Summary
Background
Intensity modulated radiotherapy for stage III lung cancer has become commonplace in the United States in the absence of randomized controlled trials. We used a large, population-based database to determine which factors led to increased utilization of IMRT and to evaluate associations of IMRT with toxicities.
Methods
The Surveillance, Epidemiology, and End Results (SEER)-Medicare records identified 3,986 individuals aged 66 years or older diagnosed with stage III lung cancer between 2001 and 2007 and treated with IMRT or 3D conformal radiotherapy. Predictors of IMRT use were determined using logistic regression. Associations of IMRT use with diagnosis codes for radiation-related toxicities were evaluated with multivariate proportional hazards regression and propensity-score matching.
Results
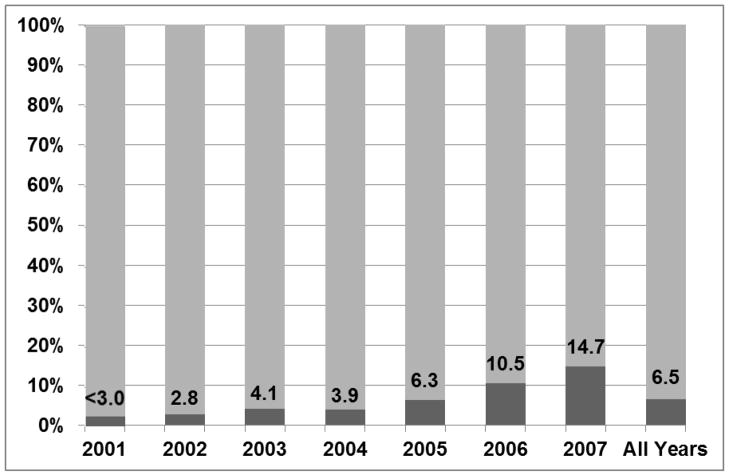
Among the 3,986 patients studied, the median age was 75 years, 54.1% were male, and 62% had IIIA disease. Two hundred fifty seven (6.5%) patients received IMRT, with use increasing from from 0.5% in 2001 to 14.7% in 2007 (P<0.001). Key predictors of IMRT delivery included increasing year of diagnosis and treatment in a freestanding center (odds ratio, 2.10; 95% confidence interval [CI], 1.59–2.77, P<0.001); tumor size, stage, and number of radiotherapy fractions delivered were not associated with IMRT use. IMRT use was not associated with a higher burden of lung or esophagus toxicities when compared to 3DCRT.
Conclusion
These findings suggest that practice environment strongly influenced adoption of IMRT for lung cancer. Patient and tumor factors were not significant predictors of IMRT use. Esophagus and lung toxicity rates were similar between IMRT and 3DCRT.
Keywords: non-small cell lung cancer, technology utilization, IMRT, radiation technique, comparative effectiveness
Introduction
The incidence of stage III non-small cell lung cancer (NSCLC) is likely to rise considerably in the coming decade due to demographic trends [1]. The majority of these patients will require radiation for definitive therapy or as an adjuvant to surgical management [2–4]. In contemporary practice, radiotherapy is mostly delivered with one of two technologies: 3D conformal radiotherapy (3DCRT) or intensity-modulated radiotherapy (IMRT). In 3DCRT, axial imaging is used to target a tumor with several radiation fields, whose sizes, shapes, and angles of entry are selected by a radiation oncologist. With IMRT, the radiation oncologist instead delineates a volume containing the tumor. This volume is then targeted by many small beamlets whose contributions are determined by computer algorithm. Given the disparity in how each technique is implemented, it cannot be taken for granted that the two technologies will yield equivalent outcomes.
There are no prospective trials comparing the two techniques for any thoracic malignancy. In the absence of phase III data, one hopes that sound clinical rationale accounts for the choice of radiotherapy, but other factors may play a role. These factors include perceived dosimetric advantages [5, 6], accessibility of technology [7], financial considerations [8, 9], a desire to escalate dose [10], or a need to meet normal organ dose constraints [11–14]. Determining which of these issues influences everyday practice is important as the selection of radiation technique can have far-reaching consequences for patients and the health care system.
Population-based data can generate hypotheses regarding the factors that promote or slow the adoption of advanced technologies. Furthermore, this data can be used to evaluate the clinical impact of those technologies after their introduction. To that end, we performed a population-based analysis using the Surveillance, Epidemiology, and End Results (SEER)-Medicare database to identify predictors of IMRT use and associations of IMRT use with radiation-related toxicities among patients with stage III NSCLC diagnosed from 2001–2007. Specifically, we sought to determine the extent to which clinical versus non-clinical factors influenced adoption of IMRT and to compare acute pulmonary and esophageal toxicities associated with IMRT versus 3DCRT.
Methods
Data Source and Study Sample
The Surveillance, Epidemiology, and End Results (SEER)-Medicare database captures claims data for cancers diagnosed in Medicare beneficiaries who reside within 16 geographic catchment areas representing 26% of the US population. The case ascertainment rate for the SEER data is approximately 98% [15]. In this study, demographic and tumor characteristics for incident malignancies diagnosed from January 1, 2001 to December 31, 2007 were linked to Medicare claims from January 1, 2000 to December 31, 2009.
From 2001–2007, 113,681 patients aged ≥ 66 years without prior malignancy were diagnosed with NSCLC and reported in the SEER-Medicare cohort. From this population, patients with pathologically-confirmed, stage III disease were selected for analysis (Supplementary Table 1). Patients were excluded from this study if they did not have complete Medicare Part A and B records from 12 months prior to diagnosis to 6 months after diagnosis (or until death); or if they had health maintenance organization (HMO) coverage within the same timeframe (Supplementary Table 1). Patients with any second cancer diagnosed within 6 months of the index lung cancer were also excluded as billing records could not discriminate between procedures performed for the index cancer versus the second cancer.
Because our goal was to compare patients treated with 3DCRT and IMRT, patients treated with other radiation modalities (proton therapy, brachytherapy, and 2-D radiotherapy) were excluded from the analysis. Finally, to ensure that radiotherapy was not directed at metastatic targets, we excluded patients with diagnosis codes for brain metastasis, adrenal, bone or liver metastases submitted in the period two weeks before the date of diagnosis until the start of radiotherapy. These criteria yielded a final sample of 3,986 patients (Supplementary Table 1).
Treatment Strategies
Medicare claims using International Classification of Diseases, 9th Revision (ICD-9) and Clinical Modification and Current Procedural Terminology/Healthcare Common Procedure Coding System (CPT) codes were utilized to extract claims for diagnostic procedures, treatments, and toxicity outcomes. Therapies occurring within 6 months of diagnosis were considered to be part of the initial treatment strategy (Supplementary Table 2). We classified patients as having received IMRT if a claims code confirming actual delivery of intensity-modulated treatment (77418, 0073T, G0174) was present. Three-dimensional conformal radiation was defined by the presence of both a claim for “three dimensional reconstruction of the tumor volume” (77295) and non-IMRT external beam radiation delivery (77402–77416)[16]. The number of radiotherapy fractions was calculated by counting the number of unique CPT codes for radiation delivery using a time window from the start of radiation delivery until 3 months thereafter. Based on treatment claims, the overall treatment strategy was stratified into four categories: trimodality therapy, chemotherapy and radiation, surgery and radiation, and radiation alone.
Other Covariates
Patient demographic variables from the SEER data included age at diagnosis, race, and gender. Baseline patient characteristics were determined using Medicare claims from an interval of 12 months before to 1 month after diagnosis [17]. The Charlson comorbidity index with Klabunde modification was determined from ICD-9 codes using published methods [18–20]. Chronic obstructive pulmonary disease (COPD) (491.2x, 493.2, 496) was not included in the index and was reported separately. Patients were classified as oxygen users if durable medical equipment claims included oxygen equipment. Using the method of Davidoff et al., a performance status covariate was generated using claims for medical assistance services or devices (canes, walkers, home hospital beds, or home health care) [21].
Tumor characteristics extracted from the SEER data included AJCC version 6 stage (IIIA, IIIB), laterality, and lung subsite [22]. Tumor size classifications are based upon maximum length of the tumor in centimeters and stratifications were applied using AJCC version 7 T-stage thresholds; invasion of local structures is not reflected in the tumor size classifications. To adjust for stage migration, the use of mediastinal sampling and positron emission tomography (PET) within a time period extending from 2 weeks prior to diagnosis to the start of radiotherapy were assessed (Supplementary Table 2).
Practice environment characteristics reflective of the patient’s county of residence were evaluated. Year of diagnosis, geographic region, and whether the setting was urban or rural were obtained from the SEER data. County-level density of radiation oncologists was determined using the Area Resource File for 2001–2005 in accordance with published methods [8]. The type of treatment center was determined from claims for radiation delivery, also in accordance with published methods [8, 23, 24].
Toxicity Outcomes
Toxicities were determined from Medicare claims (Supplementary Table 3). We evaluated the incidence of lung toxicity using two definitions. A narrow definition only included the ICD-9 diagnosis code for “unspecified acute pulmonary toxicity due to radiation”. The broad definition also included claims codes for nonspecific lung infiltrates (ie, not attributed to volume overload and no infectious organism identified). Acute esophagus toxicity was defined using diagnosis codes for esophagitis, dehydration, feeding tube placement, and mucositis. These components were analyzed individually and in aggregate.
In accordance with the natural history of radiation toxicities, toxicities were scored if the relevant claims code was submitted within 8 months after the start of radiotherapy in order to capture 2 months of radiation therapy and 6 months of followup. Sensitivity analyses were performed wherein the 8-month cutoff was shortened to 4 months and extended to 14 months. Due to a low number of toxic events, chronic toxicities were not able to be robustly studied using this data set.
Statistical Analysis
Predictors for IMRT use were determined using logistic regression. Bivariate associations at a significance level of 0.20 or less were included in an initial multivariable logistic regression model. The model was modified using backwards elimination of all predictor variables whose removal did not diminish the fit of the model (P > 0.05). Initially excluded covariates were then re-assessed using forwards elimination to generate a final model. Goodness-of-fit was assessed using the Hosmer-Lemeshow test.
In the second part of our analysis, the association of radiotherapy technique with acute toxicities was assessed. The cumulative incidence of adverse events was determined using the Kaplan-Meier method with censorship at the earliest of the following: loss of Medicare coverage, conversion to HMO coverage, death, or the end of the study period. For each endpoint, the proportional hazards assumption with respect to radiation technique was tested visually by inspection of log-log plots and analytically using Schoenfield residuals. The relationship between radiation technique and toxicity was determined with multivariate regression adjusted for factors that were considered statistically significant at P<0.20 in univariate analyses. Clinically important covariates including age, oxygen status, performance status, stage, number of radiation treatments, and treatment strategy were included in the final model regardless of their unadjusted P values. The model was stratified by SEER region and the year of diagnosis to account for variations with geography and time. Prespecified interaction terms were used to determine whether the effect of radiation technique differed with respect to the clinically important covariates listed above. The Gronnesby and Morgan test was used to assess goodness-of-fit for final models.
Propensity-score matching was also performed to validate the findings of the multivariate regression. Propensity scores were generated using a logistic model with radiation technique as the dependent variable. The independent variables included all other available covariates. Patients were matched 1:1 using nearest neighbor technique with caliper distance limited to 25% of the standard deviation of the pooled propensity scores. Standardized difference was used to assess covariate balance between cohorts with a threshold of 0.20 [25]. Proportional hazards models, stratified by matched pair [26] and adjusted for unbalanced covariates, were generated to compare the cohorts. Because combined chemotherapy and radiation is the dominant strategy for stage III NSCLC patients, we performed a sensitivity analysis wherein propensity-score matching was limited to patients treated with this strategy.
All statistical analyses were 2-sided with P ≤ 0.05 and conducted using STATA v.10 (College Station, TX). Our institutional review board granted this study exempt status.
Results
Predictors of IMRT Use
Among the 3,986 patients, median age was 75 years, 54.1% were male, and 62% had IIIA disease (Table 1). IMRT use rose from 0.5% in 2001 to 14.7% in 2007 (Figure 1) (P<0.0001). Multivariate logistic regression identified three predictors for IMRT use (Goodness-of-fit P=0.39). As expected, IMRT use rose with the year of diagnosis. IMRT use was also associated with certain SEER geographic regions. The New Jersey registry was selected as the referent group because it contained the most patients and because the proportion receiving IMRT in this registry (6.8%) approximated the proportion in the entire cohort (6.5%). Compared to New Jersey, IMRT was used less often in Connecticut, Iowa, Georgia, and Kentucky and more often in New Mexico and Los Angeles (Table 2). Treatment in a freestanding center was strongly associated with IMRT use (odds ratio, 2.10; 95% confidence interval [CI], 1.59–2.77, P<0.001). After multivariate adjustment, patient, tumor, diagnostic, and treatment covariates were not significant predictors of IMRT use.
Table 1.
Baseline Characteristics and Univariate Associations with Radiation Technique
| All Patients N=3986 | 3DCRT N=3729 | IMRT N=257 | Pa | |
|---|---|---|---|---|
|
Patient Factors
| ||||
| Age | ||||
| 66–69 | 865 (21.7%) | 810 (21.7%) | 55 (21.4%) | 0.95 |
| 70–74 | 1,186 (29.8%) | 1,111 (29.8%) | 75 (29.2%) | |
| 75–79 | 1,055 (26.5%) | 983 (26.4%) | 72 (28.0%) | |
| ≥ 80 | 880 (22.1%) | 825 (22.1%) | 55 (21.4%) | |
| Gender | ||||
| Male | 2,157 (54.1%) | 2,015 (54.0%) | 142 (55.3%) | 0.70 |
| Female | 1,829 (45.9%) | 1,714 (46.0%) | 115 (44.7%) | |
| Race | ||||
| White | 3,541 (88.8%) | 3,314 (88.9%) | 227 (88.3%) | 0.96 |
| Black | 331 (8.3%) | <320 (<10%) | <30 (<10%) | |
| Other/Unknown | 114 (2.9%) | <110 (<5%) | <11 (<5%) | |
| COPD | ||||
| No | 2,456 (61.6%) | 2,310 (61.9%) | 146 (56.8%) | 0.10 |
| Yes | 1,530 (38.4%) | 1,419 (38.1%) | 111 (43.2%) | |
| Comorbidity Score (Excluding COPD) | ||||
| 0 | 2,425 (60.8%) | 2,280 (61.1%) | 145 (56.4%) | 0.32 |
| 1 | 1,239 (31.1%) | 1,151 (30.9%) | 88 (34.2%) | |
| ≥2 | 322 (8.1%) | 298 (8.0%) | 24 (9.3%) | |
| Oxygen Use | ||||
| No | 3,478 (87.3%) | 3,265 (87.6%) | 213 (82.9%) | 0.04 |
| Yes | 508 (12.7%) | 464 (12.4%) | 44 (17.1%) | |
| Performance Score (Medical Assistance) | ||||
| 0 | 3,611 (90.6%) | 3,377 (90.6%) | 234 (91.1%) | 0.79 |
| ≥1 | 375 (9.4%) | 352 (9.4%) | 23 (8.9%) | |
|
| ||||
|
Tumor Factors
| ||||
| Stage | ||||
| IIIA | 2,469 (61.9%) | 2,301 (61.7%) | 168 (65.4%) | 0.24 |
| IIIB | 1,517 (38.1%) | 1,428 (38.3%) | 89 (34.6%) | |
| Tumor Size Classification | ||||
| T1a | 369 (9.3%) | 336 (9.0%) | 33 (12.8%) | 0.19 |
| T1b | 555 (13.9%) | 518 (13.9%) | 37 (14.4%) | |
| T2a | 1,208 (30.3%) | 1,125 (30.2%) | 83 (32.3%) | |
| T2b | 720 (18.1%) | 683 (18.3%) | 37 (14.4%) | |
| T3 | 444 (11.1%) | 414 (11.1%) | 30 (11.7%) | |
| Unknown | 689 (17.3%) | 652 (17.5%) | 37 (14.4%) | |
| Histology | ||||
| Adenocarcinoma | 1,177 (29.5%) | 1,099 (29.5%) | 78 (30.4%) | 0.34 |
| Squamous | 1,541 (38.7%) | 1,444 (38.7%) | 97 (37.7%) | |
| Large Cell | 150 (3.8%) | <150 (<5%) | <11 (<5%) | |
| NSCLC, NOS | 1,118 (28.0%) | <1100 (<30%) | <80 (<30%) | |
| Laterality | ||||
| Right | 2,318 (58.2%) | 2,165 (58.1%) | 153 (59.5%) | 0.15 |
| Left | 1,636 (41.0%) | <1,600 (<45%) | <100 (<45%) | |
| Unknown | 32 (0.8%) | <30 (<5%) | <11 (<5%) | |
| Anatomic Site | ||||
| Bronchus | 232 (5.8%) | 219 (5.9%) | 13 (5.1%) | 0.94 |
| Upper Lobe | 2,411 (60.5%) | 2,257 (60.5%) | 154 (59.9%) | |
| Middle Lobe | 136 (3.4%) | <130 (<5%) | <11 (<5%) | |
| Lower Lobe | 938 (23.5%) | 873 (23.4%) | 65 (25.3%) | |
| Overlapping/Unknown | 269 (6.7%) | <300 (<8%) | <20 (<8%) | |
|
| ||||
|
Diagnostic and Treatment Factors
| ||||
| PET Staging | ||||
| No | 1,618 (40.6%) | 1,536 (41.2%) | 82 (31.9%) | 0.003 |
| Yes | 2,368 (59.4%) | 2,193 (58.8%) | 175 (68.1%) | |
| Lymph Node Sampling | ||||
| No | 2,786 (69.9%) | 2,609 (70.0%) | 177 (68.9%) | 0.71 |
| Yes | 1,200 (30.1%) | 1,120 (30.0%) | 80 (31.1%) | |
| Treatment Strategy | ||||
| Trimodality | 436 (10.9%) | 413 (11.1%) | 23 (8.9%) | 0.59 |
| Chemotherapy & Radiation | 2,340 (58.7%) | 2,186 (58.6%) | 154 (59.9%) | |
| Surgery & Radiation | 174 (4.4%) | <170 (<5%) | <11 (<5%) | |
| Radiation Alone | 1,036 (26.0%) | <1000 (<30%) | <80 (<30%) | |
| Number of Radiotherapy Fractions | ||||
| <25 | 905 (22.7%) | 854 (22.9%) | 51 (19.8%) | 0.32 |
| 26–29 | 622 (15.6%) | 586 (15.7%) | 36 (14.0%) | |
| 30–33 | 1,079 (27.1%) | 1,011 (27.1%) | 68 (26.5%) | |
| 34–40 | 1,380 (34.6%) | 1,278 (34.3%) | 102 (39.7%) | |
|
| ||||
|
Practice Environment Factors
| ||||
| Type of Radiation Center | ||||
| Freestanding | 1,230 (30.9%) | 1,113 (29.8%) | 117 (45.5%) | <0.0001 |
| Hospital-Based | 2,739 (68.7%) | <2,650 (<70%) | <140 (<55%) | |
| Both | 17 (0.4%) | <15 (<5%) | <11 (<5%) | |
| Practice Location | ||||
| Urban | 3,630 (91.1%) | 3,389 (90.9%) | 241 (93.8%) | 0.10 |
| Rural | 356 (8.9%) | 340 (9.1%) | 16 (6.2%) | |
| Number of Radiation Oncologists per 100,000 Individuals | ||||
| 1st Quartile: < 50 | 895 (22.5%) | 841 (22.6%) | 54 (21.0%) | 0.05 |
| 2nd Quartile: 50 to 130 | 1,036 (26.0%) | 985 (26.4%) | 51 (19.8%) | |
| 3rd Quartile: 131 to 180 | 1,015 (25.5%) | 936 (25.1%) | 79 (30.7%) | |
| 4th Quartile: > 180 | 1,040 (26.1%) | 967 (25.9%) | 73 (28.4%) | |
| SEER Geographic Region | ||||
| San Francisco | 100 (2.5%) | <100 (<5%) | <11 (<5%) | <0.0001 |
| Connecticut | 316 (7.9%) | 308 (8.3%) | <11 (<5%) | |
| Detroit | 391 (9.8%) | 359 (9.6%) | 32 (12.5%) | |
| Hawaii | 18 (0.5%) | <50 (<5%) | <11 (<5%) | |
| Iowa | 191 (4.8%) | <190 (<5%) | <11 (<5%) | |
| New Mexico | 57 (1.4%) | 47 (1.3%) | 10 (3.9%) | |
| Seattle | 224 (5.6%) | 212 (5.7%) | 12 (4.7%) | |
| Utah | 40 (1.0%) | <40 (<5%) | <11 (<5%) | |
| Georgiab | 133 (3.3%) | <150 (<5%) | <11 (<5%) | |
| San Jose | 79 (2.0%) | <80 (<5%) | <11 (<5%) | |
| Los Angeles | 151 (3.8%) | 130 (3.5%) | 21 (8.2%) | |
| Greater California | 672 (16.9%) | 631 (16.9%) | 41 (16.0%) | |
| Kentucky | 492 (12.3%) | 467 (12.5%) | 25 (9.7%) | |
| Louisiana | 370 (9.3%) | 329 (8.8%) | 41 (16.0%) | |
| New Jersey | 752 (18.9%) | 701 (18.8%) | 51 (19.8%) | |
| Year of Diagnosis | ||||
| 2001 | 405 (10.2%) | <400 (<12%) | <11 (<5%) | <0.0001 |
| 2002 | 509 (12.8%) | <500 (<15%) | <20 (<10%) | |
| 2003 | 628 (15.8%) | 602 (16.1%) | 26 (10.1%) | |
| 2004 | 620 (15.6%) | 596 (16.0%) | 24 (9.3%) | |
| 2005 | 631 (15.8%) | 591 (15.8%) | 40 (15.6%) | |
| 2006 | 589 (14.8%) | 527 (14.1%) | 62 (24.1%) | |
| 2007 | 604 (15.2%) | 515 (13.8%) | 89 (34.6%) | |
P-values based on univariate logistic regression
Atlanta and rural Georgia registries combined due to geographic proximity and low number of patients (<11) in the rural Georgia registry.
Abbrev: 3DCRT, three-dimensional conformal radiotherapy; IMRT, intensity-modulated radiotherapy; PET, positron emission tomography; NOS, not otherwise specified
Figure 1.
Proportions of stage III NSCLC patients in the SEER-Medicare database who received IMRT (dark) and 3DCRT (light) between 2001 and 2007 (Ptrend<0.0001).
Table 2.
Significant Predictors of IMRT use.
| Year of Diagnosis | Odds Ratio | 95% CI | P |
|---|---|---|---|
| 2001 | 1 (Ref) | ||
| 2002 | 5.62 | 1.27 – 25.0 | 0.02 |
| 2003 | 8.79 | 2.07 – 37.3 | <0.001 |
| 2004 | 8.24 | 1.93 – 35.2 | <0.001 |
| 2005 | 14.03 | 3.36 – 58.6 | <0.001 |
| 2006 | 24.70 | 5.99 – 101.9 | <0.001 |
| 2007 | 36.14 | 8.82 – 148.1 | <0.001 |
|
| |||
|
SEER Geographic Region
| |||
| New Jersey | 1 (ref) | ||
| San Francisco | 0.37 | 0.11 – 1.23 | 0.10 |
| Connecticut | 0.34 | 0.16 – 0.73 | 0.01 |
| Detroit | 1.26 | 0.78 – 2.03 | 0.34 |
| Hawaii | 0.89 | 0.19 – 4.17 | 0.89 |
| Iowa | 0.17 | 0.05 – 0.56 | 0.00 |
| New Mexico | 2.63 | 1.22 – 5.68 | 0.01 |
| Seattle | 0.69 | 0.36 – 1.35 | 0.28 |
| Utah | 0.72 | 0.16 – 3.20 | 0.67 |
| Georgia | 0.22 | 0.07 – 0.74 | 0.01 |
| San Jose | 0.49 | 0.15 – 1.66 | 0.26 |
| Los Angeles | 1.96 | 1.11 – 3.46 | 0.02 |
| Greater California | 0.65 | 0.41 – 1.01 | 0.06 |
| Kentucky | 0.59 | 0.35 – 0.98 | 0.04 |
| Louisiana | 1.48 | 0.94 – 2.34 | 0.09 |
|
| |||
|
Type of Treatment Center
| |||
| Hospital-Based | 1 (Ref) | ||
| Freestanding | 2.10 | 1.59 – 2.77 | <0.001 |
| Both | 5.34 | 1.58 – 18.06 | 0.01 |
Proportional Hazards Analysis of Acute Toxicities
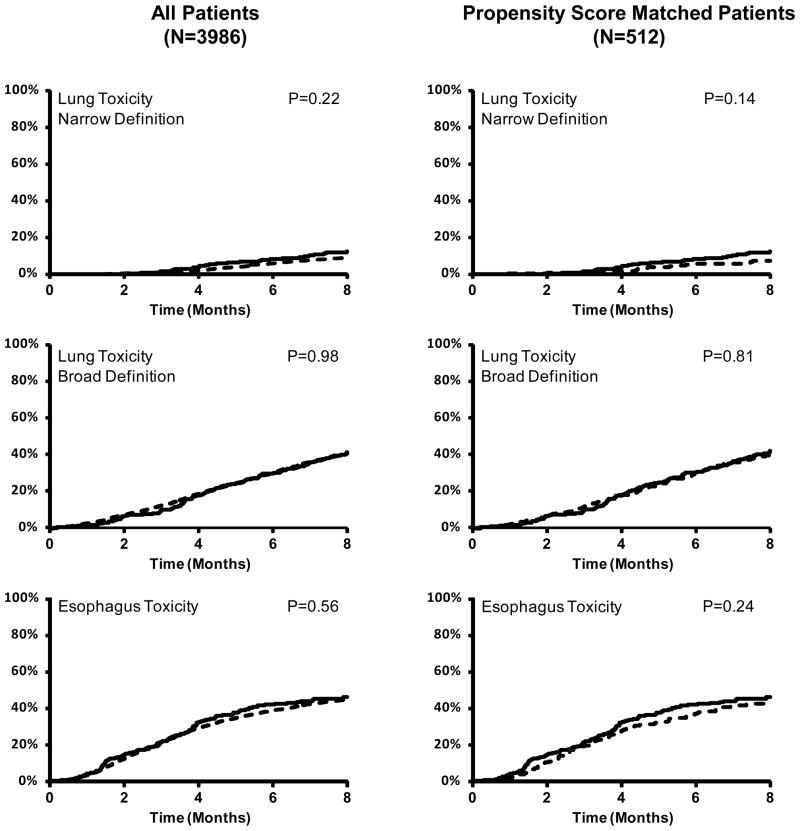
The overall incidence of acute lung toxicity within 8 months of starting radiotherapy was 10.5% in the IMRT group and 7.8% in the 3DCRT group (P=0.12) using the narrow definition, and 38.9% (IMRT) and 37.5% (3DCRT) (P=0.80) using the broad definition. In multivariate analysis, IMRT was not associated with acute pulmonary toxicity, regardless of whether the narrow (Hazard ratio [HR], 1.31; 95%CI, P=0.22) or broad (HR, 1.00; 95%CI 0.81–1.24, P=0.98) definition was used (Table 3, Figure 2). Higher comorbidity score and male sex were associated with acute lung toxicity (Supplementary Tables 4–5).
Table 3.
Association of IMRT with toxicities, grouped by analytic technique.
| Multivariate Proportional Hazards Analysisa
| |||
|---|---|---|---|
| Outcome | HR | 95%CI | P |
| Lung Toxicity (Narrow) | 1.31 | 0.86 – 1.99 | 0.22 |
| Lung Toxicity (Broad) | 1.00 | 0.81 – 1.24 | 0.98 |
| Esophagus Toxicity (All) | 1.06 | 0.87 – 1.30 | 0.56 |
| Esophagitis/Dysphagia | 1.03 | 0.78 – 1.37 | 0.82 |
| Dehydration | 1.01 | 0.80 – 1.28 | 0.91 |
| Feeding Tube Placement | 0.67 | 0.25 – 1.77 | 0.42 |
| Mucositis | 1.25 | 0.41 – 3.78 | 0.69 |
| Propensity Score Matching Analysisb
| |||
|---|---|---|---|
| Outcome | HR | 95%CI | P |
| Lung Toxicity (Narrow) | 1.64 | 0.85 – 3.19 | 0.14 |
| Lung Toxicity (Broad) | 0.96 | 0.69 – 1.32 | 0.81 |
| Esophagus Toxicity (All) | 1.21 | 0.88 – 1.64 | 0.24 |
| Esophagitis/Dysphagia | 0.98 | 0.65 – 1.47 | 0.92 |
| Dehydration | 1.13 | 0.80 – 1.59 | 0.49 |
| Feeding Tube Placement | 0.83 | 0.25 – 2.73 | 0.76 |
| Mucositis | 0.60 | 0.14 – 2.51 | 0.48 |
Hazard ratios measure the effect of IMRT compared to 3DCRT (referent) for each outcome listed after adjustment for prespecified clinically relevant covariates and covariates significant at P<0.20 in univariate analysis. A separate model was created for each of the seven outcomes listed (supplemental tables 4–10).
Hazard ratios measure the effect of IMRT compared to 3DCRT using proportional hazards regression of propensity score matched IMRT cases and 3DCRT controls, stratified by matched pair.
Abbrev: HR, hazard ratio; CI, confidence interval
Figure 2.
Cumulative incidence of acute toxicities calculated via claims for IMRT (solid line) and 3DCRT (dashed line). No statistically significant difference was observed for any of the endpoints. P-values derived from proportional hazards regression
The overall incidence of acute esophagus toxicity within 8 months of starting radiotherapy was 44% (IMRT) and 42% (3DCRT) (P=0.58). In multivariate analysis, IMRT was not associated with the cumulative endpoint of acute esophagus toxicity (HR, 1.06; 95%CI 0.87–1.30; P=0.56) (Table 3, Figure 2). IMRT was also not associated with any of the individual components of the cumulative endpoint (Table 3, Supplementary Tables 7–10). Predictors for the cumulative endpoint of esophageal toxicity included advanced age, higher comorbidity score, absence of PET staging, combining chemotherapy with radiation, and the number of radiotherapy fractions (Supplementary Table 6).
For all toxicity endpoints, the proportional hazards assumption with respect to radiation technique was satisfied, and goodness-of-fit for all final models was acceptable (P>0.05). Significant interactions between radiation technique and clinically relevant covariates were not observed in any model. Finally, all findings were unchanged if the window for acute events was shortened to 4 months or extended to 14 months.
Propensity-Score Matching Analysis of Acute Toxicities
Two hundred fifty six (99%) of the IMRT patients were successfully matched to 3DCRT control patients. The paired cohorts were well-balanced across all covariables (Supplementary Table 11). IMRT was not associated with lung toxicity using the narrow (HR, 1.64; 95%CI 0.84–3.19, P=0.14) or broad definitions (HR 0.96, 95%CI, 0.70–1.43, P=0.81) (Table 3, Figure 2). Likewise IMRT was not associated with acute esophagus toxicity using the comprehensive definition (HR, 1.21; 95%CI 0.88–1.64, P=0.24) or using the component endpoints (Table 3). These results were unchanged if the window for scoring acute events was shortened to 4 months or extended to 14 months. Propensity-score matching analysis limited to patients treated with chemotherapy and radiation yielded similar results as the entire population (Supplementary Table 12).
Discussion
Proponents of highly conformal radiotherapy, such as IMRT, for treating lung cancer have argued that these technologies can minimize normal tissue toxicity, which in turn can improve patient compliance and allow dose escalation [27–29]. However, concern has been raised that the use of advanced technologies such as IMRT can also be influenced by non-clinical factors [30–33]. Therefore, our first objective in this study was to determine whether clinical issues – such as tumor size classification, IIIB stage, or high comorbidity score – drove the increased utilization of IMRT between 2001 and 2007. Using a database of nearly 4,000 stage III lung cancer patients treated in everyday practice, we found that clinical factors did not predict for IMRT use. Aside from the year of diagnosis, the only factors that were significant predictors of IMRT use were treatment within certain geographic regions and treatment within a freestanding radiation center.
Freestanding radiotherapy centers rose in prominence beginning in the late 1980s and were supported by a higher Medicare fee schedule to compensate their higher costs of operation [34]. The purpose of these centers was to improve access to medically underserved area, but whether this has been achieved is controversial [9, 34]. In our analysis, we found that treatment at these centers was associated with twice the odds of receiving IMRT for lung cancer. One explanation is that these centers may have had greater willingness and flexibility to pursue capital equipment purchases and introduce new technology. Another factor may have been differences in reimbursement. During the study period, Medicare reimbursement at freestanding centers for IMRT was approximately twice that received by hospital-based centers [8].
Additional research is required to determine whether either of these mechanisms (or some other factor) is responsible for the increased utilization of IMRT at freestanding centers. To our knowledge, this is the first study to identify this phenomenon for lung cancer radiotherapy, but it has been observed in breast and prostate cancer practice and even in the setting of palliative care [8, 23, 24, 31, 35]. Identification of the factors that account for this effect may help to craft policies that optimize high value radiation treatment. This avenue of health services research is especially timely because the introduction of accountable care organizations in the United States will provide a framework for initiatives that foster responsible utilization of new technologies [31, 36–38].
In the second portion of our study, we compared the incidences of acute toxicities following IMRT and 3DCRT. Using two different analytic techniques, we found no difference in toxicity incidence between patients treated with 3DCRT and IMRT. Because our study period captured the earliest users of IMRT, it was reassuring to find similar rates of acute toxicity when comparing this new technology to a mature standard-of-care. For acute lung injury, our findings were consistent with prior single-institution reports which also did not find worse rates of pneumonitis despite the theoretical risks associated with increased low-dose lung exposure from intensity-modulated beams [13, 14]. These other reports actually found a lower burden of acute pneumonitis with IMRT, but it is not unexpected that outcomes in a single institution with strict quality assurance protocols would be different than those found in a diverse, population-based cohort.
With regard to esophagus toxicity, IMRT dose-painting is often used to spare the esophagus from high-dose regions, but we did not observe a clinical correlate for this practice. Again, it is possible that the early adoption phase of IMRT was not yet advanced enough to make a measurable impact on this endpoint. Another possibility, suggested by the first portion of our investigation, is that patients at high risk for esophagitis, such as those with IIIB disease, were not necessarily selected for IMRT, reducing the likelihood of detecting a benefit.
An important strength of this analysis was the implementation of new methodologies to address limitations inherent to population-based databases [21]. Though population-based analyses have emerged as powerful tools for studying radiotherapy in lung cancer [16, 39–43], a weakness is the absence of data for certain prognostic factors. For instance, the SEER-Medicare database lacks data for pulmonary function and ECOG performance status. To overcome this limitation, we generated proxy covariates including COPD status, supplemental oxygen use, and claims for medical assistance to approximate the “classic” prognostic factors. Additionally, incorporating the number of radiotherapy fractions provided some measure of adjustment for dose-escalation. We infer that adding these additional covariates to the rich set of data available in SEER-Medicare reduced the impact of hidden selection bias.
This study has several limitations. First, IMRT technique has been considerably refined since 2007 and contemporary outcomes, including acute toxicities, may be improved now when compared to the findings from our cohort. Second, our analysis is observational and may harbor bias despite adjustment for measurable confounders. Third, the use of diagnosis claims codes did not provide insight into the subjective intensity of the toxicity. Therefore, if the benefit of IMRT is to reduce the grade of toxicities rather than their incidence, our methodology may not have detected this advantage. Finally, chronic toxicities and survival were not addressed in this analysis. The combination of a relatively low number of patients in the IMRT cohort and low frequency of events prevented rigorous statistical analysis of these endpoints. Future studies with updated SEER-Medicare data will address this deficit.
Conclusion
Increased utilization of IMRT for NSCLC between 2001 and 2007 was associated with practice factors rather than clinical variables. Early use of IMRT resulted in similar rates of pulmonary and esophagus toxicity as 3DCRT. These findings will hopefully encourage confirmatory prospective trials that quantify the comparative efficacy, and morbidity of the two techniques.
Supplementary Material
Acknowledgments
Dr. Smith is supported by grants from the Cancer Prevention & Research Institute of Texas [Grant RP101207]. This work was also supported by the Department of Health and Human Services National Cancer Institute [Grants CA16672, T32CA77050].
A portion of this study was funded by a research grant from Varian Medical Systems (SR2011-00034954RG 01). This entity had no role in the study design, data analysis, or data interpretation.
Footnotes
Conflicts of Interest:
The authors have no other conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors maybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol. 2009;27:2758–2765. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 2.Stinchcombe TE, Bogart JA. Novel approaches of chemoradiotherapy in unresectable stage IIIA and stage IIIB non-small cell lung cancer. Oncologist. 2012;17:682–693. doi: 10.1634/theoncologist.2012-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scarpaci A, Mitra P, Jarrar D, Masters GA. Multimodality Approach to Management of Stage III Non-Small Cell Lung Cancer. Surg Oncol Clin N Am. 2013;22:319–328. doi: 10.1016/j.soc.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 4.Albain KS, Swann RS, Rusch VW, Turrisi AT, 3rd, Shepherd FA, Smith C, Chen Y, Livingston RB, Feins RH, Gandara DR, Fry WA, Darling G, Johnson DH, Green MR, Miller RC, Ley J, Sause WT, Cox JD. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet. 2009;374:379–386. doi: 10.1016/S0140-6736(09)60737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu HH, Wang X, Dong L, Wu Q, Liao Z, Stevens CW, Guerrero TM, Komaki R, Cox JD, Mohan R. Feasibility of sparing lung and other thoracic structures with intensity-modulated radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2004;58:1268–1279. doi: 10.1016/j.ijrobp.2003.09.085. [DOI] [PubMed] [Google Scholar]
- 6.Murshed H, Liu HH, Liao Z, Barker JL, Wang X, Tucker SL, Chandra A, Guerrero T, Stevens C, Chang JY, Jeter M, Cox JD, Komaki R, Mohan R. Dose and volume reduction for normal lung using intensity-modulated radiotherapy for advanced-stage non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2004;58:1258–1267. doi: 10.1016/j.ijrobp.2003.09.086. [DOI] [PubMed] [Google Scholar]
- 7.Mayles WP. Survey of the availability and use of advanced radiotherapy technology in the UK. Clin Oncol (R Coll Radiol) 2010;22:636–642. doi: 10.1016/j.clon.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 8.Smith BD, Pan IW, Shih YC, Smith GL, Harris JR, Punglia R, Pierce LJ, Jagsi R, Hayman JA, Giordano SH, Buchholz TA. Adoption of intensity-modulated radiation therapy for breast cancer in the United States. J Natl Cancer Inst. 2011;103:798–809. doi: 10.1093/jnci/djr100. [DOI] [PubMed] [Google Scholar]
- 9.Falit BP, Gross CP, Roberts KB. Integrated prostate cancer centers and over-utilization of IMRT: a close look at fee-for-service medicine in radiation oncology. Int J Radiat Oncol Biol Phys. 2010;76:1285–1288. doi: 10.1016/j.ijrobp.2009.10.060. [DOI] [PubMed] [Google Scholar]
- 10.Turner LM, Howard JA, Dehghanpour P, Barrett RD, Rebueno N, Palmer M, Vedam S, Klopp A, Komaki R, Welsh JW. Exploring the feasibility of dose escalation positron emission tomography-positive disease with intensity-modulated radiation therapy and the effects on normal tissue structures for thoracic malignancies. Med Dosim. 2011;36:383–388. doi: 10.1016/j.meddos.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Sura S, Gupta V, Yorke E, Jackson A, Amols H, Rosenzweig KE. Intensity-modulated radiation therapy (IMRT) for inoperable non-small cell lung cancer: the Memorial Sloan-Kettering Cancer Center (MSKCC) experience. Radiother Oncol. 2008;87:17–23. doi: 10.1016/j.radonc.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grills IS, Yan D, Martinez AA, Vicini FA, Wong JW, Kestin LL. Potential for reduced toxicity and dose escalation in the treatment of inoperable non-small-cell lung cancer: a comparison of intensity-modulated radiation therapy (IMRT), 3D conformal radiation, and elective nodal irradiation. Int J Radiat Oncol Biol Phys. 2003;57:875–890. doi: 10.1016/s0360-3016(03)00743-0. [DOI] [PubMed] [Google Scholar]
- 13.Yom SS, Liao Z, Liu HH, Tucker SL, Hu CS, Wei X, Wang X, Wang S, Mohan R, Cox JD, Komaki R. Initial evaluation of treatment-related pneumonitis in advanced-stage non-small-cell lung cancer patients treated with concurrent chemotherapy and intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2007;68:94–102. doi: 10.1016/j.ijrobp.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 14.Liao ZX, Komaki RR, Thames HD, Jr, Liu HH, Tucker SL, Mohan R, Martel MK, Wei X, Yang K, Kim ES, Blumenschein G, Hong WK, Cox JD. Influence of technologic advances on outcomes in patients with unresectable, locally advanced non-small-cell lung cancer receiving concomitant chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2010;76:775–781. doi: 10.1016/j.ijrobp.2009.02.032. [DOI] [PubMed] [Google Scholar]
- 15.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40:IV-3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 16.Chen AB, Neville BA, Sher DJ, Chen K, Schrag D. Survival outcomes after radiation therapy for stage III non-small-cell lung cancer after adoption of computed tomography-based simulation. J Clin Oncol. 2011;29:2305–2311. doi: 10.1200/JCO.2010.33.4466. [DOI] [PubMed] [Google Scholar]
- 17.Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol. 1993;46:1075–1079. doi: 10.1016/0895-4356(93)90103-8. discussion 1081–1090. [DOI] [PubMed] [Google Scholar]
- 18.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 19.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 20.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 21.Davidoff AJ, Tang M, Seal B, Edelman MJ. Chemotherapy and survival benefit in elderly patients with advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:2191–2197. doi: 10.1200/JCO.2009.25.4052. [DOI] [PubMed] [Google Scholar]
- 22.Fritz A, Percy C, Jack A, et al. International Classification of Diseases for Oncology. 3. Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]
- 23.Guadagnolo BA, Liao KP, Elting L, Giordano S, Buchholz TA, Shih YC. Use of radiation therapy in the last 30 days of life among a large population-based cohort of elderly patients in the United States. J Clin Oncol. 2013;31:80–87. doi: 10.1200/JCO.2012.45.0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roberts KB, Soulos PR, Herrin J, Yu JB, Long JB, Dostaler E, Gross CP. The Adoption of New Adjuvant Radiation Therapy Modalities Among Medicare Beneficiaries With Breast Cancer: Clinical Correlates and Cost Implications. Int J Radiat Oncol Biol Phys. 2012 doi: 10.1016/j.ijrobp.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–3107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D’Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 27.Schild SE, Curran WJ., Jr . Small Cell Lung Cancer. In: Gunderson LL, Tepper JE, editors. Clinical Radiation Oncology. Philadelphia, PA: Elsevier Churchill Livingstone; 2007. p. 1827. [Google Scholar]
- 28.Kies MS, Mira JG, Crowley JJ, Chen TT, Pazdur R, Grozea PN, Rivkin SE, Coltman CA, Jr, Ward JH, Livingston RB. Multimodal therapy for limited small-cell lung cancer: a randomized study of induction combination chemotherapy with or without thoracic radiation in complete responders; and with wide-field versus reduced-field radiation in partial responders: a Southwest Oncology Group Study. J Clin Oncol. 1987;5:592–600. doi: 10.1200/JCO.1987.5.4.592. [DOI] [PubMed] [Google Scholar]
- 29.Liengswangwong V, Bonner JA, Shaw EG, Foote RL, Frytak S, Eagan RT, Jett JR, Richardson RL, Creagan ET, Su JQ. Limited-stage small-cell lung cancer: patterns of intrathoracic recurrence and the implications for thoracic radiotherapy. J Clin Oncol. 1994;12:496–502. doi: 10.1200/JCO.1994.12.3.496. [DOI] [PubMed] [Google Scholar]
- 30.Bekelman JE, Hahn SM. The body of evidence for advanced technology in radiation oncology. J Natl Cancer Inst. 2013;105:6–7. doi: 10.1093/jnci/djs508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nguyen PL, Gu X, Lipsitz SR, Choueiri TK, Choi WW, Lei Y, Hoffman KE, Hu JC. Cost implications of the rapid adoption of newer technologies for treating prostate cancer. J Clin Oncol. 2011;29:1517–1524. doi: 10.1200/JCO.2010.31.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meropol NJ, Schrag D, Smith TJ, Mulvey TM, Langdon RM, Jr, Blum D, Ubel PA, Schnipper LE American Society of Clinical O. American Society of Clinical Oncology guidance statement: the cost of cancer care. J Clin Oncol. 2009;27:3868–3874. doi: 10.1200/JCO.2009.23.1183. [DOI] [PubMed] [Google Scholar]
- 33.Medicare Payment Advisory Commission Report to the Congress: Reforming the delivery system. 2008 Sep 16; http://www.medpac.gov/documents/20080916_Sen%20Fin_testimony%20final.pdf.
- 34.Mitchell JM, Sunshine JH. Consequences of physicians’ ownership of health care facilities--joint ventures in radiation therapy. N Engl J Med. 1992;327:1497–1501. doi: 10.1056/NEJM199211193272106. [DOI] [PubMed] [Google Scholar]
- 35.Smith GL, Xu Y, Buchholz TA, Smith BD, Giordano SH, Haffty BG, Vicini FA, White JR, Arthur DW, Harris JR, Shih YC. Brachytherapy for accelerated partial-breast irradiation: a rapidly emerging technology in breast cancer care. J Clin Oncol. 2011;29:157–165. doi: 10.1200/JCO.2009.27.0942. [DOI] [PubMed] [Google Scholar]
- 36.Iglehart JK. The ACO regulations--some answers, more questions. N Engl J Med. 2011;364:e35. doi: 10.1056/NEJMp1103603. [DOI] [PubMed] [Google Scholar]
- 37.Lieberman SM, Bertko JM. Building regulatory and operational flexibility into accountable care organizations and ‘shared savings’. Health Aff (Millwood) 2011;30:23–31. doi: 10.1377/hlthaff.2010.0928. [DOI] [PubMed] [Google Scholar]
- 38.Elnahal SM, Kerstiens J, Helsper RS, Zietman AL, Johnstone PA. Proton Beam Therapy and Accountable Care: The Challenges Ahead. Int J Radiat Oncol Biol Phys. 2012 doi: 10.1016/j.ijrobp.2012.10.038. [DOI] [PubMed] [Google Scholar]
- 39.Wanders R, Steevens J, Botterweck A, Dingemans AM, Reymen B, Baardwijk A, Borger J, Bootsma G, Pitz C, Lunde R, Geraedts W, Lambin P, De Ruysscher D. Treatment with curative intent of stage III non-small cell lung cancer patients of 75 years: a prospective population-based study. Eur J Cancer. 2011;47:2691–2697. doi: 10.1016/j.ejca.2011.06.023. [DOI] [PubMed] [Google Scholar]
- 40.Haasbeek CJ, Palma D, Visser O, Lagerwaard FJ, Slotman B, Senan S. Early-stage lung cancer in elderly patients: a population-based study of changes in treatment patterns and survival in the Netherlands. Ann Oncol. 2012;23:2743–2747. doi: 10.1093/annonc/mds081. [DOI] [PubMed] [Google Scholar]
- 41.Palma D, Visser O, Lagerwaard FJ, Belderbos J, Slotman B, Senan S. Treatment of stage I NSCLC in elderly patients: a population-based matched-pair comparison of stereotactic radiotherapy versus surgery. Radiother Oncol. 2011;101:240–244. doi: 10.1016/j.radonc.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 42.Shirvani SM, Jiang J, Chang JY, Welsh JW, Gomez DR, Swisher S, Buchholz TA, Smith BD. Comparative effectiveness of 5 treatment strategies for early-stage non-small cell lung cancer in the elderly. Int J Radiat Oncol Biol Phys. 2012;84:1060–1070. doi: 10.1016/j.ijrobp.2012.07.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palma D, Visser O, Lagerwaard FJ, Belderbos J, Slotman BJ, Senan S. Impact of introducing stereotactic lung radiotherapy for elderly patients with stage I non-small-cell lung cancer: a population-based time-trend analysis. J Clin Oncol. 2010;28:5153–5159. doi: 10.1200/JCO.2010.30.0731. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.