified to be cystic by EUS-FNA. This would potentially raise concerns about the quality of CT scans and/or their interpretation. However, these 51 patients comprise <10% of all patients with pancreatic cysts in our database. It is not unusual for cystic lesions with thick mucus or thick debris to not be identified as cystic on CT scan. Serous cystadenomas that are predominantly microcystic are commonly diagnosed as solid focal lesions on CT scan. It is conceivable that a small number of these lesions would be diagnosed to be cystic by rigorous CT parameters applied retrospectively. We, however, used radiologic interpretations that were actually used in the clinical management of these patients. The present data are reflective of the real-life scenario in majority of large referral practices where patients are referred for EUS based on findings in CT scans performed in community hospitals and interpreted by non-academic radiologists.
This study has limitations inherent to its retrospective design, although it is based on data from a prospectively maintained database and used objective easily verifiable data. We excluded patients with obstructive jaundice from the present cohort because the pre-test probability of neoplasm in patients with identifiable pancreatic mass lesion on CT with obstructive jaundice is much higher. We also excluded patients with lesions that were identified as cystic on CT scan, because they have a much lower likelihood of malignancy than those with solid pancreatic lesions. Furthermore, dilation of PD beyond a focal cystic lesion in pancreas is less common and does not have the same significance as in patients with solid lesion. The performance characteristics of EUS-FNA in study patients reflect the results of a high volume referral center with availability of dedicated on-site attending cytopathologist for immediate cytological interpretation of the EUS-FNA specimens. We did not test for autoimmune pancreatitis in the EUS-FNA specimens as a potential etiology of the focal pancreatic lesions nor did we evaluate if the focal pancreatic lesion that yielded lymphoid tissue on EUS-FNA was an accessory intrapancreatic spleen not an embedded lymph node.8 Another potential limitation is that the follow-up of 12 months is not sufficient to reliably exclude a missed neuroendocrine tumor. However, there is no consensus on what is adequate length of follow-up for excluding missed non-functioning neuroendocrine tumors. In patients with a focal lesion with ‘small blue cells' on cytology but negative for neuroendocrine markers, we test for lymphocyte markers to confirm that these cells are lymphoid in origin. These patients also undergo a follow-up EUS-FNA in 12–24 weeks to re-evaluate the lesion. The negative predictive value when no mass is noted by EUS is close to 100% in patients without chronic pancreatitis.9 The major strength of the study is that it is based on data that was actually used in patient management. With widespread use of CT/magnetic resonance imaging, more and more patients are incidentally being found to have focal pancreatic ‘mass' lesions and dilation of PD is often used to guide further management of these patients. Our data on the differential diagnosis of focal pancreatic lesions with and without PD dilation can provide basis to devise optimal strategies for further diagnostic evaluation and management of these patients.
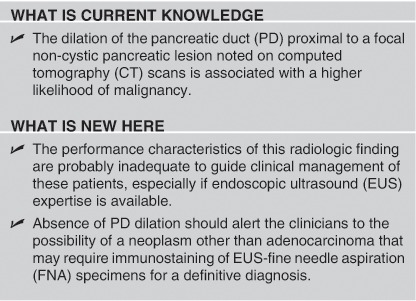
To conclude, the dilation of the PD proximal to a focal non-cystic pancreatic lesion noted on CT scans is associated with a higher likelihood of malignancy but the performance characteristics of this radiologic finding are probably inadequate to guide clinical management of these patients, especially if EUS expertise is available. Absence of PD dilation should alert the clinicians to the possibility of a neoplasm other than adenocarcinoma that may require immunostaining of EUS-FNA specimens for a definitive diagnosis.
Study Highlights
Guarantor of the article: Banke Agarwal, MD.
Specific author contributions: Data collection and analysis and drafting the manuscript: Pavan Tummala; data collection and analysis and manuscript review/drafting: Savitha Rao; and conceived the study idea, data analysis, critical revisions, and final draft of the manuscript: Banke Agarwal.
Financial support: None.
Potential competing interests: None.
References
- Prokesch RW, Chow LC, Beaulieu CF, et al. Isoattenuating pancreatic adenocarcinoma at multi-detector row CT: secondary signs. Radiology. 2002;224:764–768. doi: 10.1148/radiol.2243011284. [DOI] [PubMed] [Google Scholar]
- Yoon SH, Lee JM, Cho JY, et al. Small (≤20 mm) pancreatic adenocarcinomas: analysis of enhancement patterns and secondary signs with multiphasic multidetector CT. Radiology. 2011;259:442–452. doi: 10.1148/radiol.11101133. [DOI] [PubMed] [Google Scholar]
- Lin F, Staerkel G. Cytologic criteria for well differentiated adenocarcinoma of the pancreas in fine-needle aspiration biopsy specimens. Cancer. 2003;99:44–50. doi: 10.1002/cncr.11012. [DOI] [PubMed] [Google Scholar]
- Brugge WR, Lewandrowski K, Lee-Lewandrowski E, et al. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330–1336. doi: 10.1053/j.gastro.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Sahai AV, Zimmerman M, Aabakken L, et al. Prospective assessment of the ability of endoscopic ultrasound to diagnose, exclude, or establish the severity of chronic pancreatitis found by endoscopic retrograde cholangiopancreatography. Gastrointest Endosc. 1998;48:18–25. doi: 10.1016/s0016-5107(98)70123-3. [DOI] [PubMed] [Google Scholar]
- Rodriguez S, Faigel D. Absence of a dilated duct predicts benign disease in suspected pancreas cancer: a simple clinical rule. Dig Dis Sci. 2010;55:1161–1166. doi: 10.1007/s10620-009-0889-y. [DOI] [PubMed] [Google Scholar]
- Padilla-Thornton AE, Willmann JK, Jeffrey RB. Adenocarcinoma of the uncinate process of the pancreas: MDCT patterns of local invasion and clinical features at presentation. Eur Radiol. 2012;22:1067–1074. doi: 10.1007/s00330-011-2339-4. [DOI] [PubMed] [Google Scholar]
- Schreiner AM, Mansoor A, Faigel DO, et al. Intrapancreatic accessory spleen: mimic of pancreatic endocrine tumor diagnosed by endoscopic ultrasound guided fine needle aspiration biopsy. Diagn Cytopathol. 2008;36:262–265. doi: 10.1002/dc.20801. [DOI] [PubMed] [Google Scholar]
- Bhutani MS, Gress FG, Giovannini M, et al. The No Endosonographic Detection of Tumor (NEST) Study: a case series of pancreatic cancers missed on endoscopic ultrasonography. Endoscopy. 2004;36:385–389. doi: 10.1055/s-2004-814320. [DOI] [PubMed] [Google Scholar]


