Abstract
The influence of topography on the biogeochemical cycle of mercury (Hg) has received relatively little attention. Here, we report the measurement of Hg species and their corresponding isotope composition in soil sampled along an elevational gradient transect on Mt. Leigong in subtropical southwestern China. The data are used to explain orography-related effects on the fate and behaviour of Hg species in montane environments. The total- and methyl-Hg concentrations in topsoil samples show a positive correlation with elevation. However, a negative elevation dependence was observed in the mass-dependent fractionation (MDF) and mass-independent fractionation (MIF) signatures of Hg isotopes. Both a MIF (Δ199Hg) binary mixing approach and the traditional inert element method indicate that the content of Hg derived from the atmosphere distinctly increases with altitude.
Mercury (Hg) is a persistent, semi-volatile element that can be ubiquitously detected throughout the world. Gaseous compounds dominate the pool of atmospheric Hg, with elemental Hg (Hg0) as the major constituent (>95%) and with gaseous oxidised compounds (GOM) and Hg bound to aerosols (Hg-p) as minor constituents1. Hg0 has a residence time in the atmosphere of 0.5 to 2 years1 and can be transported through the atmosphere far beyond the regions where it was emitted and thus deposited into pristine environments. Tremendous effort has been exerted in recent decades to understand the fate, transport and behaviour of Hg on both regional and global scales due to its potential adverse impacts on the health of humans and the environment1,2,3. In general, inorganic species predominate in the biogeochemical cycle of Hg, but various abiotic- and biotic-mediated pathways convert small amounts of Hg into neurotoxic methylated Hg (MeHg) species. Such organomercurials can effectively be bio-accumulated through aquatic food webs and even in some terrestrial plants (e.g., rice3), eventually posing a serious threat to humans through the consumption of fish and/or rice2.
Atmospheric Hg deposition is dominated largely by the physical scavenging of GOM and Hg-p1. Hg0 is known to exhibit bi-directional flux patterns and is associated with deposition velocities in the range of up to only a few mm s−1 (ref 4). In sharp contrast to Hg0, inorganic GOM species have intermediate vapour pressures (log10p (Pa) < 2) and partition favourably (i.e., Henry's law coefficient of ≥104 M atm−1) to the aqueous phase. The dominant global sink for atmospheric Hg0 is gas-phase oxidation yielding GOM species, with most of the oxidation occurring in the middle and upper troposphere5. This tropospheric pool of GOM may, through subsidence and other down flow processes, be an important source of Hg2+ deposition in high-altitude surface sites6. For example, recent studies have provided evidence of a free tropospheric source of Hg in wet deposition to the western United States7,8.
Alpine regions are generally considered to be vulnerable ecological environments because of their weak capabilities for self-purification and self-recovery. Previous studies have shown that environments in these regions are critically sensitive to atmospheric Hg deposition, especially topsoil and vegetation, which are regarded as effective carriers of atmospheric Hg deposition9,10. Furthermore, alpine regions exhibit substantial differences in their climatic, biological and environmental characteristics with altitude, such as an increased atmospheric deposition due to high surface roughness as well as increased precipitation and cloud water interception and lower soil/foliage emissions due to low temperatures11,12. Mechanisms driven by specific orographic conditions may thus act together to cause mountainous areas to become convergence zones for Hg (the ‘mountain trapping effect’).
Nevertheless, few studies investigating Hg have been conducted in appropriate mountainous areas along well-defined elevational transects9,10,13. Consequently, in some respects, our knowledge of the biogeochemical cycling of Hg in mountainous ecosystems remains limited. In recent decades, there has been great progress in simulations of the global/hemispheric or regional Hg distribution and examinations of the source-receptor relationship using various modelling systems (e.g., CMAQ-Hg14 and GEOS-Chem15), although surprisingly little attention appears to have been given to orographic effects. Ignoring the possibility of a mountain trapping effect on Hg may, however, hamper the validity of modelling results. Broadly defined, 27% of the Earth's landmass can be classified as mountainous, including plateaus and hills, and is inhabited by 22% of the world's population16. In China, mountains account for two-thirds of the total land area, with >50% composed of mountains and plateaus with elevations > 1,000 m a.s.l16.. Therefore, studies supplying the missing Hg pollution data for the mountain ecosystems in China and worldwide are of particular importance for evaluating the roles of these areas in global Hg distribution and cycling.
Soil compartments have typically been used to determine atmospheric contaminant deposition because the soil is the major terrestrial repository of contaminants, reflecting decades to centuries of wet and dry deposition8,17. A recent study on areal Hg mass conducted in 14 forests across the U.S. has shown that soil is the biggest terrestrial repository for Hg (90%), followed by litter (8%) and aboveground biomass (<1%)18. However, quantifying the Hg sources in soil contaminant pools from atmospheric input remains challenging because mountain soil Hg concentrations stem from both local mineral composition, as a natural background, and atmospheric input of natural and anthropogenic origins9.
Of potential benefit to our understanding of Hg sources has been research, in the past decade, into the isotopic dimension of environmental Hg cycling of Hg. This research has proven to be a powerful approach in tracing the sources of Hg and quantifying the physicochemical processes that affect Hg cycling. Recent studies have demonstrated that Hg isotope ratios vary widely among different source materials and that Hg isotopes can be systematically fractionated during specific environmental processes19,20,21. In addition to mass-dependent fractionation (MDF), mass-independent fractionation (MIF) of the odd-mass Hg isotopes (199Hg and 201Hg) may occur as a consequence of mechanisms such as the magnetic isotope effect (MIE)22,23 and nuclear volume effect (NVE)20,24. A combined analysis of MDF and MIF Hg signatures in topsoil samples can be used as an effective tracer for atmospheric sources25.
Mt. Leigong (‘god of thunder’) is the highest peak (2,179 m a.s.l., 26.39°N, 108.20°E) within the Miaoling Range of Guizhou Province, China (Fig. S1) and was selected for the present study. The mountain, which is regarded as holy by local ethnic minorities, is located within a National Nature Reserve (473 km2). Because the southwestern slope is the only slope with a relatively consistent gradient along its length11, it was used for sample collection. The bedrock on Mt. Leigong is primarily composed of low-grade metamorphic rock from the Pre-Sinian Age, and the soil type is dominated by ultisol26. The relative proximity to the South China Sea (~750 km) causes Mt. Leigong to be greatly affected by the summer monsoon, resulting in abundant rainfall (annual precipitation ranges from ~1250 mm in the lowland to >1600 mm in the summit zone). Following an altitudinal gradient, the mean annual temperature decreases by 0.46°C per 100 m of elevation gain to a low of 9.2°C in the highest zone12. The summit is frequently shrouded in persistent fog (~300 days yr−1), and the piedmont experiences fog on <25% of the days in the year11,26.
The present study was conducted principally to test the hypothesis that mountainous regions act as regional convergence zones for atmospheric Hg, analogous to classes of organic persistent pollutants27. The survey of Hg in the topsoil along an elevational transect is linked to a seasonally resolved monitoring program conducted at the summit that examines Hg in the air and Hg deposition10. Hg isotope signatures and inert element tracers were used to identify and quantify the Hg sources and to explore the possible underlying mechanisms of ‘mountain trapping effect’ of Hg.
Results
Hg levels and distribution along the elevation gradient
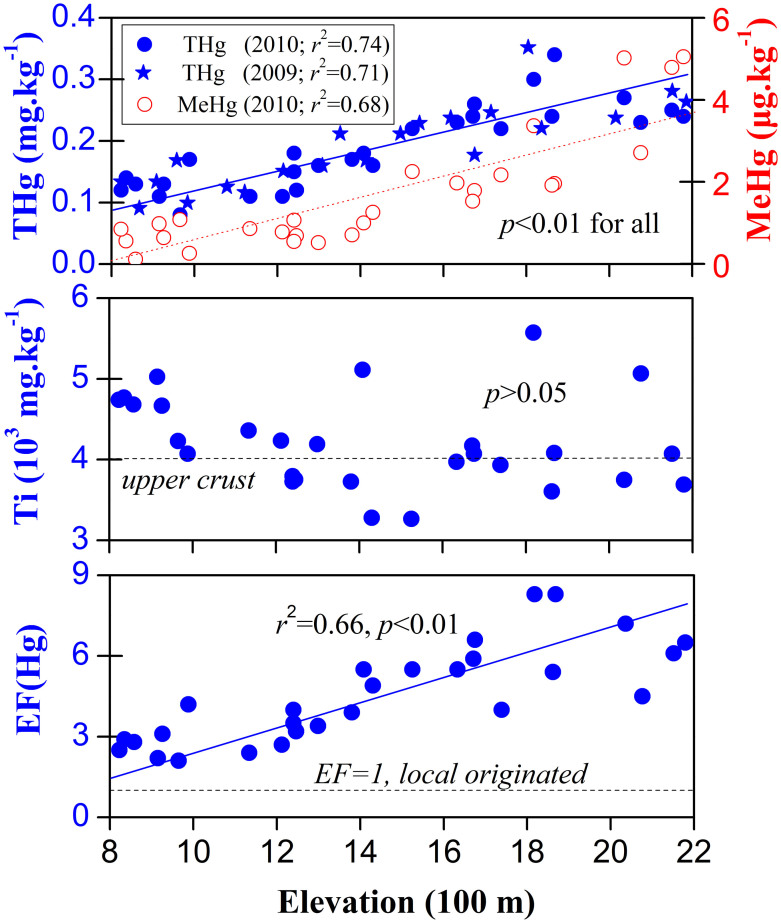
Elevated total Hg (THg) levels were observed in the topsoil samples compared with the gross background average of China (0.052 mg·kg−1 (ref 28)), with a mean (range) of 0.18 (0.07–0.34) mg·kg−1 and 0.20 (0.08–0.38) mg·kg−1 in samples collected in September in 2009 and 2010, respectively (Fig. 1). The mean (range) of the MeHg content in the corresponding samples for 2010 was 2.16 (0.26–5.05) μg.kg−1. No significant difference (ANOVA, p > 0.05) in the THg levels was observed between the samples collected at equal altitudes in 2009 and 2010; therefore, these data will be combined in the following discussion. The soil THg level (mean 0.19 mg·kg−1) for Mt. Leigong compares favourably with the distributions reported for forest soils from remote areas in the U.S. (0.15 mg·kg−1), Norway (0.19 mg·kg−1) and Sweden (0.25 mg·kg−1)9.
Figure 1. Scatter plots of soil THg and MeHg contents (upper panel), soil Ti content (centre panel) and calculated enrichment factors (EF(Hg) = (Hg/Ti)soil/(Hg/Ti)crust) (lower panel) versus elevation.
The THg and MeHg concentrations in the topsoil show an increasing trend with altitude (r2 = 0.68–0.71, p < 0.01 for both) (Fig. 1). The linear fits resulted in slopes of 0.12 μg.kg−1 m−1 and 3.1 ng.kg−1 m−1 for THg and MeHg, respectively. Both THg and MeHg concentrations in the soils near the summit increased to approximately three times higher than those in the piedmont areas. Furthermore, the THg concentration in the moss and litterfall samples exhibited similar trends with elevation (r2 = 0.33–0.39, p < 0.01 for both) (Fig. S2–S3). These results may indicate an altitudinal magnification effect (i.e., mountain trapping effect) of atmospheric Hg on Mt. Leigong. Several studies in high-altitude regions have revealed enhanced concentrations and deposition rates of Hg10,17,18. Hence, a ubiquitous phenomenon in which these regions function as regional convergence zones for atmospheric Hg may exist.
Hg isotope ratios distribution along the elevation gradient
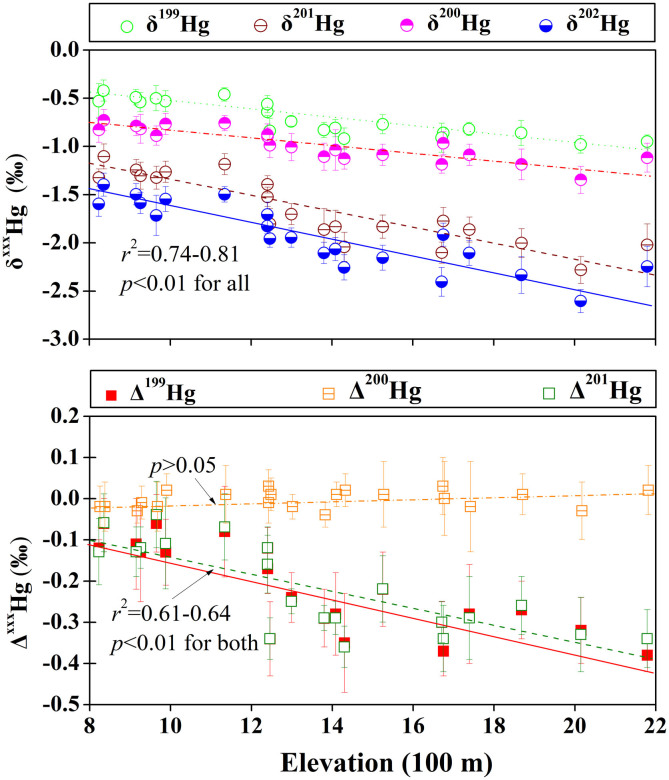
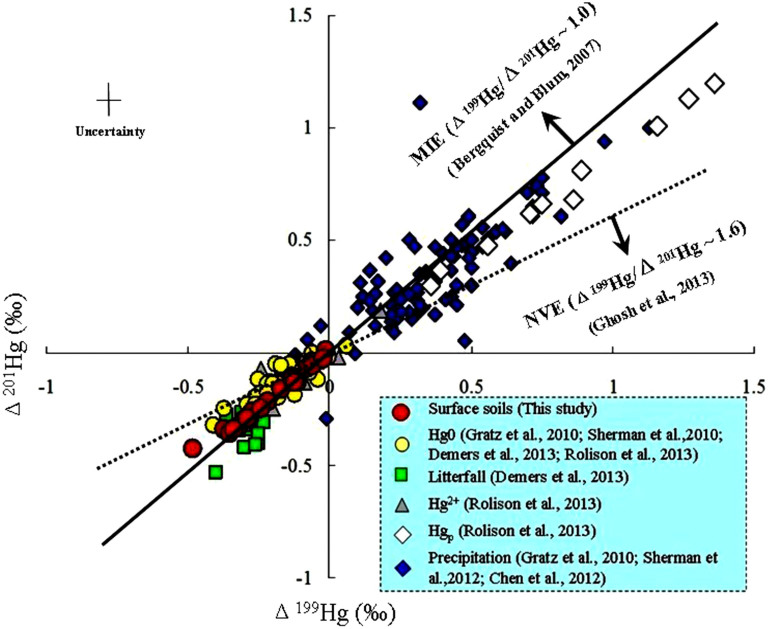
Significant MDF (within a 1.2‰ range for δ202Hg) and MIF (a 0.3‰ range for both Δ201Hg and Δ199Hg) signatures were observed in all soil samples (Fig. 2). A consistent negative trend with elevation was observed for the soil THg isotope ratio values (r2 = 0.61–0.82, p < 0.01) (Fig. 2), with the corresponding slopes (‰ per 100 m elevational gain) obtained by the linear fit of −0.039, −0.040, −0.083 and −0.083 for δxxxHg (xxx = 199, 200, 201 and 202, respectively) and of −0.020 and −0.021 for Δ199Hg and Δ201Hg, respectively. Furthermore, the Δ201Hg values were well correlated with the Δ199Hg values (r2 = 0.98, p < 0.01) (Fig. 3). No significant MIF of even isotopes (e.g., 200Hg and 204Hg) was observed in any of our investigated samples (lichen, soil and rock samples).
Figure 2. Scatter plots of mean δXXXHg (upper panel) and mean ΔxxxHg (MIF, lower panel) isotope ratios in surface soil versus elevation.
All error bars represent ± 2 s.d.
Figure 3. A comparison of the relationship between Δ199Hg and Δ201Hg from various studies (MIE = magnetic isotope effect; NVE = nuclear volume effect).
Tracing and quantifying the atmospheric Hg inputs in soil samples
(1). Inert element method. The inert element method was employed to differentiate between Hg sources in the Mt. Leigong soil samples. Titanium (Ti), a conservative element in the chemical weathering process, was selected as the reference element to calculate the Hg enrichment factor (EF) (defined as EF[Hg]) based on the surface soil and upper crust concentrations28 according to the following equations:
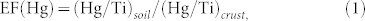 |
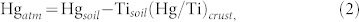 |
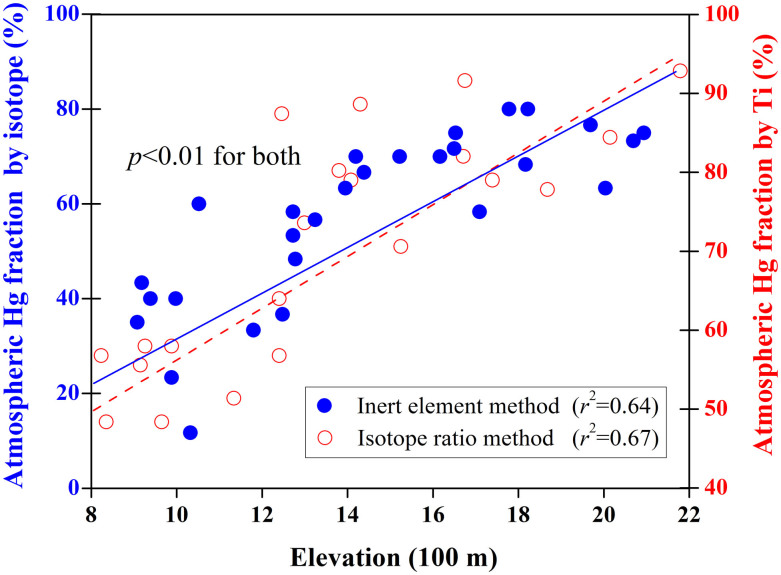
As Fig. 1 illustrates, the EF(Hg) was positively correlated with elevation (r2 = 0.66, p < 0.01). An EF(Hg) value closer to 1 indicates that the Hg in the soil is of geogenic origin. The trend of increasing EF(Hg) values with increased elevation suggests that non-geogenic sources of Hg (assumed to be primarily Hgatm, or atmospheric input) increase with elevation. The ratio of atmospheric Hg to THg in the soil progressively decreased from approximately 90% at the summit to less than 50% at the base of the mountain (r2 = 0.64, p < 0.01) (Fig. 4), suggesting a significant contribution from non-geogenic sources (largely atmospheric input) to the surface soil Hg on Mt. Leigong, particularly at higher elevations.
Figure 4. Predicted fractional contribution (%) of atmospheric input to the soil THg level as a function of elevation using the isotope ratio method (closed circles, left ordinate axis) and the inert element method (open circles, right ordinate axis).
(2). Hg isotope signature method. As suggested by Bergquist and Blum19, the application of stable Hg isotopes as a tool to discriminate the sources and transformations of Hg can be challenging, due to the fact that MDF signatures can result from numerous processes. Compared with MDF, MIF signatures are more specific to certain geochemical processes, and these might be identifiable by their Δ199Hg/Δ201Hg ratios. According to current knowledge, the MIF is expected to be unaltered by MDF processes, but can be changed by other MIF processes or through the mixing of Hg pools with different MIF signatures. Several studies have observed significant negative Δ199Hg values in epiphytic lichens used to identify atmospheric Hg deposition29,30,31. However, as shown in Fig. 3, recent studies on the direct analysis of atmospheric Hg species have showed positive Δ199Hg in particulate mercury (Hgp) andprecipitation, and slightly negative Δ199Hg in gaseous elemental Hg and its oxidised form (Hg2+)25,32,33,34,35. For example, Gratz et al32. collected precipitation and Hg0 samples in the Great Lakes region of the U.S. The precipitation samples showed a positive MIF (Δ199Hg: +0.04‰ to +0.52‰), whereas the Hg0 samples showed a negative MIF (Δ199Hg:−0.21‰ to +0.06‰). Sherman et al25. observed a slightly negative MIF (Δ199Hg: −0.11‰ to −0.22‰) in Hg0 collected from Arctic areas. Rolison et al35. investigated the isotopic composition of species-specific atmospheric Hg in a coastal environment of Grand Bay, Mississippi, U.S. According to their study, particulate Hg (Hgp) samples displayed a significant positive MIF (Δ199Hg: +0.36‰ to +1.36‰), reactive gaseous Hg2+ displayed an intermediate MIF (Δ199Hg: −0.28‰ to 0.18‰) and gaseous Hg0 (which contributed >95% of the total gaseous Hg) displayed a negative MIF (Δ199Hg: −0.41‰ to −0.03‰). These findings raise the question of the integrity of the Hg isotopes measured in lichens relative to atmospheric Hg, with the possibility of MIF during bioaccumulation. Terrestrial vegetation (including lichens) can accumulate Hg through the absorption of wet (e.g., precipitation) and dry (e.g., particulate Hg) atmospheric Hg deposition and through the incorporation of Hg0 through the stoma31. Atmospheric Hg can be fractionated during the process of incorporation by plants31,34, mosses and lichens29,36, with the lighter isotopes preferentially binding within the foliage. However, empirical evidence demonstrating a lack of MIF during metabolic processes has been shown in fish, microorganisms and terrestrial plants31,37,38. For example, Demers et al34. investigated the Hg isotopic composition in foliage samples of Aspen trees in a pristine forest in NE Wisconsin, U.S. They demonstrated an MDF of ~−3.0% in the δ202Hg values and a slight shift of ~−0.1% in the Δ199Hg values (perhaps due to the influence of the deposited Hgp) between the total atmospheric Hg pool (δ202Hg: −0.94‰ ± 0.35‰, 2 s.d., n = 12; Δ199Hg: −0.19‰ ± 0.04‰, 2 s.d., n = 12) and the foliage (δ202Hg: −2.14‰ ± 0.19‰, 2 s.d., n = 18; Δ199Hg: −0.30‰ ± 0.05‰, 2 s.d., n = 18). Yin et al31. measured the Hg isotopic composition in the rice foliage near Wanshan Mercury Mine in southwestern China. According to their study, an MDF of ~−3.0% in the δ202Hg value and insignificant MIF were observed between the Hg0 pool (δ202Hg: −2.15‰ ± 0.21‰, 2 s.d., n = 4; Δ199Hg: −0.29‰ ± 0.04‰, 2 s.d., n = 4) and the foliage (δ202Hg: −3.18‰ ± 0.21‰, 2 s.d., n = 6; Δ199Hg: −0.24‰ ± 0.08‰, 2 s.d., n = 6).
Litterfall is an important source of Hg in forest organic soils17,18. The uptake of isotopically lighter atmospheric Hg by plant leaves, followed by litterfall, has been suggested to be important for understanding Hg sources in forest regions34. Published data on forest organic soils demonstrates significant negative δ202Hg values and a negative MIF, similar to the Hg isotopic compositions of the organic soils on Mt. Leigong34,39. A recent study showed that the decomposition of foliage does not generate significant changes in the Hg isotopic composition34. Indeed, the lush vegetation in the Mt. Leigong forest areas is likely to have sequestered Hg through complexation with organic matter. On Mt. Leigong, the surface soils generally contain moderately high organic matter (6.1 ± 3.9%, with a maximum of 18%) resulting from partially decayed plant matter. The organic matter contents were significantly correlated with both THg and MeHg in the topsoil (r2 = 0.16, p < 0.05 for THg; r2 = 0.51, p < 0.01 for MeHg; the r2 for THg can be increased to 0.41 if the outliers are excluded; Fig. S4) and this is presumably due to the well-documented strong affinity of terrestrial Hg for organic matter3,17,18. The bedrocks of Mt. Leigong generally have a negligible OM content, suggesting that the Hg associated with organic matter in the soil mostly originates from the decomposition of foliage34. On Mt. Leigong, the surface soils (δ202Hg: −2.63‰ to −1.42‰; Δ199Hg: −0.38‰ to −0.06‰) fall in between the bedrock (δ202Hg: −0.92‰ to −0.86‰; Δ199Hg: −0.04‰ to +0.01) and moss samples (δ202Hg: −2.37‰ to −2.09‰; Δ199Hg: −0.48‰ to −0.39‰) with respect to isotopic signature. Hg in the moss samples favourably corresponds to recent data on lichens29,40 and plant leaves31,34, and this may indicate the mixing of decaying foliage with geogenic Hg sources. Hence, the application of a simple MIF mass balance model to the soil system using the following equations is considered to be valid:
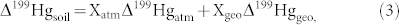 |
 |
where the ‘atm’ and ‘geo’ subscripts refer to the atmospheric (i.e., litterfall) and geogenic sources, respectively. The Δ199Hggeo is assumed to be zero given that MIF does not occur during the rock weathering process. According to the above-estimated isotopic compositions of the two end-members (the atmospheric and geogenic sources), the fraction of Hg that originated from the atmospheric source (Xatm) and “the geogenic source (Xgeo)” can be evaluated using equations 3 and 4.
As indicated in Fig. 4, the isotope method demonstrated a very similar trend to that of the inert element method and revealed a significant atmospheric fractionation (>80%) at high elevations, with less than 40% in the foothills (r2 = 0.64–0.67, p < 0.01 for both). However, the inert element method revealed a higher atmospheric fraction at low elevations. These results confirm our hypothesis that there is an altitudinal magnification effect (i.e., mountain trapping effect) on atmospheric Hg inputs in montane soils, which may be indicative of the fate and transport of Hg at a regional or global scale.
Simple MDF (δ202Hg) binary mixing models have been successfully used to estimate Hg pollution sources in many ecosystems40,41. In the present study, the plot of δ202Hg and 1/THg displayed a positive linear correlation (r2 = 0.48, p < 0.001) (Fig. S5), indicating the binary mixing of atmospheric Hg and geogenic Hg. A significant correlation (r2 = 0.68, p < 0.001) between δ202Hg and Xatm in the soil was observed (Fig. S6). Based on this correlation, the MDF signatures of the two end-members were obtained (Fig. S6). The δ202Hg from geogenic origin was approximately −1.38‰, and that from atmospheric deposition was approximately −2.50‰. The δ202Hg of the atmospheric Hg MDF compared to the observed δ202Hg in moss samples (δ202Hg of −2.21 ± 0.14‰, 2 s.d., n = 3) demonstrats that an MDF of −0.29 ± 0.14‰ may have occurred during the absorption of Hg by moss. Moss is an epiphytic plant that incorporates atmospheric Hg predominantly through the stoma. Isotope fractionations of heavy metals (e.g., Cu, Zn and Fe) have been demonstrated to occur, with a preferential translocation of light Hg isotopes to plants. Recent studies also demonstrated that plants (e.g., rice31 and lichen36) can preferentially incorporate light Hg isotopes during growth. The geogenic source of Hg is primarily derived from the weathering of bedrock. The type of bedrock in Mt. Leigong is uniform, and the average δ202Hg in the rock samples is −0.89 ± 0.10‰ (2 s.d., n = 2), which indicates that an MDF of −0.49 ± 0.10‰ in δ202Hg may occur during weathering. Mt. Leigong has a sub-tropical climate with an annual precipitation of 1250–1700 mm11. In such a wet climate, intense weathering may involve leaching. Recent leaching experiments of soils and Hg mine wastes clearly suggested that the more soluble Hg fractions are generally enriched with heavier Hg isotopes42. The δ202Hg of the atmospheric Hg (−2.50‰) is comparable with previous data on plants (e.g., rice31, aspen trees34 and lichen36), indicating that plants can preferentially incorporate light Hg isotopes during growth.
Discussion
Potential mechanisms for Hg isotope signatures in montane soils
Vertical variations of Hg isotopic composition in topsoils recorded in this study can be explained by isotope fractionation during Hg cycling in the forest ecosystem and/or mixing of Hg from different sources (e.g., atmospheric and geogenic origins). In mountain forest areas such as Mt. Leigong, the steep environmental gradients (e.g., temperature, precipitation and solar radiation) very likely influence the biogeochemical behaviour of Hg and lead to Hg isotope fractionation. The isotope fractionation of Hg in the Mt. Leigong elevation gradient may be a function of multiple physico-chemical processes, such as the evasion of Hg0 from soils, deposition of atmospheric Hg (e.g., precipitation, dry deposition and litterfall) and re-emission of wet-deposition Hg. To the best of our knowledge, the evasion of Hg from soils mainly involves processes such as photo-reduction22, volatilisation24,43 and the microbial reduction of soil Hg37. Generally, all these processes induce typical kinetic MDF values of Hg isotopes and produce Hg0 with significantly lower δ202Hg values than the original Hg2+. The photo-reduction of Hg may lead to the MIF of odd Hg isotopes20,22,44, whereas no significant MIF is recorded to be induced during volatilisation and microbial reduction processes24,37,43. A recent study by Demers et al34. also indicated that photo-reduction, volatilisation and microbial reduction could not be the major processes for the evaded Hg pool in forest areas. In soil humus such as that on Mt. Leigong, Hg binds strongly with thiols and other reduced sulphur groups associated with organic matter9. Soil evasion fluxes in pristine forest areas are generally extremely low because of the high organic matter content, suppression by leaf litter cover, and canopy shading34.
On Mt. Leigong, the surface soils have received Hg from geological sources (e.g., weathering) and atmospheric sources (dry and wet deposition). In this study, the Hg levels in two rock samples (0.10 ± 0.02 mg·kg−1, 2 s.d., n = 2) were lower than those in the soil samples. The δ202Hg (−0.89‰ ± 0.04, 2 s.d., n = 2) and the Δ199Hg (−0.02‰ ± 0.04, 2 s.d., n = 2) in the rock samples are consistent with the data from previous studies, as Hg in geogenic material (e.g., mineral deposits45, hydrothermal emissions45 and volcanoes46) generally have δ202Hg values of approximately −0.60‰, with no evidence of a significant MIF (Δ199Hg < 0.2‰)19.
Despite the proposed geogenic sources of Hg, atmospheric sources of Hg could also have been incorporated into the organic soils through wet (e.g., precipitation) and dry (e.g., particulate Hg and litterfall) atmospheric Hg deposition. Few studies have focused on the Hg isotope composition of precipitation and direct atmospheric Hg species25,32,33. It is worth noting that all of the described studies have demonstrated a significant MIF of even isotopes (e.g., 200Hg and 204Hg) in precipitation and direct atmospheric Hg samples25,32,33,34. Generally, Hg0 is characterised by negative Δ200Hg values, and precipitation (which contains mainly Hg2+ and Hgp) displays positive Δ200Hg values. In the present study, the absence of any MIF of even Hg isotopes in surface soils could be explained by the mixing of different atmospheric Hg species and precipitation. Alternatively, Chen et al33. suggested that the MIF of even Hg isotopes is likely linked to photo-initiated Hg0 oxidation, being controlled by stratosphere incursion, the presence of aerosols, oxidant intensity, solar irradiation and air mass movement. Several studies have reported near-zero Δ200Hg values in ambient gaseous Hg in the Great Lakes region32, the Arctic25 and near the Wanshan Mercury Mine31, indicating that photo-initiated Hg0 oxidation occurring in certain areas may not induce a significant MIF of even Hg isotopes. The mechanism for the MIF of even Hg isotopes is still unclear33, and further studies are needed.
In the present study, the significant negative MIF of odd Hg isotopes is established as an important feature of our investigated samples (i.e., lichens and surface soils). Two plausible mechanisms that might explain the odd-N MIF in this Hg isotope system include (1) the magnetic isotope effect (MIE)47 and (2) the nuclear volume effect (NVE)48. The Δ199Hg/Δ201Hg ratios of MIF produced by different mechanisms may be diagnostic. According to several recent studies, MIF occurring due to the NVE (e.g., Hg0 evaporation, abiotic dark reduction of Hg2+ and equilibrium Hg2+-thiol complexation) was estimated to result in a Δ199Hg/Δ201Hg ratio of 1.5 to 2.020,23,24. The MIE has been documented during photochemical reactions of aqueous Hg species (e.g., MeHg and Hg2+). When Δ199Hg and Δ201Hg values are plotted for each of these photochemical processes, CH3Hg+ and Hg2+ photo-reduction have slopes of 1.36 and 1.00, respectively22. The sign of MIF produced by MIE is dependent upon the type of organic ligand involved20,22,44. As shown in Fig. 3, the negative Δ199Hg values of the surface soils from Mt. Leigong indicate a deficit of odd isotopes, which is in close agreement with the direct/indirect air samples and surface soils from other regions in the world23. The slope of approximately 0.98 that was obtained here for Δ201Hg/Δ199Hg (which is not compatible with the NVE) indicates that a portion of Hg in the soil samples in this region may have undergone photo-reduction processes before being stored in continental pristine soils.
Potential mechanisms for Hg magnification in montane soils
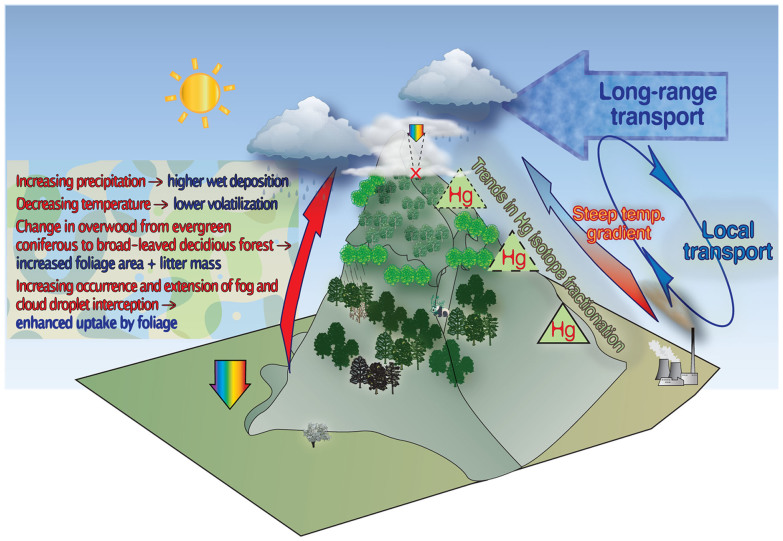
Whether the levels of Hg, a typical volatile pollutant, are increased in the montane soils at higher elevations in colder zones depends primarily on the distinction of the relationship between the ‘source’ and the ‘sink’ of Hg and the corresponding geochemical processes that are influenced by the elevation difference. A suite of controls that may cause the preferential accumulation of Hg at higher altitudes in the investigated montane area are depicted in Fig. 5 and are addressed in the following discussion.
Figure 5. Illustration of potential mechanisms for mercury magnification in montane soils (by Hua Zhang and Jonas Sommar).
(1). Litterfall. Litterfall is a critical Hg input to mountain forest ecosystems in autumn, when deciduous trees enter dormancy and their leaves senesce17. Hg0 flux measurements over deciduous forest ecosystems have indicated growing seasonal patterns from significant net deposition following leafing to net emission towards the end of the foliar season49,50. This finding primarily reflects the assimilation of Hg0 by the foliage via the stomata and cuticle over time. Furthermore, Hg tends to become enriched in forest litter compared with aboveground fresh foliage (50%–800%)17,18. Dry deposition (litterfall) can account for 40% and 80% of the total Hg mass entrainment in the forest soil in winter and spring, respectively17,18. In a previous communication10, we reported that Hg depositional fluxes in the summit zone of Mt. Leigong were, on a yearly basis, substantially dominated by litterfall with minor contributions from precipitation and throughfall (39.5, 6.1 and 10.5 μg.m−2.yr−1, respectively).
Moreover, the foliage/air partition coefficient increases with lower temperatures and higher elevations51 (i.e., plant leaves may retain more atmospheric Hg at higher elevations). In a broad survey of background US forests, Obrist et al. identified latitude, in addition to factors such as precipitation, as a suitable predictor of the Hg burden in litter17. The foliar uptake of atmospheric Hg mediated by intercepting cloud/fog water may also constitute a viable pathway for Hg accumulation. Although a firm conclusion is inhibited by the scarcity of observations, the Hg concentration in clouds and fogs has been observed to be elevated compared with that in precipitation8,52, especially in persisting and stationary fog52. The positive correlation (r2 = 0.33, p < 0.01; Fig. S3) between the Hg concentrations in leaf litter samples and elevation may reflect increased foliage uptake promoted by lower temperatures and extensive cloud water contact at higher elevations.
In the canopy flora of Mt. Leigong, there is a transition (1400–1800 m, evergreen coniferous species mixed with broad-leaved deciduous) from a domination of evergreen coniferous species in the foothills (<1400 m) to broad-leaved deciduous forests in the higher elevation zones (>1800 m)11,12 (Fig. 5), which suggests an increase in areal litterfall mass at higher elevations. Therefore, the higher litterfall mass combined with the higher Hg concentrations in the leaf litter at high elevations described above further suggest that litterfall decomposition may play an important role in the amplification of soil Hg on Mt. Leigong.
(2). Temperature. A certain proportion of the Hg deposited into the forests is in labile forms that can be re-emitted (as Hg0 after reduction) in direct competition to the process of incorporation with the soil matrix through complexation18. Hg0 emission fluxes from terrestrial surfaces are influenced by the substrate (e.g., soil) temperature7,17,18,53, with high temperatures facilitating Hg0 volatilisation53,54. In addition, the aqueous (photo-)reduction of Hg2+ to Hg0 is facilitated in warmer vegetation zones exposed to more abundant sunlight55. Hence, decreasing temperature at high elevations16 supports a suppression of the Hg0 air–surface exchange, thereby indirectly enhancing the retention of Hg in the soil compartment.
One process affecting certain persistent organic pollutants, termed the ‘grasshopper effect’, may also be engaged in the Hg enrichment process in montane soils. In this process, Hg evaporates from warmer zones in neighbouring lowlands (especially where pollution sources exist), travels through the atmosphere and is deposited in cooler, higher montane zones when the temperature drops. This process can be repeated in ‘hops’ (Fig. 5). However, the repeated mountain ‘hops’ (local transport) may be relatively limited and might insignificantly contribute to Hg enrichment in remote montane soils compared with long-range transport processes1,53. Additionally, a diurnal variation of wind patterns controlled by temperature has previously been proposed51 as an important driver of volatile pollutants in mountain regions. Specifically, more volatile contaminants are carried by upslope winds in warmer daytime temperatures than by downslope winds in cooler night-time temperatures.
(3) Precipitation. Orographic effects driven by temperature at higher elevations would result in greater Hg wet deposition due to higher precipitation relative to neighbouring lowlands8. Atmospheric hydrometeors (rain drops and snow) are very efficient scavengers of aerosol particles and ionic Hg species8. In the context of quantifying atmospheric wet deposition processes, the ratio of a chemical's concentration in precipitation to its concentration in the air is known as the scavenging ratio, W. The W values reported in the literature for Hg-p span a large range from 300 to 150056. Compared with Hg0 and Hg-p, GOM exhibits greater dry deposition velocities57. Even without experimental evidence, the W value of GOM applied in models is usually treated as that of an acidic gas (e.g., HNO3). Simple theoretical considerations have indicated that W is a function of inverse temperature. Drevnick et al. (2010)13 reported a significant increase in W with increasing altitude in the western U.S., which is consistent with our recent observations in southwestern China58. A recent study by Huang and Gustin (2012)7 also indicated higher levels of Hg wet deposition at sites with higher elevations.
On Mt. Leigong, precipitation increases with the altitudinal decrease of temperature (detailed in the Supporting Information)11,12. Significantly positive correlations between the soil Hg levels and precipitation or the inverse of temperature (r2 = 0.67–0.69, p < 0.01 for both) (Fig. S7) were observed in the present study, and these relationships may be indicative of the enhanced retention/deposition of Hg in high-elevation soils due to the temperature- and precipitation-related mechanisms described above. However, other mechanisms may also contribute to the altitudinal enrichment processes. For example, solar radiation has been suggested as a significant factor controlling the Hg flux between the soil and atmosphere59. Solar radiation decays with rising elevation on Mt. Leigong26 and in most alpine regions (due to increased cloud cover and an increased number of rainy days)16, thereby limiting direct photolytic degradation1, which, in turn, reduces the Hg emissions from the land surface to the atmosphere.
On Mt. Leigong, the temperature and solar radiation decrease and the precipitation, fog/cloud and air humidity increase with increasing elevation11,12,26. Hence, re-emission (the ‘grasshopper effect’) produces a negative influence on the sequestration of atmospheric Hg and decreases the Hg concentration, especially at low elevations. In contrast, scavenging of atmospheric Hg by precipitation and enhanced litterfall provoke increases in the soil Hg concentration at higher altitudes. These processes may be the main reasons that explain the increased Hg concentrations in soil samples with rising elevation.
Implications for regional or global Hg cycling
A negative elevational dependence was observed in the MDF and MIF signatures of Hg isotopes. The application of a MIF (Δ199Hg) binary mixing approach and the traditional inert element method unanimously indicated that the fraction of Hg derived from the atmosphere distinctly increased with altitude. Our study, for the first time, demonstrates that a ‘mountain trapping effect’ of semi-volatile Hg can occurs in montane environments and provides a systematic discussion of the possible mechanisms. Mercury magnification in high-elevation montane soils is likely driven by the altitudinal dependence of temperature, precipitation, litterfall and other factors (e.g., solar radiation), Of these factors, litterfall may be the most critical. Our observations infer that previous studies on regional or global Hg cycles/distribution may have significantly underestimated the Hg mass trapped by mountainous regions, as mountains account for a significant proportion of the global terrestrial area. Our study shows that Hg stable isotope ratios can be used to track atmospheric Hg deposition in upland forest systems. This technique may be useful in future studies that assess environmental changes in montane forest ecosystems.
Methods
The THg concentrations were measured in the soil and rock samples using cold-vapour atomic absorption spectrometry (CVAAS), and the THg concentrations in the moss and leaf litter samples were determined using the dual-stage gold amalgamation method and cold-vapour atomic fluorescence spectrometry (CVAFS) detection following USEPA method 1631. The soil MeHg concentrations were determined using aqueous ethylation, purge, trap and GC CVAFS detection following USEPA method 1630. The Hg isotopic ratios were determined with MC-ICP-MS using a Nu-Plasma mass spectrometer equipped with 12 Faraday cups. The quality-control system for the Hg concentration analyses consisted of method blanks, blank spikes, matrix spikes, certified reference materials and blind duplicates. The reproducibility of the isotopic data was assessed after measuring replicate sample digests (typically n = 2). We also analysed the UM-Almadén as a secondary standard (once every 10 samples) in addition to the bracketing standard NIST 3133. The Hg concentration in the UM-Almadén was measured with the same method as other samples in each analytical session.
Detailed information regarding the site description, sample collection and preparation, analysis methods for determining the Hg species concentration and the Hg isotope ratio, quality assurance and control, and climatic parameter estimation methods is provided in the Supplementary Information.
Author Contributions
H.Z., R.S.Y., X.B.F. and T.L. conceived the project. H.Z., R.S.Y., X.W.F. and A.S. organised the sampling. H.Z. and R.S.Y. measured the Hg species concentrations and the Hg isotope ratios. H.Z., R.S.Y., X.B.F., T.L., C.W.N.A. and J.S. analysed and interpreted the data. H.Z. and R.S.Y. wrote the paper with comments from all authors.
Supplementary Material
Supplemental Material
Acknowledgments
This work was funded by the “973” Program (2013CB430003), the Youth Innovation Promotion Association from the Chinese Academy of Sciences, the Natural Science Foundation of China (40825011 and 41203092) and the Sino-Norwegian cooperation project ‘Capacity building for reducing mercury pollution in China - a case study in Guizhou province’, which was funded by the Norwegian Government.
References
- Schroeder W. H. & Munthe J. Atmospheric mercury - An overview. Atmos. Environ. 32, 809–822 (1998). [Google Scholar]
- Zhang H., Feng X., Larssen T., Qiu G. & Vogt R. D. In Inland China, Rice, Rather than Fish, Is the Major Pathway for Methylmercury Exposure. Environ. Health Perspect. 118, 1183–1188 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Feng X., Larssen T., Shang L. & Li P. Bioaccumulation of Methylmercury versus Inorganic Mercury in Rice (Oryza sativa L.) Grain. Environ. Sci. Technol. 44, 4499–4504 (2010). [DOI] [PubMed] [Google Scholar]
- Sommar J., Zhu W., Lin C.-J. & Feng X. Field approaches to measure mercury exchange between natural surfaces and the atmosphere–a review. Cri. Rev.Env. Sci. Tec. 43, 1657–1739 (2013). [Google Scholar]
- Holmes C. D. et al. Global atmospheric model for mercury including oxidation by bromine atoms. Atmo. Chem. Phys. 10, 12037–12057 (2010). [Google Scholar]
- Fain X., Obrist D., Hallar A. G., McCubbin I. & Rahn T. High levels of reactive gaseous mercury observed at a high elevation research laboratory in the Rocky Mountains. Atmo. Chem. Phys. 9, 8049–8060 (2009). [Google Scholar]
- Huang J. Y. & Gustin M. S. Evidence for a Free Troposphere Source of Mercury in Wet Deposition in the Western United States. Environ. Sci. Technol. 46, 6621–6629 (2012). [DOI] [PubMed] [Google Scholar]
- Stankwitz C., Kaste J. M. & Friedland A. J. Threshold Increases in Soil Lead and Mercury from Tropospheric Deposition Across an Elevational Gradient. Environ. Sci. Technol. 46, 8061–8068 (2012). [DOI] [PubMed] [Google Scholar]
- Szopka K., Karczewska A. & Kabala C. Mercury accumulation in the surface layers of mountain soils: A case study from the Karkonosze Mountains, Poland. Chemosphere 83, 1507–1512 (2011). [DOI] [PubMed] [Google Scholar]
- Fu X. W. et al. Atmospheric gaseous elemental mercury (GEM) concentrations and mercury depositions at a high-altitude mountain peak in south China. Atmos. Chem. Phys. 10, 2425–2437 (2010). [Google Scholar]
- Jin H. T. Distribution Characteristics of Precipitation in Mt. Leigong. Journal of Guizhou Meteorology 2, 34–38 (1990). [Google Scholar]
- Xiong Y. H., Yang S. Z. & Yang J. Temperature trend in recent 46a years in Mt. Leigong. Journal of Guizhou Meteorology 32, 21–23 (2008). [Google Scholar]
- Drevnick P. E. et al. Mercury Flux to Sediments of Lake Tahoe, California-Nevada. Water Air Soil Poll. 210, 399–407 (2010). [Google Scholar]
- Lin C. J. et al. Estimating mercury emission outflow from East Asia using CMAQ-Hg. Atmos. Chem. Phys. 10, 1853–1864 (2010). [Google Scholar]
- Jaeglé L., Strode S. A., Selin N. E. & Jacob D. J. (The Geos-Chem model). in Mercury Fate and Transport in the Global Atmosphere (eds Pirrone N., & Mason R.) 533–545 (Springer, 2009). [Google Scholar]
- Fu B. & Yu J. Mountain climate Resources and Development 93–126 (Nanjing University Press, Nanjing, 1996). [Google Scholar]
- Obrist D. et al. Mercury Distribution Across 14 U.S. Forests. Part I: Spatial Patterns of Concentrations in Biomass, Litter, and Soils. Environ. Sci. Technol. 45, 3974–3981 (2011). [DOI] [PubMed] [Google Scholar]
- Obrist D. Mercury Distribution across 14 U.S. Forests. Part II: Patterns of Methyl Mercury Concentrations and Areal Mass of Total and Methyl Mercury. Environ. Sci. Technol. 46, 5921–5930 (2012). [DOI] [PubMed] [Google Scholar]
- Bergquist R. A. & Blum J. D. The Odds and Evens of Mercury Isotopes: Applications of Mass-Dependent and Mass-Independent Isotope Fractionation. Elements 5, 353–357 (2009). [Google Scholar]
- Zheng W. & Hintelmann H. Nuclear Field Shift Effect in Isotope Fractionation of Mercury during Abiotic Reduction in the Absence of Light. J. Phys. Chem. A 114, 4238–4245 (2010). [DOI] [PubMed] [Google Scholar]
- Blum J. in Handbook of Environmental Isotope Geochemistry Advances in Isotope Geochemistry(ed Baskaran) Ch. 12, 229–245 (Springer Berlin Heidelberg, 2012). [Google Scholar]
- Bergquist B. A. & Blum J. D. Mass-dependent and -independent fractionation of Hg isotopes by photoreduction in aquatic systems. Science 318, 417–420 (2007). [DOI] [PubMed] [Google Scholar]
- Sonke J. E. A global model of mass independent mercury stable isotope fractionation. Geochim. Cosmochim. Acta 75, 4577–4590 (2011). [Google Scholar]
- Estrade N., Carignan J., Sonke J. E. & Donard O. F. X. Mercury isotope fractionation during liquid-vapor evaporation experiments. Geochim. Cosmochim. Acta 73, 2693–2711 (2009). [Google Scholar]
- Sherman L. S. et al. Mass-independent fractionation of mercury isotopes in Arctic snow driven by sunlight. Nature Geoscience 3, 173–177 (2010). [Google Scholar]
- Yang S. L. Protection and Rational Utilization of Natural Resources from the vertical natural zones in Mt. Leigong. J. Guizhou Normal Univ. (Natural Sciences) 2, 34–38 (1990). [Google Scholar]
- Loewen M. D. et al. Persistent organic pollutants and mercury in the Himalaya. Aquat. Ecosyst. Health Manag. 8, 223–233 (2005). [Google Scholar]
- Wedepohl K. H. The compositon of the continental-crust. Geochim. Cosmochim. Acta 59, 1217–1232 (1995). [Google Scholar]
- Carignan J., Estrade N., Sonke J. E. & Donard O. F. X. Odd Isotope Deficits in Atmospheric Hg Measured in Lichens. Environ. Sci. Technol. 43, 5660–5664 (2009). [DOI] [PubMed] [Google Scholar]
- Estrade N., Carignan J. & Donard O. F. X. Isotope tracing of atmospheric mercury sources in an urban area of northeastern France. Environ. Sci. Technol. 44, 6062–6067 (2010). [DOI] [PubMed] [Google Scholar]
- Yin R., Feng X. & Meng B. Stable Mercury Isotope Variation in Rice Plants (Oryza sativa L.) from the Wanshan Mercury Mining District, SW China. Environ. Sci. Technol. 47, 2238–2245 (2013). [DOI] [PubMed] [Google Scholar]
- Gratz L. E., Keeler G. J., Blum J. D. & Sherman L. S. Isotopic composition and fractionation of mercury in Great Lakes precipitation and ambient air. Environ. Sci. Technol. 44, 7764–7770 (2010). [DOI] [PubMed] [Google Scholar]
- Chen J., Hintelmann H., Feng X. & Dimock B. Unusual fractionation of both odd and even mercury isotopes in precipitation from Peterborough, ON, Canada. Geochim. Cosmochim. Acta 90, 33–46 (2012). [Google Scholar]
- Demers J. D., Blum J. D. & Zak D. R. Mercury isotopes in a forested ecosystem: Implications for air-surface exchange dynamics and the global mercury cycle. Global Biogeochemical Cycles 27, 222–238 (2013). [Google Scholar]
- Rolison J. M., Landing W. M., Luke W., Cohen M. & Salters V. J. M. Isotopic composition of species-specific atmospheric Hg in a coastal environment. Chemical Geology 336, 37–49 (2013). [Google Scholar]
- Estrade N., Carignan J. & Cloquet C. Mercury isotope fractionation during bio-accumulation in lichens. Goldschmidt Conference Abstracts. A818. (2011).Available: http://goldschmidt.info/2011/abstracts/finalPDFs/818.pdf [accessed 31 Oct. 2013]. [Google Scholar]
- Kritee K., Barkay T. & Blum J. D. Mass dependent stable isotope fractionation of mercury during mer mediated microbial degradation of monomethylmercury. Geochim. Cosmochim. Acta 73, 1285–1296 (2009). [Google Scholar]
- Kwon S. Y. et al. Absence of fractionation of mercury isotopes during trophic transfer of methylmercury to freshwater fish in captivity. Environ. Sci. Technol. 46, 7527–7534 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas A., Blum J. D., Bergquist B. A., Keeler G. J. & Xie Z. Natural Mercury Isotope Variation in Coal Deposits and Organic Soils. Environ. Sci. Technol. 42, 8303–8309 (2008). [DOI] [PubMed] [Google Scholar]
- Estrade N., Carignan J. & Donard O. F. X. Tracing and Quantifying Anthropogenic Mercury Sources in Soils of Northern France Using Isotopic Signatures. Environ. Sci. Technol. 45, 1235–1242 (2011). [DOI] [PubMed] [Google Scholar]
- Feng X. et al. Tracing Mercury Contamination Sources in Sediments Using Mercury Isotope Compositions. Environ. Sci. Technol. 44, 3363–3368 (2010). [DOI] [PubMed] [Google Scholar]
- Yin R. et al. Mercury isotope variations between bioavailable mercury fractions and total mercury in mercury contaminated soil in Wanshan Mercury Mine, SW China. Chem. Geol. 336, 80–86 (2013). [Google Scholar]
- Zheng W., Foucher D. & Hintelmann H. Mercury isotope fractionation during volatilization of Hg(0) from solution into the gas phase. J. Anal. At. Spectrom. 22, 1097–1104 (2007). [Google Scholar]
- Zheng W. & Hintelmann H. Mercury isotope fractionation during photoreduction in natural water is controlled by its Hg/DOC ratio. Geochim. Cosmochim. Acta 73, 6704–6715 (2009). [Google Scholar]
- Smith C. N., Kesler S. E., Klaue B. & Blum J. D. Mercury isotope fractionation in fossil hydrothermal systems. Geology 33, 825–828 (2005). [Google Scholar]
- Zambardi T., Sonke J. E., Toutain J. P., Sortino F. & Shinohara H. Mercury emissions and stable isotopic compositions at Vulcano Island (Italy). Earth. Planet. Sci. Lett. 277, 236–243 (2009). [Google Scholar]
- Buchachenko A. L., Lukzen N. N. & Pedersen J. B. On the magnetic field and isotope effects in enzymatic phosphorylation. Chem. Phys. Lett. 434, 139–143 (2007). [Google Scholar]
- Schauble E. A. Role of nuclear volume in driving equilibrium stable isotope fractionation of mercury, thallium, and other very heavy elements. Geochim. Cosmochim. Acta 71, 2170–2189 (2007). [Google Scholar]
- Poissant L., Pilote M., Yumvihoze E. & Lean D. Mercury concentrations and foliage/atmosphere fluxes in a maple forest ecosystem in Québec, Canada. J. Geophys. Res. Atmos 113, D10307 (2008). [Google Scholar]
- Bash J. O. & Miller D. R. Growing season total gaseous mercury (TGM) flux measurements over an Acer rubrum L. stand. Atmos. Environ. 43, 5953–5961 (2009). [Google Scholar]
- Daly G. L. & Wania F. Organic contaminants in mountains. Environ. Sci. Technol. 39, 385–398 (2005). [DOI] [PubMed] [Google Scholar]
- Ritchie C. D., Richards W. & Arp P. A. Mercury in fog on the Bay of Fundy (Canada). Atmospheric Environment 40, 6321–6328 (2006). [Google Scholar]
- Fu X., Feng X. & Wang S. Exchange fluxes of Hg between surfaces and atmosphere in the eastern flank of Mount Gongga, Sichuan province, southwestern China. J. Geophys. Res. 113, D20306 (2008). [Google Scholar]
- Moore C. & Carpi A. Mechanisms of the emission of mercury from soil: role of UV radiation. J. Geophys. Res. Atmos 110, 9 pp.–9 pp (2005). [Google Scholar]
- Schluter K. Review: evaporation of mercury from soils. An integration and synthesis of current knowledge. Environ. Geol. 39, 249–271 (2000). [Google Scholar]
- Steding D. J. & Flegal A. R. Mercury concentrations in coastal California precipitation: Evidence of local and trans-Pacific fluxes of mercury to North America. J. Geophys. Res. 107, 4764 (2002). [Google Scholar]
- Zhang L., Wright L. P. & Blanchard P. A review of current knowledge concerning dry deposition of atmospheric mercury. Atmos. Environ. 43, 5853–5864 (2009). [Google Scholar]
- Fu X. et al. Elevated atmospheric deposition and dynamics of mercury in a remote upland forest of southwestern China. Environ. Pollut. 158, 2324–2333 (2010). [DOI] [PubMed] [Google Scholar]
- Wang S. F., Feng X. B., Qiu G. L., Wei Z. Q. & Xiao T. F. Mercury emission to atmosphere from Lanmuchang Hg-Tl mining area, Southwestern Guizhou, China. Atmos. Environ. 39, 7459–7473 (2005). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material