Abstract
Background
Mesenchymal stem cells (MSCs) are key to regenerative wound healing. MSCs have spatial memory and respond to local environment. MSCs orchestrate wound repair by: (1) structural repair via cellular differentiation; (2) immune-modulation; (3) secretion of growth factors that drive neovascularization and re-epithelialization; and (4) mobilization of resident stem cells.
The Problem
Autologous bone-marrow-derived cells and MSCs demonstrate improved healing and tissue-integrity in animal models and clinical trials. However, the effects are variable and the mechanisms of MSC-mediated wound healing are not fully understood. The mammalian MSC niche and signaling sequences and factors affecting their homing, differentiation, viability, and safety need to be characterized to get full benefits of MSC cellular therapy.
Basic/Clinical Science Advances
MSCs can be isolated from bone-marrow, and less-invasive tissues such as adipose, gingiva, muscle, and umbilical cord, with similar functional effects. However, isolation, culture conditions, and markers used to identify and trace the lineage of these MSCs have not been standardized, which is crucial to determine the extent to which MSCs act as multipotent stem cells or sources of secreted factors in wounds.
Clinical Care Relevance
In chronic nonhealing wounds, where efficacy of conventional therapies is unsatisfactory, autotransplantation of MSCs could accelerate wound healing, promote regeneration and restoration of tissue integrity, and reduce recurrence of wounds at characteristically predisposed sites.
Conclusion
Regenerative medicine and novel wound therapies using autologous stem cells holds great promise for clinical management of difficult wounds. The ideal candidate stem cells can be used to repopulate the wound bed to mediate appropriate epidermal and dermal regeneration and promote efficient wound repair, while modulating the immune system to prevent infection.

Timothy M. Crombleholme
Background
Wound healing proceeds through a complex and dynamic, yet highly orchestrated interactive sequence of events. Different cells, growth factors, and cytokines coordinate at the cellular and molecular levels to influence wound repair and re-establish barrier function,1 which in mammalian postnatal setting occurs through characteristic scar formation. In stark contrast, the mid-gestational mammalian fetus and numerous other less-evolved species (such as hydra, planarians, and amphibians) heal with an attenuated inflammatory response and a more regenerative pattern of wound healing. One of the key elements of this regenerative wound healing phenotype is believed to be their undifferentiated mesenchymal elements. The mesenchymal elements or adult stromal stem cells are a population of cells that self renew and differentiate into multiple cell types and play an important role in tissue regeneration after injury; yet the underlying mechanisms are not completely elucidated. We can utilize the existing genomic information and latest advances in cell and molecular biology and bioinformatics tools to more completely understand the intrinsic properties of the mesenchymal and stromal stem cells. This can potentially elucidate the mechanisms that regulate their role in regenerative wound healing.2 Understanding the role of these effector cell types within the wound will identify targeted manipulations of their functions to favorably influence the postnatal mammalian wound healing outcome and minimize scar formation. This review aims to evaluate the current knowledge of the role of mesenchymal stem cells (MSCs) and their contribution in wound repair, and their potential as a cellular therapeutic to produce a postnatal regenerative phenotype.
Target Articles.
1. Stappenbeck TS and Miyoshi H: The role of stromal stem cells in tissue regeneration and wound repair. Science 2009; 324: 1666.
2. Ko SH, Nauta A, Wong V, Glotzbach J, Gurtner GC, and Longaker MT: The role of stem cells in cutaneous wound healing: what do we really know? Plast Reconstruct Surg 2011; 127 Suppl 1: 10S.
3. Singer NG and Caplan AI: Mesenchymal stem cells: mechanisms of inflammation. Annu Rev Pathol 2011; 6: 457.
Clinical Problem Addressed
Several chronic diseases such as diabetes, peripheral vascular disease, and venous stasis and pressure ulcers are characterized by impaired wound healing. Current wound management focuses on wound coverage to facilitate repair but does not address the underlying pathophysiologic mechanisms that predisposes these wounds to impaired healing. This strategy has yielded less than ideal results. Another major problem is recurrence of wounds at characteristically predisposed sites, as a direct consequence of less than satisfactory repair of the initial wound. Cellular therapy using MSCs has the potential to address the underlying pathogenesis of impaired wound healing and accelerate tissue repair with more durable tissue integrity. This strategy may result in a more regenerative form of wound repair, with obvious implications for cutaneous wound healing, and any disease characterized by increased fibroplasia, such as intra-abdominal adhesions, keloids, scleroderma, pulmonary/renal fibrosis, and hepatic cirrhosis.
Relevant Basic Science Context
Mammalian MSCs are similar to undifferentiated mesenchymal elements that regulate regeneration after injury/amputation in simpler animals such as planarians and amphibians.3 Depending on the cellular environment, MSCs can differentiate into multiple mesenchymal lineages including muscle, bone, cartilage, and fat.4 The major mechanisms of MSC's contribution to wound repair process are thought to be: (1) structural repair of wounds via cellular differentiation; (2) immune-modulation; (3) production of growth factors that drive neovascularization and re-epithelialization; and (4) mobilization of resident stem cell niche. It is also well established that proper signal transmission between epithelium and mesenchyme is important for epithelial differentiation/re-epithelialization and wound regeneration.5 MSCs appear to be a key cell type in the mesenchyme that coordinates the wound repair response.
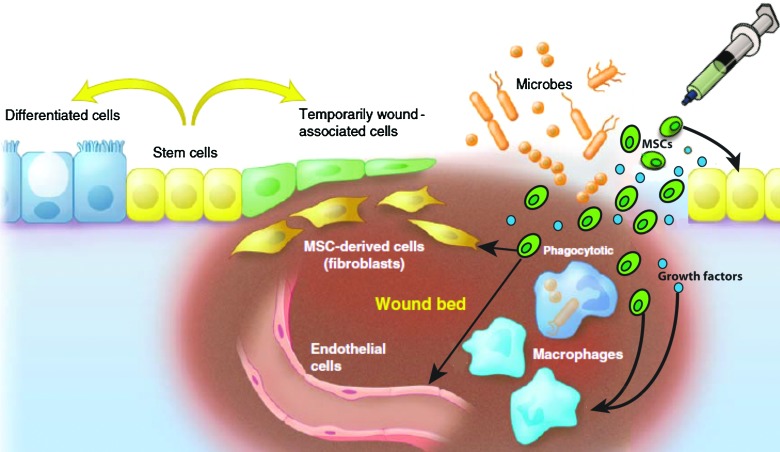
Several studies have demonstrated that systemically injected MSCs migrate to various sites of injury in the host.6 The wound local environment influences the homing and behavior of the MSCs. These cells respond favorably to the local inflammatory and hypoxic conditions of the wound environment, and they support important wound healing events such as matrix deposition and blood vessel formation by stimulating increased rate of proliferation, differentiation, and growth factor production (such as vascular endothelial growth factor [VEGF] and fibroblast growth factor [FGF]).7 Recent evidence indicates that VEGF not only supports neovascularization but also enhances keratinocyte proliferation, suggesting a further paracrine role for MSCs in wound re-epithelialization.8 Other studies demonstrate that MSCs and their secreted factors can inhibit T-lymphocyte activation and proliferation (reviewed in Ref.4). MSCs secrete prostaglandin E2, which downregulates inflammatory cytokines produced by macrophages.9 MSCs can themselves secrete and can induce macrophages to secrete anti-inflammatory cytokines, such as interleukin 10, implicated in postnatal tissue regeneration.10 Taken together, these data suggest an immunomodulatory role for MSCs,11 which may also function as gatekeepers in preventing excessive inflammation. Further, these data support the concept that exogenous MSCs can be inserted into a wound to communicate with other resident cell populations in the epithelium and mesenchyme and immune cells to orchestrate wound repair events, as shown in Fig. 1.1,2
Figure 1.
Model for stem cell-based therapy for wound healing in epithelium lined organs. The cellular component of the wound bed consists primarily of fibroblasts, endothelial cells, and macrophages. The epithelial response consists of stem cells that produce less-differentiated cells that are temporarily associated with the wound bed. Immediately after injury, there is a surge in the inflammatory response at the wound site to eliminate microbes and activate the healing process. The timing and balance between wound repair and elimination of microbes in the wound bed must be orchestrated. MSCs are uniquely poised in the wound to communicate with the overlying epithelial cells and the inflammatory cells within wound bed to orchestrate their coordinated efforts toward wound healing. Stem cells applied to wounds can contribute to healing by: (1) directly differentiating into daughter cells that form building blocks for tissue renewal (such as keratinocytes, fibroblasts, and pericytes); (2) immune-modulation; (3) secretion of growth factors that mediate wound healing by paracrine signaling; and (4) mobilization of resident stem cell niche to participate in wound healing response. MSCs=mesenchymal stem cells. Color image available online at www.liebertpub.com/wound
Experimental Model or Material: Advantages and Limitations
MSCs can be isolated from bone marrow, and a variety of tissues (including adipose, gingiva, muscle, and umbilical cord) with functional effects similar to bone -marrow-derived MSCs. Utilization of MSCs derived from these sources are even more attractive due to the less invasive nature of harvest and the potential for distinct site of origin-specific properties.4 However, isolation and culture conditions of these MSCs have not been standardized and there is no consensus on the markers used to identify them and trace their lineage. The development of such methods will enable us to truly determine the extent to which MSCs act as multipotent stem cells or sources of secreted factors in wounds, and dissect this cell type into distinct and functional subpopulations. The function of MSCs in wound repair depends on the site and type of injury; the optimization of the appropriate population/dose and the route of administration of MSCs may be critical for optimal results. Because MSCs respond to the wound microenvironment, there is a need to extensively study the other supporting cells and factors that compose the wound milieu, which determines the survival and differentiation of MSCs. Lastly, in their in vivo niches, stem cells are present under hypoxic conditions. Change in oxygen levels can induce oxidative stress, which can influence stem cell phenotype, proliferation, fate, pluripotency etc.12 Therefore in vitro culture conditions used to study MSCs should replicate those found in their in vivo niches.
Discussion of Findings and Relevant Literature
Regeneration after resection in various model organisms (less-evolved organisms such as planarian-head or tail; fish-fins; and amphibians-limbs) requires mesenchymal elements, which proliferate and migrate to the site of injury to form the blastema (mass of undifferentiated cells capable of growth and regeneration into organs).2,13 These cells require instructive cues from the local environment. For example loss of Wnt signaling14 or bone morphogenetic protein15 affects axis recognition and results in improper regeneration in planarians and hydra. In amphibians, denervated limbs do not regenerate, reflecting dependence on signaling between blastemal stem cells and the nearby nervous system.16 The amphibian blastemal cells, although morphologically indistinct, appear to be compartmentalized and functionally distinct with the ability to regenerate only specific compartments.17 These data indicate that even in these simpler organisms, stem cells have a spatial memory, and their position, spatial orientation, and cues from local environment are critical for coordinated progression of events in regeneration.
This concept appears to have application in adult mammalian organs as well, if we extend the concept of regeneration to restoration of morphologically distinct epithelial stem cell-containing “mini-organs” located within an organ (including hair follicles in skin; crypts in intestine; and limbus of cornea). Wnt signaling is required for the patterning of hair follicle during its regeneration.18 In the cornea, Notch function in the epithelium is critical for transmission of appropriate signals between the epithelium and mesenchyme that regulate epithelial differentiation and corneal wound regeneration.5 In contrast to simpler organisms, there is no evidence of blastema formation in mammalian wounds. This raises the question, which cell type in the mesenchyme of the mammalian organ system communicates with the epithelium to modulate wound repair? A possible candidate cell that coordinates wound repair in mammals may be the MSC. Due to the crude methods of isolation of MSCs, there is significant interest in characterizing the cell-types that they represent. In parallel to amphibian blastemal cells, the stromal fibroblasts from adult mammalian skin have geographic specificity and distinct properties depending on the site of isolation, (superficial vs. deeper within the organ; reviewed in Ref.2). Recent evidence also demonstrates that fibroblasts isolated from different tissues appear to have properties similar to MSCs, including the ability to differentiate into multiple mesenchymal lineages. This has led to the recent suggestion that MSCs, when injected into bloodstream of mammals, may represent a mobilized form of tissue resident fibroblasts. Another intriguing suggestion is that MSCs are pericytes, a supporting cell for blood vessels, raising interesting connection between MSCs and angiogenesis, a key component in wound repair. However, it is not known whether MSCs dedifferentiate during injury repair or they proliferate and increase their representation in the wound bed. Multiple transplantation studies demonstrate that MSCs differentiate to directly participate in structural repair. But due to low engraftment and viability of MSCs in the wound bed, paracrine signaling from the released soluble factors (such as FGF, VEGF, and angiopoietin 1), is thought to play a major role in MSC-mediated wound healing. This is also supported by studies that demonstrate the vulnerary effects of MSC-conditioned media. MSCs have been shown to enhance wound healing and closure through increased angiogenesis, re-epithelialization, granulation tissue formation, and decreased inflammatory cell infiltration in challenging situations such as deep burn wounds in rats and excisional splinted wound healing model in both normal and diabetic mice (reviewed in Ref.1). In another study, when human bone marrow-derived MSCs were applied to full-thickness skin defects in mice, both topically and intravenously, all skin wounds healed without scar or retraction.19
In mammals, injury is followed by a rapid response aimed at hemostasis and inflammatory process to clean the wound bed, which is a prerequisite for healing. chronic inflammation, such as in diabetes mellitus, derails the healing cascade resulting in impaired wound closure and nonhealing ulcers. Recent evidence suggests that hMSCs have the ability to modulate tolerance or attenuate inflammation from injury or autoimmune diseases by both contact-dependant and -independent mechanisms (reviewed in Ref.4). This concept raises the question: do MSC-activated macrophages secrete factors that promote wound healing and a more regenerative phenotype, in contrast to conventionally activated macrophages that either inhibit repair or lead to scar formation?
There are encouraging results from human studies using multipotent stem cells as therapeutic agents for tissue repair. Application of autologous bone marrow cells results in complete wound closure with dermal rebuilding and decreased scar in chronic nonhealing wounds that were refractory to conventional therapy.20 While these results are indeed promising, certain questions must be addressed: what part of the BM cells do the MSCs constitute?; do MSCs act primarily as factories of growth factor production or undergo cellular differentiation?; what is the ultimate fate of MSCs in the wound?; and what is their oncologic potential, if any, which would limit their use in clinical applications?.
Innovation
Major advantages of cellular therapy are: (1) cells can be isolated from patients and amenable to autologous transplantation, which theoretically attenuates the host's immune response; (2) using high-throughput technology, a single isolation can provide a lifetime repository of cells for the patient; and (3) these cells can also be genetically modified to overexpress vulnerary transgenes that can potentially augment the wound milieu and redirect chronic wounds toward a regenerative wound healing progression. Combining cellular therapy with advances in tissue engineering strategies can result in: (1) efficient delivery of cells; (2) development of smart biomaterials that can conform to any wound and direct the recruitment of the patient's native stem cells from their niche to participate in wound healing. This accomplishment will enable significant therapeutic advances in the management of chronic wounds.
Caution, Critical Remarks, and Recommendations
The rationale and goals of using stem cells as a cell source for regenerative therapy is clearly worth pursuing. Animal transplantation models and small clinical trials with autologous bone marrow-derived cells and tissue-engineered skin constructs with MSCs have yielded some encouraging data. The effects of MSCs in vivo appear to be pleiotropic and influenced by their local environment, however this dimension of cell–cell interaction is missing from in vitro experiments, making it difficult to interpret how a population of culture expanded MSCs may behave in vivo. Despite rapid progress in evaluating the efficacy of MSC transplantation on wound healing, many questions still need to be addressed. Safety in stem cell therapy must be a paramount concern. The stem cell niche and signaling sequences and factors that affect stem cell homing, differentiation, and viability need to be characterized. Likewise, the heterogeneous cell type that we have at our disposal today should be divided into more distinct and functional subpopulations to be used in future applications in a more targeted fashion. The ideal stem cell subpopulations should be comprised of autologous cells that can be used to repopulate the wound bed to mediate appropriate epidermal and dermal regeneration and promote efficient wound repair, while modulating the immune system to prevent infection. Regenerative medicine and novel wound therapies using stem cells holds great promise for clinical management of difficult wounds.
Take-Home Message.
Basic science advances
• Mammalian MSCs are similar to undifferentiated mesenchymal elements in less-evolved organisms (planaria, hydra, and amphibians) that form blastema that regenerates lost tissue after injury.
• The mesenchymal cells have a spatial memory and their position, spatial orientation, and cues from local environment are critical for coordinated progression of events in wound regeneration.
• MSCs can be isolated from BM and a variety of tissues including adipose, muscle, umbilical cord, peripheral blood, gingiva etc.
• MSCs can orchestrate wound repair by: (1) structural repair of wounds via cellular differentiation; (2) immune-modulation; (3) production of growth factors that drive neovascularization and re-epithelialization; and (4) communicating with resident stem cells to participate in wound repair.
• Mammalian MSCs may be a form of tissue-specific fibroblasts or pericytes. However, the relative number and source of MSCs (tissue resident vs. mobilized cells from remote sites) are an ongoing investigation.
• Although MSCs are originally hypothesized to be the panacea for all regenerating tissues, they appear to have a much more potent immune regulatory role.
• In their in vivo niches, stem cells are present under hypoxic conditions. Change in oxygen levels can induce oxidative stress, which can influence stem cell phenotype, proliferation, fate, pluripotency etc. Therefore, in vitro culture conditions used to study MSCs should be maintained similar to their in vivo niches.
Clinical science advances
• Preliminary evidence from the clinical trials utilizing hMSCs for wound treatment has been favorable. Because these trials are primarily therapeutic innovations, they do not provide mechanistic information of how MSCs promote wound healing.
• Appropriate disease targets for MSC-based therapy needs to be fully defined.
• There is a need to develop: (1) in vitro readouts to positively predict the effects of MSCs in vivo; and (2) potency assays for MSCs from different sources to elucidate their actions in vivo.
Relevance to clinical care
-
• Major advantages of MSC-based cellular therapy are:
(1) These cells can be isolated from patient and amenable to autologous transplantation or developing tissue engineered matrices to cover the wounds, without risk of infection associated with the introduction of foreign bodies or rejection of allografted cells.
(2) Using high-throughput technology, a single isolation can be a lifetime repository of cells for the patient.
(3) These cells can also be genetically modified to overexpress vulnerary transgenes that can potentially augment the wound milieu and redirect chronic wound toward-s a regenerative wound healing progression.
• There are diverse possibilities for future of this research, including developing sophisticated delivery systems for MSC therapy and growth factors and biological matrices with embedded cells and growth factors.
Future Development of Interest
Continued investigations will need to be carried out to not only develop the ideal stem cell populations, but also sophisticated delivery systems of these cells. Diverse cell types such as embryonic stem cells, MSCs, tissue-resident stem cells, and induced pluripotent stem cells are currently under intense investigation (reviewed in Ref.1). Identifying the molecular mechanisms involved in acute healing and the pathogenic mechanisms of the chronic disease states is essential to continue to identify model candidate stem cells to address these mechanistic differences. The complex mammalian wound environment may require a mixture of different cell populations to fully restore the wounds in many of these conditions. As the understanding of stem cell biology grows through basic science research and the pathways for vulnerary mechanisms will be revealed, translational stem cell-based therapies should be tested in larger clinical trials to determine the true efficacy of cellular therapy.
Abbreviations and Acronyms
- BM
bone marrow
- FGF
fibroblast growth factor
- h-MSCs
human mesenchymal stem cells
- MSCs
mesenchymal stem cells
- VEGF
vascular endothelial growth factor
Acknowledgments and Funding Sources
Wound healing research in our laboratory is supported by: NIH-NIDDK awards R01-DK072446 and R01-DK074055 (T.M.C.); Wound Healing Foundation 3M award and NIH-NIGMS award K08 GM098831-01 (S.G.K).
Author Disclosure and Ghostwriting
There are no conflicts of interests for S.B., S.G.K., and T.M.C. This article was not written by any writer other than the authors.
References
- 1.Ko SH. Nauta A. Wong V. Glotzbach J. Gurtner GC. Longaker MT. The role of stem cells in cutaneous wound healing: what do we really know? Plast Reconstruct Surg. 2011;127(Suppl 1):10S. doi: 10.1097/PRS.0b013e3181fbe2d8. [DOI] [PubMed] [Google Scholar]
- 2.Stappenbeck TS. Miyoshi H. The role of stromal stem cells in tissue regeneration and wound repair. Science. 2009;324:1666. doi: 10.1126/science.1172687. [DOI] [PubMed] [Google Scholar]
- 3.Pittenger MF. Mackay AM. Beck SC. Jaiswal RK. Douglas R. Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 4.Singer NG. Caplan AI. Mesenchymal stem cells: mechanisms of inflammation. Annu Rev Pathol. 2011;6:457. doi: 10.1146/annurev-pathol-011110-130230. [DOI] [PubMed] [Google Scholar]
- 5.Vauclair S. Majo F. Durham AD. Ghyselinck NB. Barrandon Y. Radtke F. Corneal epithelial cell fate is maintained during repair by Notch1 signaling via the regulation of vitamin A metabolism. Dev Cell. 2007;13:242. doi: 10.1016/j.devcel.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 6.Fu X. Fang L. Li X. Cheng B. Sheng Z. Enhanced wound-healing quality with bone marrow mesenchymal stem cells autografting after skin injury. Wound Repair Regen. 2006;14:325. doi: 10.1111/j.1743-6109.2006.00128.x. [DOI] [PubMed] [Google Scholar]
- 7.Lee EY. Xia Y. Kim WS. Kim MH. Kim TH. Kim KJ, et al. Hypoxia-enhanced wound-healing function of adipose-derived stem cells: increase in stem cell proliferation and up-regulation of VEGF and bFGF. Wound Repair Regene. 2009;17:540. doi: 10.1111/j.1524-475X.2009.00499.x. [DOI] [PubMed] [Google Scholar]
- 8.Brem H. Kodra A. Golinko MS. Entero H. Stojadinovic O. Wang VM, et al. Mechanism of sustained release of vascular endothelial growth factor in accelerating experimental diabetic healing. J Invest Dermatol. 2009;129:2275. doi: 10.1038/jid.2009.26. [DOI] [PubMed] [Google Scholar]
- 9.Nemeth K. Leelahavanichkul A. Yuen PS. Mayer B. Parmelee A. Doi K, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gordon A. Kozin ED. Keswani SG. Vaikunth SS. Katz AB. Zoltick PW, et al. Permissive environment in postnatal wounds induced by adenoviral-mediated overexpression of the anti-inflammatory cytokine interleukin-10 prevents scar formation. Wound Repair Regen. 2008;16:70. doi: 10.1111/j.1524-475X.2007.00326.x. [DOI] [PubMed] [Google Scholar]
- 11.Maggini J. Mirkin G. Bognanni I. Holmberg J. Piazzon IM. Nepomnaschy I, et al. Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. PLoS One. 2010;5:e9252. doi: 10.1371/journal.pone.0009252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohyeldin A. Garzon-Muvdi T. Quinones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell. 2010;7:150. doi: 10.1016/j.stem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Birnbaum KD. Sanchez Alvarado A. Slicing across kingdoms: regeneration in plants and animals. Cell. 2008;132:697. doi: 10.1016/j.cell.2008.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Broun M. Gee L. Reinhardt B. Bode HR. Formation of the head organizer in hydra involves the canonical Wnt pathway. Development. 2005;132:2907. doi: 10.1242/dev.01848. [DOI] [PubMed] [Google Scholar]
- 15.Reddien PW. Bermange AL. Kicza AM. Sanchez Alvarado A. BMP signaling regulates the dorsal planarian midline and is needed for asymmetric regeneration. Development. 2007;134:4043. doi: 10.1242/dev.007138. [DOI] [PubMed] [Google Scholar]
- 16.Kumar A. Godwin JW. Gates PB. Garza-Garcia AA. Brockes JP. Molecular basis for the nerve dependence of limb regeneration in an adult vertebrate. Science. 2007;318:772. doi: 10.1126/science.1147710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Echeverri K. Tanaka EM. Proximodistal patterning during limb regeneration. Dev Biol. 2005;279:391. doi: 10.1016/j.ydbio.2004.12.029. [DOI] [PubMed] [Google Scholar]
- 18.Ito M. Yang Z. Andl T. Cui C. Kim N. Millar SE, et al. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007;447:316. doi: 10.1038/nature05766. [DOI] [PubMed] [Google Scholar]
- 19.Mansilla E. Marin GH. Sturla F. Drago HE. Gil MA. Salas E, et al. Human mesenchymal stem cells are tolerized by mice and improve skin and spinal cord injuries. Transplant Proc. 2005;37:292. doi: 10.1016/j.transproceed.2005.01.070. [DOI] [PubMed] [Google Scholar]
- 20.Falanga V. Iwamoto S. Chartier M. Yufit T. Butmarc J. Kouttab N, et al. Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng. 2007;13:1299. doi: 10.1089/ten.2006.0278. [DOI] [PubMed] [Google Scholar]