Abstract
The tumorigenicity of DNA from polyoma virus after cleavage with a variety of restriction enzymes was evaluated in suckling hamsters. Cleavage with enzymes that interrupt the region of the genome coding for the large tumor (T) antigen of polyoma virus markedly enhanced the tumorigenicity above that observed with DNA I of the virus. Cell lines established in vitro from tumors induced by polyoma virions, polyoma virus DNA I, or polyoma virus DNA that had been cleaved with restriction endonucleases in the early region all contain the polyoma virus middle and small T antigens but not the large T antigen of polyoma virus is not required for maintenance of the transformed state and probably not for initiation of tumorigenesis by viral DNA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLACK P. H., ROWE W. P., TURNER H. C., HUEBNER R. J. A SPECIFIC COMPLEMENT-FIXING ANTIGEN PRESENT IN SV40 TUMOR AND TRANSFORMED CELLS. Proc Natl Acad Sci U S A. 1963 Dec;50:1148–1156. doi: 10.1073/pnas.50.6.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin T. L. Host range mutants of polyoma virus. Proc Natl Acad Sci U S A. 1970 Sep;67(1):394–399. doi: 10.1073/pnas.67.1.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
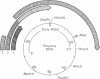
- Chen M. C., Chang K. S., Salzman N. P. Studies of polyoma virus DNA: cleavage map of the polyoma virus genome. J Virol. 1975 Jan;15(1):191–198. doi: 10.1128/jvi.15.1.191-198.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhart W. Complementation and transformation by temperature-sensitive mutants of polyoma virus. Virology. 1969 May;38(1):120–125. doi: 10.1016/0042-6822(69)90133-0. [DOI] [PubMed] [Google Scholar]
- Eckhart W. Complementation between temperature-sensitive (ts) and host range nontransforming (hr-t) mutants of polyoma virus. Virology. 1977 Apr;77(2):589–597. doi: 10.1016/0042-6822(77)90484-6. [DOI] [PubMed] [Google Scholar]
- FRIED M. CELL-TRANSFORMING ABILITY OF A TEMPERATURE-SENSITIVE MUTANT OF POLYOMA VIRUS. Proc Natl Acad Sci U S A. 1965 Mar;53:486–491. doi: 10.1073/pnas.53.3.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIED M. ISOLATION OF TEMPERATURE-SENSITIVE MUTANTS OF POLYOMA VIRUS. Virology. 1965 Apr;25:669–671. doi: 10.1016/0042-6822(65)90098-x. [DOI] [PubMed] [Google Scholar]
- Feunteun J., Sompayrac L., Fluck M., Benjamin T. Localization of gene functions in polyoma virus DNA. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4169–4173. doi: 10.1073/pnas.73.11.4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluck M. M., Staneloni R. J., Benjamin T. L. Hr-t and ts-a: two early gene functions of polyoma virus. Virology. 1977 Apr;77(2):610–624. doi: 10.1016/0042-6822(77)90486-x. [DOI] [PubMed] [Google Scholar]
- Francke B., Eckhart W. Polyoma gene function required for viral DNA synthesis. Virology. 1973 Sep;55(1):127–135. doi: 10.1016/s0042-6822(73)81014-1. [DOI] [PubMed] [Google Scholar]
- Griffin B. E., Fried M., Cowie A. Polyoma DNA: a physical map. Proc Natl Acad Sci U S A. 1974 May;71(5):2077–2081. doi: 10.1073/pnas.71.5.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HABEL K. SPECIFIC COMPLEMENT-FIXING ANTIGENS IN POLYOMA TUMORS AND TRANSFORMED CELLS. Virology. 1965 Jan;25:55–61. doi: 10.1016/0042-6822(65)90251-5. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
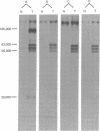
- Hutchinson M. A., Hunter T., Eckhart W. Characterization of T antigens in polyoma-infected and transformed cells. Cell. 1978 Sep;15(1):65–77. doi: 10.1016/0092-8674(78)90083-1. [DOI] [PubMed] [Google Scholar]
- Israel M. A., Chan H. W., Hourihan S. L., Rowe W. P., Martin M. A. Biological activity of polyoma viral DNA in mice and hamsters. J Virol. 1979 Mar;29(3):990–996. doi: 10.1128/jvi.29.3.990-996.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamen R., Lindstrom D. M., Shure H., Old R. W. Virus-specific RNA in cells productively infected or transformed by polyoma virus. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):187–198. doi: 10.1101/sqb.1974.039.01.025. [DOI] [PubMed] [Google Scholar]
- Lund E., Fried M., Griffin B. E. Polyoma virus defective DNAs. I. Physical maps of a related set of defective molecules (D76, D91, D92). J Mol Biol. 1977 Dec 5;117(2):473–495. doi: 10.1016/0022-2836(77)90138-3. [DOI] [PubMed] [Google Scholar]
- Miller L. K., Fried M. Construction of the genetic map of the polyoma genome. J Virol. 1976 Jun;18(3):824–832. doi: 10.1128/jvi.18.3.824-832.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POPE J. H., ROWE W. P. DETECTION OF SPECIFIC ANTIGEN IN SV40-TRANSFORMED CELLS BY IMMUNOFLUORESCENCE. J Exp Med. 1964 Aug 1;120:121–128. doi: 10.1084/jem.120.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prives C., Gilboa E., Revel M., Winocour E. Cell-free translation of simian virus 40 early messenger RNA coding for viral T-antigen. Proc Natl Acad Sci U S A. 1977 Feb;74(2):457–461. doi: 10.1073/pnas.74.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prives C., Gluzman Y., Winocour E. Cellular and cell-free synthesis of simian virus 40 T-antigens in permissive and transformed cells. J Virol. 1978 Feb;25(2):587–595. doi: 10.1128/jvi.25.2.587-595.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROWE W. P., HARTLEY J. W., ESTES J. D., HUEBNER R. J. Studies of mouse polyoma virus infection. 1. Procedures for quantitation and detection of virus. J Exp Med. 1959 Apr 1;109(4):379–391. doi: 10.1084/jem.109.4.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassoulzadegan M., Seif R., Cuzin F. Conditions leading to the establishment of the N (a gene dependent) and A (a gene independent) transformed states after polyoma virus infection of rat fibroblasts. J Virol. 1978 Nov;28(2):421–426. doi: 10.1128/jvi.28.2.421-426.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif R., Cuzin F. Temperature-sensitive growth regulation in one type of transformed rat cells induced by the tsa mutant of polyoma virus. J Virol. 1977 Dec;24(3):721–728. doi: 10.1128/jvi.24.3.721-728.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D. T., Chang C., Martin M. A. Multiple forms of polyoma virus tumor antigens from infected and transformed cells. J Virol. 1979 Mar;29(3):881–887. doi: 10.1128/jvi.29.3.881-887.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D. T., Takemoto K. K., Martin M. A. Properties of simian virus 40 and BK virus tumor antigens form productively infected and transformed cells. Virology. 1978 Mar;85(1):137–145. doi: 10.1016/0042-6822(78)90418-x. [DOI] [PubMed] [Google Scholar]
- Simmons D. T., Takemoto K. K., Martin M. A. Relationship between the methionine tryptic peptides of simian virus 40 and BK virus tumor antigens. J Virol. 1977 Oct;24(1):319–325. doi: 10.1128/jvi.24.1.319-325.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto K. K., Malmgren R. A., Habel K. Heat-labile serum factor required for immunofluorescence of polyoma tumor antigens. Science. 1966 Sep 2;153(3740):1122–1123. doi: 10.1126/science.153.3740.1122. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P. Altered patterns of protein synthesis in infection by SV40 mutants. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):9–15. doi: 10.1101/sqb.1974.039.01.004. [DOI] [PubMed] [Google Scholar]
- VOGT M., DULBECCO R. Studies on cells rendered neoplastic by polyoma virus: the problem of the presence of virus-related materials. Virology. 1962 Jan;16:41–51. doi: 10.1016/0042-6822(62)90200-3. [DOI] [PubMed] [Google Scholar]