Abstract
Background
There are limited data assessing the predictive value of fraction of exhaled nitric oxide (FENO) for persistence of wheezing, exacerbations, or lung function change over time in infants/toddlers with recurrent wheezing.
Objectives
In an ongoing longitudinal cohort of infants and toddlers with recurrent wheezing compare predictive values of single-breath FENO (SB-FENO), tidal-breathing mixed-expired FENO (tidal-FENO), bronchodilator responsiveness (BDR), and the Castro-Rodriquez asthma predictive index (API) for persistence of wheezing, exacerbations, and lung function change through age 3 yrs.
Methods
Enrollment forced expiratory flows and volumes (iPFTs) were measured in 44 infants/toddlers using the raised-volume rapid thoracoabdominal compression method. SB-FENO was measured at 50 mL/sec, and tidal-FENO was measured during awake tidal breathing. Clinical outcomes were assessed at age 3 yrs. in 42 infants. Follow-up iPFTs were completed between ages 2.5-3 yrs. in 32 subjects.
Results
An enrollment SB-FENO concentration ≥30 ppb predicted persistence of wheezing at age 3 years with a sensitivity of 77%, a specificity of 94%, and an area under the curve (AUC) of 0.86 (95% CI: 0.74 – 0.98). The sensitivity, specificity, positive predictive, and negative predictive values of SB-FENO for persistence of wheezing and exacerbations were superior to tidal-FENO, BDR, and the API. SB-FENO ≥30 ppb and tidal FENO ≥7 ppb measured at enrollment was associated with a decline in both FEV0.5 and FEF25-75 between enrollment and age 3 years.
Conclusions
In wheezy infants/toddlers SB-FENO was superior to tidal-FENO, BDR, and the API in predicting future exacerbations and persistence of wheezing at age 3 years. Both SB-FENO and tidal FENO were associated with lung function decline over time.
Keywords: exhaled nitric oxide, FENO, recurrent wheezing, infants, pulmonary function, raised-volume rapid thoracic compression
INTRODUCTION
More than half of school age children with persistent asthma were symptomatic during their preschool years, usually presenting with recurrent episodes of wheezing.[1] Recurrent wheezing affects up to 30% of infants and toddlers, yet resolves in at least 50% of these children by school age. An accurate predictor of asthma does not currently exist for infants/toddlers; hampering decision making as clinicians generally cannot differentiate infants/toddlers with transient wheezing from those with persistent asthma. NHLBI guidelines currently recommend daily use of inhaled corticosteroids (ICS) for children under age 5 yrs. with > 3 prior episodes of wheezing and epidemiologic risk factors for asthma.[2] These guidelines are based primarily on the Asthma Predictive Index (API) proposed by Castro-Rodriquez et al[3]. However, the API has not been assessed prospectively in unselected infants/toddlers with recurrent wheeze. When applied to the birth cohort from which it was developed[3], and to a large independent population-based cohort,[4] the API had a high negative predictive value yet a sensitivity of only 28-37% and positive predictive value of only 40-48% for asthma at age 6 yrs.
Many parents are reluctant to treat their children with inhaled steroids if the diagnosis of asthma is uncertain. Concern about treating infants/toddlers with inhaled or systemic steroids has further increased with recent trials suggesting that treatment of viral-induced wheezing with oral steroids in preschool children is ineffective [5], and that prevention of such attacks with inhaled steroids may be associated with reduced growth velocity.[6] A biomarker with a high positive predictive value for atopic asthma among infants and toddlers with recurrent wheezing would be useful to investigators testing potential asthma controller therapies in very young children by allowing for more targeted enrollment of young asthmatics in clinical trials, and helpful to clinicians as an adjunctive predictor of patients likely to benefit from current and future asthma controller regimens. The fractional concentration of exhaled nitric oxide (FENO) is a biomarker of airway inflammation repeatedly shown to be elevated in adults and children with allergic asthma[7], yet there is a paucity of data assessing FENO prospectively among wheezy infants/toddlers.[8-12] In adults and school-age children with atopic asthma FENO is correlated with sputum and bronchial eosinophils, peak flow variability, and bronchial reactivity[13-18], and decreases following systemic or inhaled steroid treatment.[19-21] In atopic infants at risk for asthma, FENO was associated with airway reactivity.[22] Furthermore, among adults with chronic respiratory symptoms FENO has been shown to be a superior predictor of steroid responsiveness than spirometry, BDR, or airway reactivity to methacholine or adenosine.[23] ATS FENO Clinical Practice Guidelines [24] recommend use of FENO to diagnose eosinophilic airway inflammation, determine likelihood of steroid responsiveness, and support the diagnosis of asthma when objective evidence is lacking. Data supporting these recommendations is most robust in steroidnaïve eosinophilic asthmatics. [24] Most wheezy infants/toddlers who go on to develop persistent childhood asthma can be characterized as having an atopic phenotype of asthma[25]; therefore FENO may be well suited as a biomarker to separate infants/toddlers with asthma from those with wheezing due to other etiologies.
In one recent study of early school-age and preschool children, FENO measured during tidal breathing was modestly associated with a greater risk of respiratory illness over one year.[26] However, the mean enrollment age was >3 years, and most children < 3yrs. could not perform acceptable tidal breathing FENO measurements, indicating that unsedated tidal-breathing FENO may be technically challenging to measure in infants/toddlers.[26] Additional limitations of tidal breathing-FENO in infants include: potential nasal NO contamination as nasal NO production far exceeds lower airway production, and variable expiratory flow which is problematic due the highly flow dependent nature of FENO[27, 28]. Despite these potential limitations, it is easier to measure FENO in infants/toddlers during tidal breathing than to measure FENO using a flow-regulated exhalation method.
Previously we reported that single-breath flow-regulated FENO (SB-FENO) was predictive of subsequent wheezing, exacerbations, and lung function decline in a cohort of wheezy infants and toddlers (mean enrollment age of 15.8 mos.) over a 6 month follow-up period.[29] We now report follow-up of this cohort through age 3 years. Our objective was to compare enrollment SB-FENO, mixed expired FENO measured during tidal breathing, and the Asthma Predictive Index (API)[3] as predictors of persistence of wheezing at age 3 years, exacerbations of wheezing during follow-up, and changes in lung function. We hypothesized that FENO would be a superior to the Asthma Predictive Index (API)[3] as a predictor of these outcomes. Some of these results have been reported in abstract form.[30]
METHODS
Subjects
Children 6-24 months of age with ≥ 3 episodes of physician-diagnosed wheezing treated with bronchodilators or corticosteroids were recruited for a single-center longitudinal study. Subjects with birth < 36 weeks gestation, congenital heart disease, dysphagia, severe gastroesophageal reflux, or upper airway obstruction were excluded. Systemic or inhaled corticosteroid treatment was not permitted for at least 3 weeks prior to baseline lung function and FENO measurement. Thereafter, corticosteroid treatment was at the discretion of the subject’s primary care or emergency department provider who did not have access to FENO data. Written consent was obtained from parents of subjects. The study was approved by the Seattle Children’s Hospital Institutional Review Board.
Study Visits
Study visits occurred a minimum of 3 weeks following resolution of an upper or lower respiratory infection, or acute exacerbation of wheezing. At enrollment medical history was reviewed, length and weight were measured with a calibrated stadiometer and digital scale, SB-FENO and tidal-FENO was measured, and lung function testing performed. Between enrollment and follow-up visits parents completed monthly symptom and medication diaries that recorded: wheezing episodes lasting ≥ 3 days treated with at least a bronchodilator, use of systemic (oral or intravenous) corticosteroids, and use of inhaled corticosteroids. Diaries were returned to study personnel on a monthly basis. Interval medical history and medication use was reviewed and lung function tests repeated at a follow-up visit 6 months after enrollment and at age 3 years.
Lung Function Measurements
Forced expiratory volumes and flows (FVC, FEV0.5, and FEF25-75) were measured using the RVRTC technique according to ATS/ERS guidelines for raised volume forced expirations in infants[31] via the InSpire® Infant Pulmonary Lab (IPL) at enrollment, 6 months post enrollment, and age 3 years. Lung function tests were performed at least 3 weeks after resolution of acute exacerbations of wheezing or respiratory illnesses. Lung function parameters were analyzed as z-scores calculated from published normative data. [32] Measurement of forced expiratory flows and volumes were repeated 10 minutes after administration of 4 puffs of albuterol. Bronchodilator responsiveness (BDR) was defined as ≥ 12% improvement in FEV0.5, or ≥ 25% improvement in FEF25-75.
Exhaled Nitric Oxide Measurements
At each testing session, at least 3 flow-regulated SB-FENO measurements were performed in each subject immediately prior to lung function testing using a method we have previously described.[29] Mixed expired FENO measurements during tidal breathing (tidal-FENO) were obtained prior to administration of chloral hydrate while subjects were awake and seated in parent’s lap watching a video for distraction. Mixed expired collections were obtained over 30 seconds of quiet regular breathing with a full facemask over the mouth and nose following 5 breaths to washout circuit dead space. Nitric oxide free air (<2 ppb) was provided during inspiration via a filter attached to the collection circuit with a one-way valve. Nitric oxide was measured using a Sievers® NOA 280 chemiluminescence analyzer (GE Analytical Instruments; Boulder, CO, USA).
Asthma Predictive Index (API)
Using data collected at enrollment a positive or negative API was determined for each subject using criteria modified slightly from criteria proposed by Castro-Rodriquez et al. [3] A positive API was defined as meeting 1 of 2 major, or 2 minor criteria. Major Criteria included a history of parental physician-diagnosed asthma or a history of physician-diagnosed eczema in the subject. Minor Criteria included a history of physician-diagnosed allergic rhinitis or a history of wheezing apart from colds.
Statistical Analysis
Forty subjects were estimated to provide 80% power to identify SB-FENO as a significant predictor of FEV0.5 if SB-FE accounted for ≥15% of the total variance in FEV0.5 (i.e. r2 NO =0.15). SB-FENO and lung function data were determined to be normally distributed by the D’Agostino and Pearson as well as Kolmogorov-Smirnov tests. Summary statistics, Pearson’s correlation coefficients and independent t-tests were used to describe and differentiate subjects by their wheeze and exacerbation status at 3 years of age.
Multivariate logistic regression was used to assess associations between enrollment FENO measures (SB-FENO and tidal-FENO) and: 1) acute exacerbations of wheezing (defined as ≥ 1 episode of wheezing lasting ≥ 3 days treated with both albuterol and systemic corticosteroids) during the 6 months prior to the age 3 year follow-up visit, and 2) persistence of wheezing at age 3 years (defined as ≥1 episode of any wheezing treated with albuterol during the 6 months prior to age 3 years). Persistence of wheezing was further characterized by parental report into multi-triggered persistent wheezing if wheezing was triggered by more than viral respiratory tract infections (e.g. vigorous play/exercise, food, pets, or other inhalant exposures per parent observation), or URI-triggered persistent wheezing if triggered exclusively by viral upper respiratory tract infections. Enrollment age, gender, family history of asthma, eczema, sustained inhaled corticosteroid treatment, and tobacco smoke exposure were included as covariates in the logistic models. Receiver operating characteristics (ROC) analyses were performed to determine the enrollment SB-FENO and tidal-FENO values that best predicted persistence of wheezing at age 3 years as well as exacerbations of wheezing. Using the best cutoff points for SB-FENO and tidal-FENO, the sensitivity, specificity, positive predictive value, and negative predictive value of enrollment SB-FENO and tidal-FENO, bronchodilator responsiveness at enrollment, a positive API, and SB-FENO combined with a positive API, were calculated as predictors of persistence of wheezing and exacerbations of wheezing.
Generalized estimating equations (GEE) regression models were used to assess associations between enrollment SB-FENO and tidal FENO, baseline subject characteristics, and changes in lung function during follow-up utilizing RVRTC lung function data from each visit at which it was obtained (enrollment, 6 month follow-up, and age 3 years). An ROC analysis was performed to determine the enrollment SB-FENO and tidal-FENO concentrations that best predicted a decline in FEV0.5 or FEF25-75. All reported p-values are two sided. Data analyses were conducted using GraphPad® Prism 4.0 (La Jolla, CA, USA), Intercooled Stata for Windows® version 10.1 (College Station, Texas, USA), and SAS version 9.2 (Cary, NC, USA).
RESULTS
Forty-seven infants and toddlers were recruited with a mean age of 15.6 (SD ± 5.2) months at enrollment. At enrollment technically acceptable RVRTC lung function tests were performed in 44 subjects, and FENO measurements obtained in 45 subjects. Baseline characteristics of the cohort are presented in Table 1. Forty subjects completed clinical follow-up 6 months following enrollment, of which 32 completed RVRTC lung function testing. At age 3 years, 42 subjects completed clinical follow-up (95% of subjects who completed enrollment lung function testing), and 34 subjects completed RVRTC lung function measurements (77% of subjects who completed enrollment testing). The mean duration between subject enrollment visits and follow-up at age 3 years was 19 months.
TABLE 1.
Baseline Characteristics of Subjects Completing Enrollment FENO Measurement
| Subjects (n) | 45 |
| Gender (male) | 30 (67%) |
| Race (Caucasian) | 33 (73%) |
| Mean age at enrollment (months)* | 15.7 ± 5.2 |
| Eczema (physician-diagnosed and steroid treated) | 16 (36%) |
| Family history asthma (1st degree relative) |
24 (53%) |
| Enviromental tobacco smoke exposure | 12 (27%) |
| Prior treatment with systemic steroids for wheezing | 34 (76%) |
| History of ED visit for wheezing | 34 (76%) |
| History of hospitalization for wheezing | 16 (36%) |
| History of wheezing apart from viral infections | 16 (36%) |
| Enrollment SB-FENO (ppb)* | 28.7 ppb ± 16.7 |
| Enrollment tidal-FENO (ppb)* | 7.8 ppb ± 4.9 |
| Enrollment FVC (z-score)** | −0.04 (−0.42 to 0.35) |
| Enrollment FEV0.5 (z-score)** | −0.42 (−0.78 to −0.07) |
| Enrollment FEF25-75 (z-score)** | −0.67 (−1.10 to −0.25) |
Definition of Abbreviations: ED = emergency department; SB-FENO = single-breath exhaled nitric oxide; Tidal-FENO = mixed expired exhaled nitric oxide during tidal breathing; FVC = forced vital capacity; FEV0.5 = forced expiratory volume in 0.5 seconds; FEF25-75 = forced expiratory flow between 25% and 75% of forced expiratory maneuver.
Data are presented as the mean ± SD
Data are presented as the mean ± 95% CI; n=44 subjects
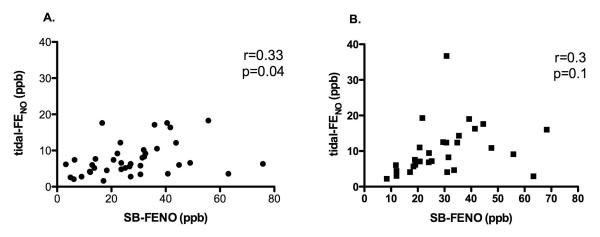
The mean FEV0.5 and FEF25-75 for the cohort at enrollment were significantly less than zero (a z-score equal to zero represents the mean value of published normative data). [29, 32] Enrollment of subjects was evenly distributed across seasons.[29] Eight subjects used inhaled corticosteroids on a sustained basis between the enrollment and 6 month follow-up visits, and 9 of the 34 subjects who completed lung function testing at age 3 years used inhaled steroids on a sustained basis during the 6 months prior to the visit at age 3 years. We previously reported that SB-FENO measured at enrollment was associated with both age and a history of eczema, but was not associated with gender, family history of asthma, environmental tobacco smoke exposure, wheezing apart from viral respiratory infections, enrollment lung function measures, hospitalization for wheezing, or oral steroid treatment prior to enrollment.[29] At enrollment the mean SB-FENO for the cohort was 28.7 ppb (s.d. 16.7) and mean awake tidal-FENO was 7.8 ppb (s.d. 4.9). SB-FENO and awake tidal-FENO measurements at enrollment were weakly correlated (r=0.33, p=0.04; Figure 1, panel A.), however, at the final visit during which SB-FENO was measured prior to age 3 years, there was no longer a significant correlation between SB-FENO and tidal-FENO measurements (r=0.3, p=0.1; Figure 1, panel B.).
FIGURE 1.
Panel A: correlation between enrollment single-breath exhaled nitric oxide (SB-FENO) concentrations and mixed-expired exhaled nitric oxide concentrations collected during tidal breathing (tidal-FENO) (r=0.33, p=0.04). Panel B: correlation between SB-FENO and tidal-FENO concentrations collected at age 3 years (r=0.3, p=0.1). Correlations determined using the Pearson correlation coefficient.
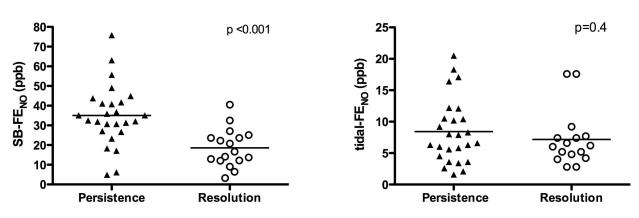
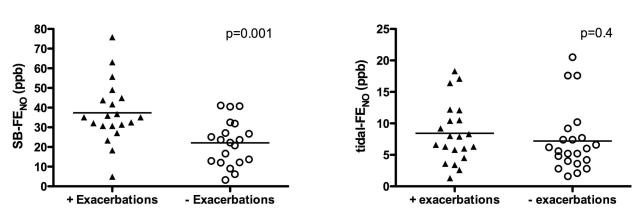
Twenty-five subjects had persistence of wheezing at age 3 years, and during the 6 months prior to age 3 years, 19 subjects experienced an acute exacerbation of wheezing. Enrollment SB-FENO concentrations were significantly higher among subjects who experienced persistence of wheezing at age 3 years than among those subjects who did not (mean 35.1 ppb vs. 18.6 ppb, p < .001; Figure 2, panel A.). The odds of persistence of wheezing was three times higher (OR 3.1; 95% CI 1.5 – 6.4) for every 10 ppb increase in enrollment SB-FENO (Table II). Enrollment SB-FENO concentrations were also significantly higher among subjects who experienced acute exacerbations of wheezing than among those subjects who did not (37.4 ppb vs. 22.1 ppb, p = .001; Figure 3, panel A.). The odds of an acute exacerbation were 2.6 times higher (OR 2.6; 95% CI 1.4 – 5.2) for every 10ppb increase in enrollment SB-FENO (Table 2.). Enrollment tidal-FENO concentrations were not significantly different between children with persistence of wheezing and among those whose wheezing resolved (8.4 ppb vs. 7.1 ppb, p = 0.4: Figure 2, panel B.), or between those who experienced an acute exacerbation and those that did not (8.4 ppb vs. 7.2 ppb, p=0.4; Figure 3, panel B.).
FIGURE 2.
Panel A: enrollment single-breath exhaled nitric oxide (SB-FENO) concentrations were significantly higher among subjects who experienced persistence of wheezing (closed triangles) at age 3 years than among those subjects who experience resolution (open circles) of wheezing (35.1 ppb vs. 18.6 ppb, p < .001). Analysis conducted using an unpaired t test. Panel B: enrollment tidal-FENO concentrations were not significantly different between subjects with persistence of wheezing (closed triangles) and among those whose wheezing resolved (open circles) (8.4 ppb vs. 7.1 ppb, p = 0.4). Analysis conducted using the Mann-Whitney test.
TABLE 2.
Associations between Enrollment SB-FENO and Persistence of Wheezing and Exacerbations of Wheezing
| Persistence of Wheezing | Exacerbations of Wheezing | |||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Enrollment SB-FENO (10 ppb increments) |
3.1 | 1.5 – 6.4 | 2.6 | 1.4 – 5.2 |
| Age at enrollment (mos.) | 1.0 | 0.8 – 1.1 | 1.0 | 0.8 – 1.2 |
| Gender (female) | 0.9 | 0.2 – 4.8 | 1.5 | 0.3 – 7.5 |
| FH Asthma (yes) | 3.7 | 0.6 – 21.5 | 2.4 | 0.5 – 12.2 |
| Eczema (yes) | 0.4 | 0.1 – 2.0 | 0.5 | 0.1 – 2.8 |
| Sustained ICS use during follow-up (yes) |
5.3 | 0.2 - 140 | 9.3 | 0.5 - 186 |
| Tobacco Smoke Exposure (yes) |
2.3 | 0.3 – 16.8 | 2.3 | 0.3 – 16.8 |
Definition of Abbreviations: SB-FENO = single-breath exhaled nitric oxide; ICS = inhaled corticosteroids; FH = family history; OR = odds ratio; CI = confidence interval. N=42 subjects. Bold type denotes a statistically significant association.
FIGURE 3.
Panel A: enrollment single-breath exhaled nitric oxide (SB-FENO) concentrations were significantly higher among subjects who experienced an acute exacerbation of wheezing (closed triangles) between ages 2.5 and 3 years than among those subjects who did not experience an exacerbation (open circles) of wheezing (37.4 ppb vs. 22.1 ppb, p = .001). Analysis conducted using an unpaired t test. Panel B: enrollment tidal-FENO concentrations were not significantly different between subjects who experienced an acute exacerbation (closed triangles) and those that did not (open circles) (8.4 ppb vs. 7.2 ppb, p=0.4). Analysis conducted using the Mann-Whitney test.
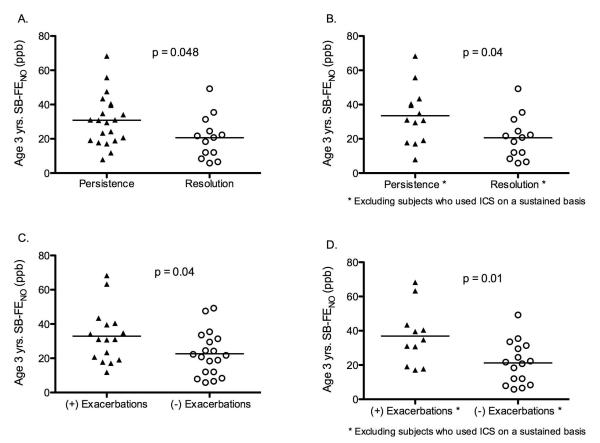
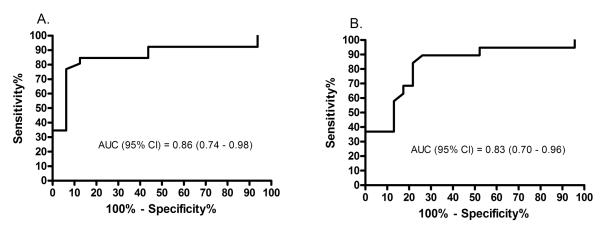
An ROC analysis showed that an enrollment SB-FENO concentration > 30 ppb predicted persistence of wheezing (regardless of trigger) with a sensitivity of 77%, a specificity of 94%, and an area under the curve (AUC) of 0.86 (95% CI: 0.74 – 0.98) (Figure 4; Table 3). A SB-FENO concentration > 30 ppb predicted the prevalence of multi-trigger persistent wheezing prior to age 3 years (n=15 subjects) with a sensitivity of 93%, a specificity of 75%, a positive predictive value of 67%, and negative predictive value of 95%. In contrast, a SB-FENO concentration > 30 ppb predicted the prevalence of URI-triggered persistent wheezing (n=12 subjects) with a sensitivity of 58%, a specificity of 59%, a positive predictive value of 37%, and negative predictive value of 77%. A SB-FENO concentration > 30 ppb predicted acute exacerbations of wheezing with a sensitivity of 84%, a specificity of 78%, and an area under the curve (AUC) of 0.83 (95% CI: 0.7 – 0.96) (Figure 4). The sensitivity, specificity, positive predictive value, and negative predictive values of enrollment SB-FENO for persistence of wheezing and acute exacerbations were superior to enrollment tidal-FENO, bronchodilator responsiveness, or a positive API (Table 3.). The combination of a positive API and a SB-FENO concentration > 30 ppb yielded a modestly improved specificity for predicting persistence of wheezing and exacerbations, however, the sensitivity was much lower than for SB-FENO or the API alone (Table 3).
FIGURE 4.
Single-breath exhaled nitric oxide (SB-FENO) concentrations measured at age 3 years were significantly greater among subjects with persistence of wheezing at age 3 years (closed triangles) as compared to subjects with resolution of wheezing (open circles), both among all subjects (panel A.; 30.8 ppb vs. 20.7 ppb, p=0.048) and when subjects using inhaled corticosteroids on a sustained basis were excluded (panel B.; 33.5 ppb vs. 20.7 ppb, p=0.036). SB-FENO concentrations were also significantly higher among subjects who experienced exacerbations prior to age 3 years (closed triangles) then among subjects who did not experience exacerbations prior to age 3 years (open circles), both among all subjects (panel C.; 32.9 ppb vs. 22.6 ppb, p=0.04) and when subjects using inhaled corticosteroids on a sustained basis were excluded (panel D.; 36.9 ppb vs. 22.2 ppb, p=0.036). All analyses conducted using unpaired t tests.
TABLE 3.
Sensitivity, Specificity, PPV, and NPV of Enrollment SB-FENO, Bronchodilator Responsiveness, and the Asthma Predictive Index for Persistence of Wheezing and Acute Exacerbations of Wheezing
| Persistence of Wheezing at Age 3 Years | ||||
| Predictor | Sensitivity | Specificity | PPV | NPV |
| SB-FENO (>30ppb) | 77 | 94 | 95 | 73 |
| Tidal-FENO (>6.2ppb) | 48 | 56 | 61 | 43 |
| BDR | 32 | 91 | 85 | 43 |
| API | 46 | 63 | 67 | 42 |
| SB-eNO (>30ppb) + positive API |
33 | 100 | 100 | 45 |
| Acute Exacerbations of Wheezing between Ages 2.5 and 3 Years | ||||
| Predictor | Sensitivity | Specificity | PPV | NPV |
| SB-FENO (>30ppb) | 84 | 78 | 76 | 86 |
| Tidal-FENO (>6.2ppb) | 53 | 61 | 52 | 60 |
| BDR | 38 | 88 | 71 | 65 |
| API | 47 | 61 | 50 | 58 |
| SB-eNO (>30ppb) + positive API |
37 | 91 | 78 | 64 |
Definition of Abbreviations: PPV = positive predictive value; NPV = negative predictive value; SB-FENO = single-breath exhaled nitric oxide; Tidal-FENO = mixed expired exhaled nitric oxide during tidal breathing; BDR = bronchodilator responsive; API = Asthma Predictive Index (modified) defined as 1 of 2 major, or 2 minor criteria (major criteria = parental physician diagnosed asthma, or physician-diagnosed and treated eczema; minor criteria = physician diagnosed atopic dermatitis, or wheezing apart from colds).
Increased SB-FENO concentration at enrollment was correlated with a decline in both FEV0.5 (r = −0.67, p = 0.01) and FEF25-75 (r = −0.4, p = 0.02) between enrollment and age 3 years. An ROC analysis showed that an enrollment SB-FENO concentration ≥ 30 ppb predicted a decline in FEV0.5 between enrollment and age 3 years with a sensitivity of 74% and a specificity of 87%, with an area under the curve (AUC) of 0.86 (95% CI: 0.77 , 0.99). Using GEE modeling to analyze lung function data from all three study time points and adjust for age at enrollment, gender, family history of asthma, and sustained use of inhaled corticosteroids during follow-up, we found that an enrollment SB-FENO ≥ 30ppb was associated with a −1.55 z-score decline in FEV0.5 from baseline to age 3 (95% CI: −2.18, −0.93), and a −1.01 z-score decline in FEF25-75 (95% CI: −1.74, −0.28) compared to no change or increase in those with enrollment SB-FENO <30 ppb (Table 4.). There was a significant interaction effect of baseline SB-FENO by FEV05 change (p = 0.013) and FEF25-75 change at 3 years (p = 0.032). Sustained use of inhaled corticosteroids was associated with an overall higher FEV0.5 (0.52 z-score, 95% CI: −0.02, 1.06) and FEF25-75 z-score (0.61, 95% CI: 0.15, 1.08) whereas female gender was associated with a lower lung function (FEV05: −0.68, 95% CI: −1.16, −0.22 and FEF25-75: −0.98, 95% CI: −1.51, −0.46). Tidal-FENO ≥ 7 ppb was associated with a −1.44 z-score decline in FEV05 (95% CI: −2.08, −0.81) and a −0.91 decline in FEF25-75 z-score (95% CI: −1.69, −0.12); again the change in FEV05 (p = 0.014) and FEF25-75 was significantly different for the two levels of baseline tidal FENO. While not statistically significant, baseline lung function z-scores were slightly higher in those with high SB-FENO or tidal FENO than the others; an analyses adjusting for baseline FEV05 and FEF25-75 did not alter the conclusions or substantially alter the change estimates. At the final visit during which SB-FENO was measured prior to age 3 years, SB-FENO was significantly higher among subjects with BDR than among those without BDR (mean 39.2 ppb +/− s.d. 14.6 vs. 27.9 ppb +/− s.d. 13; p = 0.05). In contrast, tidal-FENO was similar among subjects with and without BDR (mean 11.9 ppb +/− s.d. 9.5 vs. 8.5 ppb +/− s.d. 9.6; p = 0.4).
Table 4.
Associations between Enrollment SB-FENO and tidal FENO and Change in LungFunction through Age 3 Years, Adjusted for Gender, Age, FH Asthma, and Sustained ICS use in Follow-up.
| FEV0.5 | FEF25-75 | |||
|---|---|---|---|---|
| ß Coefficient (Δ FEV0.5 z-scores) |
P value | ß Coefficient (Δ FEF25-75 z-score) |
P value | |
| Enrollment SB-FENO (<30 ppb) | −0.02 | 0.94 | 0.95 | .009 |
| Enrollment SB-FENO (≥30 ppb) | − 1.55 | <0.0001 | − 1.01 | 0.006 |
| Enrollment tidal FENO (<7 ppb) | 0.02 | 0.9 | 1.13 | 0.0007 |
| Enrollment tidal FENO (≥7 ppb) | − 1.44 | <0.0001 | − 0.91 | 0.02 |
Definition of Abbreviations: SB-FENO = single-breath exhaled nitric oxide; ICS = inhaled corticosteroids; FH = family history; Δ = change between enrollment and age 3 years, FEV0.5 = forced expiratory volume in 0.5 seconds; FEF25-75 = forced expiratory flow between 25% and 75% of forced expiratory maneuver. N=34 subjects. Associations determined using generalized estimating equation (GEE) regression modeling. P value for test of parameter estimate (β coefficient) differing from zero. Bold type denotes a statistically significant association.
After excluding subjects who used inhaled corticosteroids on a sustained basis during the final 6 months prior to age 3 years (n=9), there was a significant direct correlation between SB-FENO concentrations measured at enrollment and age 3 years (r = 0.6, P = 0.004). SB-FENO concentrations at enrollment were not significantly different from concentrations at age 3 years (mean 28.7 vs. 31.2 ppb, p = 0.2).
DISCUSSION
In a cohort of infants and toddlers (mean age 15.6 months) with a history of recurrent episodes of physician-diagnosed and treated wheezing we found that SB-FENO measured at enrollment was associated with the persistence of wheezing at age 3 years. Furthermore, higher SB-FENO concentrations measured at study entry were associated with an increased risk of exacerbations prior to age 3 years. SB-FENO concentration at enrollment was superior to both the Asthma Predictive Index and the presence of BDR as predictors of the persistence of wheezing at age 3 years and acute exacerbations during the 6 months prior to age 3 years. Both SB-FENO and tidal-FENO measurements at enrollment were associated with a decline in lung function between enrollment and age 3 years. However, in contrast to flow-controlled SB-FENO measurements, we found no association between tidal-FENO measurements at enrollment and persistence of wheezing at age 3 years or exacerbations of wheezing prior to age 3 years. Finally, SB-FENO, but not tidal-FENO, measured prior to age 3 years was associated with BDR.
Our SB-FENO results are consistent with a recent report from a prospective cohort of infants with atopic dermatitis but without prior wheezing wherein SB-FENO measured at enrollment (mean age 10.7 months) was predictive of asthma as well as airway reactivity at age 5 years.[33] However, to our knowledge, our study is the first to perform SB-FENO measurements and obtain longitudinal lung function measurements in a cohort of infants and toddlers with recurrent physician-diagnosed wheezing. Although our finding that SB-FENO is associated with persistence of wheezing at age 3 years is consistent with our hypothesis, follow-up of this cohort into the school-age years will be essential to determine if SB-FENO robustly predicts which wheezy infants will develop atopic asthma and which will experience resolution of wheezing.
Our observed lack of an association between tidal-FENO measured at enrollment and persistence of wheezing at age 3 years or exacerbations of wheezing is consistent with other recent studies of FENO measured during tidal breathing in young children with recurrent wheezing.[34] [35] There are several potential explanations for the poor predictive value of FENO measured during tidal breathing. First, there is potential for contamination of sampled lower airway NO by nasal NO when measurements are performed during tidal breathing given that the soft palate is not closed and nasal NO concentrations are frequently at least 100-fold greater than lower airway NO concentrations.[36] Contamination of measurements by nasal NO therefore would tend to obscure inter-individual differences in lower airway FENO and bias towards a lack of association between tidal-FENO and our clinical outcomes. Secondly, expiratory flow is often variable during tidal breathing measurements conducted in awake young children. Variable expiratory flow is problematic due the highly flow dependent nature of FENO.[27, 28]
Although the prevalence of recurrent wheezing among infants/toddlers is high, this group of children is heterogeneous. The majority of wheezy infants/toddlers do not have asthma, but rather wheeze for a variety of anatomic and/or pathophysiologic reasons, including but not limited to post-viral airway inflammation and injury, small airway caliber, central airway malacia, or dysphagia. For most of these young children episodic wheezing resolves by school age. However, discriminating between infants/toddlers with transient wheezing and young children with evolving asthma is a major challenge for clinicians. Castro-Rodriquez et al proposed the Asthma Predictive Index, derived from epidemiologic asthma risk factors, to assist in predicting asthma in wheezy infants/toddlers.[3] While the API has a high negative predictive value for asthma when applied to recurrently wheezy infants/toddlers, the clinical utility of the API is limited by its poor positive predictive value.[4] Our data demonstrating that the predictive value of SB-FENO is markedly superior to the API through age 3 years suggests that this biomarker holds promise as an adjunctive diagnostic test for asthma in wheezy infants and toddlers. Diagnosing asthma at an early age using an objective test such as FENO could greatly facilitate more targeted use of asthma therapies, prevent under- and over-treatment with corticosteroids, and reduce preventable emergency department visits and hospitalization for asthma exacerbations. However, the lack of an association between tidal-FENO and persistence of wheezing or acute exacerbations dampens enthusiasm for FENO as practical biomarker that could be used clinically on a large scale given that SB-FENO measurements require that children undergo sedation and measurements require specialized equipment as well as technical expertise to perform. Alternatively, SB-FENO measurements may have utility in future clinical trials involving infants and toddlers with recurrent wheezing.
There are several limitations to this study. First, although we obtained clinical follow-up through age 3 years in 42 of the 45 subjects (93%) who completed enrollment FENO measurements, we were unable to obtain infant lung function data at age 3 years in 28% of subjects. Although, a higher percentage of subjects completing follow-up measurements would be preferable, the failure rate of sedation with chloral hydrate increases with age.[37] Chloral hydrate is used to sedate infants for lung function testing by nearly all centers using the RVRTC method, and our overall success rate is comparable to other centers.[38] [39] Second, we measured SB-FENO at an expiratory flow of 50 mL/sec. to be consistent with ATS recommendations for FENO measurements in older children and adults.[36] A positive association between age and FENO has been reported in several large studies of healthy school age children.[40] [41] [42] Possible mechanisms for the age dependency of FENO include a higher relative flow in young subjects with smaller airways compared to older subjects with larger airways, changes in NO diffusion coefficients, changes in nitric oxide synthase expression with age, or changes in S-nitrosoglutathione (GSNO) kinetics. [43] Measurement at 50 mL/sec may not be the ideal flow to maximize the predictive value of this biomarker, therefore future studies in this age group should assess FENO at several different flows. Variable patterns of inhaled corticosteroid use during follow-up limited our ability to assess associations between change in FENO over time and longitudinal lung function and clinical outcomes. Finally, the normative data used to calculate lung function z-scores was obtained from children ranging in age from 1 month to 2.8 years, with a mean age of 1 year [32], while the final lung function measurements in our cohort were obtained near age 3 years. Although the normative data used in our analysis could potentially over- or under-estimate normal flows for children near age 3 years, given that the same reference data was applied to all subjects any potential effect on our results should be non-differential.
An important limitation of this study is that we did not perform skin or radioallergosorbent testing of subjects to food or inhalant allergens at enrollment. Kulig et demonstrated in a birth cohort that development of allergic sensitization to inhalant allergens occurs mostly beyond age 2 years[44], however, ideally we would have objective data characterizing the sensitization status of our subjects at enrollment. Future studies evaluating the predictive value of FENO must include prospective serial measures of allergic sensitization.
Subjects were not receiving any corticosteroid treatment when enrollment SB-FENO concentrations were measured. Although by study design corticosteroids did not bias enrollment SB-FENO concentrations, 9 subjects in our study did use inhaled steroids on a sustained basis during follow-up, and therefore it is possible that inhaled steroid use prior to age 3 years could decrease the incidence of wheezing exacerbations or persistence of wheezing. This effect could have biased our results toward decreased specificity and positive predictive values of enrollment SB-FENO. Another limitation of this study is an insufficient sample size to allow adequate statistical power to determine the sensitivity, specificity and predictive values of FENO for persistence of wheezing independently among the subjects who used inhaled steroids on a sustained basis during follow-up. However, only nine subjects used inhaled steroids on a sustained basis prior to age 3 years. Among those nine subjects, all had persistence of wheezing at age 3 years, and enrollment SB-FENO values were greater than 30ppb in eight of the nine subjects.
In conclusion, we have demonstrated that SB-FENO measurements in infants and toddlers with a history of recurrent episodes of physician-diagnosed wheezing is associated with the persistence of wheezing and risk of acute exacerbations of wheezing through age 3 years, and a decline in lung function between age 15 months and age 3 years. Furthermore, SB-FENO was a superior predictor of these clinical outcomes than the API or documented BDR during lung function testing. In contrast, tidal-FENO, although associated with decline in lung function, was a poor predictor of future clinical outcomes in wheezy infants/toddlers. A larger multi-center study with follow-up into the school-age years will be required to definitely evaluate the predictive value of SB-FENO for asthma in wheezy infants and toddlers.
FIGURE 5.
Receiver Operator Curves (ROC) of enrollment single-breath exhaled nitric oxide (SB-FENO) concentrations as predictors of persistence of wheezing at age 3 yrs. (panel A.) and acute exacerbations of wheezing (panel B.) between ages 2.5 and 3 years.
Acknowledgments
Supported by: NHLBI: K23HL077626; CTSA Grant Number I ULI RR025014-01
Abbreviations
- FENO
fractional concentration of exhaled nitric oxide
- SB-FENO
single-breath exhaled nitric oxide
- Tidal-FENO
mixed expired exhaled nitric oxide during tidal breathing
- BDR
bronchodilator responsive
- API
Asthma Predictive Index
- FVC
forced vital capacity
- FEV0.5
forced expiratory volume in 0.5 seconds
- FEF25-75
forced expiratory flow 25-75% of expiration
- RVRTC
raised volume rapid thoracic compression
- ROC
receiver operating characteristics
- AUC
area under the curve
- NHLBI
National Heart Lung Blood Institute
- ATS
American Thoracic Society
- ERS
European Respiratory Society
References
- 1.Martinez FD. Development of wheezing disorders and asthma in preschool children. Pediatrics. 2002;109:362–7. [PubMed] [Google Scholar]
- 2.NIH/NHLBI National Asthma Education and Prevention Program Expert Panel Report 3 (EPR3): Guidelines for the Diagnosis and Management of Asthma. http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm.
- 3.Castro-Rodriguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med. 2000;162:1403–6. doi: 10.1164/ajrccm.162.4.9912111. [DOI] [PubMed] [Google Scholar]
- 4.Leonardi NA, Spycher BD, Strippoli MP, Frey U, Silverman M, Kuehni CE. Validation of the Asthma Predictive Index and comparison with simpler clinical prediction rules. J Allergy Clin Immunol. 2011;127:1466–72. doi: 10.1016/j.jaci.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Panickar J, Lakhanpaul M, Lambert PC, Kenia P, Stephenson T, Smyth A, Grigg J. Oral prednisolone for preschool children with acute virus-induced wheezing. N Engl J Med. 2009;360:329–38. doi: 10.1056/NEJMoa0804897. [DOI] [PubMed] [Google Scholar]
- 6.Ducharme FM, Lemire C, Noya FJ, Davis GM, Alos N, Leblond H, Savdie C, Collet JP, Khomenko L, Rivard G, Platt RW. Preemptive use of high-dose fluticasone for virus-induced wheezing in young children. N Engl J Med. 2009;360:339–53. doi: 10.1056/NEJMoa0808907. [DOI] [PubMed] [Google Scholar]
- 7.Taylor DR, Pijnenburg MW, Smith AD, De Jongste JC. Exhaled nitric oxide measurements: clinical application and interpretation. Thorax. 2006;61:817–27. doi: 10.1136/thx.2005.056093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wildhaber JH, Hall GL, Stick SM. Measurements of exhaled nitric oxide with the single-breath technique and positive expiratory pressure in infants. Am J Respir Crit Care Med. 1999;159:74–8. doi: 10.1164/ajrccm.159.1.9805021. [DOI] [PubMed] [Google Scholar]
- 9.Baraldi E, Dario C, Ongaro R, Scollo M, Azzolin NM, Panza N, Paganini N, Zacchello F. Exhaled nitric oxide concentrations during treatment of wheezing exacerbation in infants and young children. Am J Respir Crit Care Med. 1999;159:1284–8. doi: 10.1164/ajrccm.159.4.9807084. [DOI] [PubMed] [Google Scholar]
- 10.Gabriele C, Nieuwhof EM, Van Der Wiel EC, Hofhuis W, Moll HA, Merkus PJ, De Jongste JC. Exhaled nitric oxide differentiates airway diseases in the first two years of life. Pediatr Res. 2006;60:461–5. doi: 10.1203/01.pdr.0000238242.39881.64. [DOI] [PubMed] [Google Scholar]
- 11.Moeller A, Diefenbacher C, Lehmann A, Rochat M, Brooks-Wildhaber J, Hall GL, Wildhaber JH. Exhaled nitric oxide distinguishes between subgroups of preschool children with respiratory symptoms. J Allergy Clin Immunol. 2008;121:705–9. doi: 10.1016/j.jaci.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Meyts I, Proesmans M, Van Gerven V, Hoppenbrouwers K, De Boeck K. Tidal off-line exhaled nitric oxide measurements in a pre-school population. Eur J Pediatr. 2003;162:506–10. doi: 10.1007/s00431-003-1215-x. [DOI] [PubMed] [Google Scholar]
- 13.Jatakanon A, Lim S, Kharitonov SA, Chung KF, Barnes PJ. Correlation between exhaled nitric oxide, sputum eosinophils, and methacholine responsiveness in patients with mild asthma. Thorax. 1998;53:91–5. doi: 10.1136/thx.53.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kharitonov SA, Yates DH, Chung KF, Barnes PJ. Changes in the dose of inhaled steroid affect exhaled nitric oxide levels in asthmatic patients. Eur Respir J. 1996;9:196–201. doi: 10.1183/09031936.96.09020196. [DOI] [PubMed] [Google Scholar]
- 15.Lim S, Jatakanon A, John M, Gilbey T, O’Connor BJ, Chung KF, Barnes PJ. Effect of inhaled budesonide on lung function and airway inflammation. Assessment by various inflammatory markers in mild asthma. Am J Respir Crit Care Med. 1999;159:22–30. doi: 10.1164/ajrccm.159.1.9706006. [DOI] [PubMed] [Google Scholar]
- 16.Jatakanon A, Kharitonov S, Lim S, Barnes PJ. Effect of differing doses of inhaled budesonide on markers of airway inflammation in patients with mild asthma. Thorax. 1999;54:108–14. doi: 10.1136/thx.54.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gibson PG, Henry RL, Thomas P. Noninvasive assessment of airway inflammation in children: induced sputum, exhaled nitric oxide, and breath condensate. Eur Respir J. 2000;16:1008–15. [PubMed] [Google Scholar]
- 18.Dupont LJ, Rochette F, Demedts MG, Verleden GM. Exhaled nitric oxide correlates with airway hyperresponsiveness in steroid-naive patients with mild asthma. Am J Respir Crit Care Med. 1998;157:894–8. doi: 10.1164/ajrccm.157.3.9709064. [DOI] [PubMed] [Google Scholar]
- 19.Massaro AF, Gaston B, Kita D, Fanta C, Stamler JS, Drazen JM. Expired nitric oxide levels during treatment of acute asthma. Am J Respir Crit Care Med. 1995;152:800–3. doi: 10.1164/ajrccm.152.2.7633745. [DOI] [PubMed] [Google Scholar]
- 20.Yates DH, Kharitonov SA, Robbins RA, Thomas PS, Barnes PJ. Effect of a nitric oxide synthase inhibitor and a glucocorticosteroid on exhaled nitric oxide. Am J Respir Crit Care Med. 1995;152:892–6. doi: 10.1164/ajrccm.152.3.7663801. [DOI] [PubMed] [Google Scholar]
- 21.Lehtimaki L, Kankaanranta H, Saarelainen S, Turjanmaa V, Moilanen E. Inhaled fluticasone decreases bronchial but not alveolar nitric oxide output in asthma. Eur Respir J. 2001;18:635–9. doi: 10.1183/09031936.01.00000201. [DOI] [PubMed] [Google Scholar]
- 22.Tepper RS, Llapur CJ, Jones MH, Tiller C, Coates C, Kimmel R, Kisling J, Katz B, Ding Y, Swigonski N. Expired nitric oxide and airway reactivity in infants at risk for asthma. J Allergy Clin Immunol. 2008;122:760–5. doi: 10.1016/j.jaci.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith AD, Cowan JO, Brassett KP, Filsell S, McLachlan C, Monti-Sheehan G, Peter Herbison G, Robin Taylor D. Exhaled nitric oxide: a predictor of steroid response. Am J Respir Crit Care Med. 2005;172:453–9. doi: 10.1164/rccm.200411-1498OC. [DOI] [PubMed] [Google Scholar]
- 24.Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, Olin AC, Plummer AL, Taylor DR. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184:602–15. doi: 10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guilbert TW, Morgan WJ, Zeiger RS, Bacharier LB, Boehmer SJ, Krawiec M, Larsen G, Lemanske RF, Liu A, Mauger DT, Sorkness C, Szefler SJ, Strunk RC, Taussig LM, Martinez FD. Atopic characteristics of children with recurrent wheezing at high risk for the development of childhood asthma. J Allergy Clin Immunol. 2004;114:1282–7. doi: 10.1016/j.jaci.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 26.Beigelman A, Mauger DT, Phillips BR, Zeiger RS, Taussig LM, Strunk RC, Bacharier LB, Childhood Asthma R, Education N, National Heart L, Blood I. Effect of elevated exhaled nitric oxide levels on the risk of respiratory tract illness in preschool-aged children with moderate-to-severe intermittent wheezing. Ann Allergy Asthma Immunol. 2009;103:108–13. doi: 10.1016/S1081-1206(10)60162-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franklin PJ, Turner SW, Mutch RC, Stick SM. Measuring exhaled nitric oxide in infants during tidal breathing: methodological issues. Pediatr Pulmonol. 2004;37:24–30. doi: 10.1002/ppul.10382. [DOI] [PubMed] [Google Scholar]
- 28.Franklin PJ, Turner SW, Mutch RC, Stick SM. Comparison of single-breath and tidal breathing exhaled nitric oxide levels in infants. Eur Respir J. 2004;23:369–72. doi: 10.1183/09031936.04.00084604. [DOI] [PubMed] [Google Scholar]
- 29.Debley JS, Stamey DC, Cochrane ES, Gama KL, Redding GJ. Exhaled nitric oxide, lung function, and exacerbations in wheezy infants and toddlers. J Allergy Clin Immunol. 2010;125:1228–34. e13. doi: 10.1016/j.jaci.2010.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Debley JSS, Cochrane E, Elliot M, Redding G. Exhaled Nitric Oxide Predicts Persistence Of Wheezing, Exacerbations, And Decline In Lung Function In Wheezy Infants And Toddlers. Am J Respir Crit Care Med. 2011;183:A1033. doi: 10.1111/cea.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.ATS/ERS statement: raised volume forced expirations in infants: guidelines for current practice. Am J Respir Crit Care Med. 2005;172:1463–71. doi: 10.1164/rccm.200408-1141ST. [DOI] [PubMed] [Google Scholar]
- 32.Jones M, Castile R, Davis S, Kisling J, Filbrun D, Flucke R, Goldstein A, Emsley C, Ambrosius W, Tepper RS. Forced expiratory flows and volumes in infants. Normative data and lung growth. Am J Respir Crit Care Med. 2000;161:353–9. doi: 10.1164/ajrccm.161.2.9903026. [DOI] [PubMed] [Google Scholar]
- 33.Chang DVSE, Mattiello R, Tiller CJ, Kisling J, Tabbey R, Yu Z, Yao W, Kaplan MH, Tepper RS. Relationship Of Atopy, Airway Function, Exhaled Nitric Oxide And Cytokine Production Early In Life To Airway Function And Current Asthma At 5-Years Of Age. Am J Respir Crit Care Med. 2012;185:A2339. [Google Scholar]
- 34.Gabriele C, Jaddoe VW, van Mastrigt E, Arends LR, Hofman A, Moll HA, de Jongste JC. Exhaled nitric oxide and the risk of wheezing in infancy, the generation R study. Eur Respir J. 2011 doi: 10.1183/09031936.00151010. [DOI] [PubMed] [Google Scholar]
- 35.van de Kant KD, Koers K, Rijkers GT, Lima Passos V, Klaassen EM, Mommers M, Dagnelie PC, van Schayck CP, Dompeling E, Jobsis Q. Can exhaled inflammatory markers predict a steroid response in wheezing preschool children? Clin Exp Allergy. 2011;41:1076–83. doi: 10.1111/j.1365-2222.2011.03774.x. [DOI] [PubMed] [Google Scholar]
- 36.American Thoracic S, European Respiratory S. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171:912–30. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 37.Greenberg SB, Faerber EN, Radke JL, Aspinall CL, Adams RC, Mercer-Wilson DD. Sedation of difficult-to-sedate children undergoing MR imaging: value of thioridazine as an adjunct to chloral hydrate. AJR Am J Roentgenol. 1994;163:165–8. doi: 10.2214/ajr.163.1.8010205. [DOI] [PubMed] [Google Scholar]
- 38.Borrego LM, Stocks J, Leiria-Pinto P, Peralta I, Romeira AM, Neuparth N, Rosado-Pinto JE, Hoo AF. Lung function and clinical risk factors for asthma in infants and young children with recurrent wheeze. Thorax. 2009;64:203–9. doi: 10.1136/thx.2008.099903. [DOI] [PubMed] [Google Scholar]
- 39.Lum S, Gustafsson P, Ljungberg H, Hulskamp G, Bush A, Carr SB, Castle R, Hoo AF, Price J, Ranganathan S, Stroobant J, Wade A, Wallis C, Wyatt H, Stocks J. Early detection of cystic fibrosis lung disease: multiple-breath washout versus raised volume tests. Thorax. 2007;62:341–7. doi: 10.1136/thx.2006.068262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buchvald F, Baraldi E, Carraro S, Gaston B, De Jongste J, Pijnenburg MW, Silkoff PE, Bisgaard H. Measurements of exhaled nitric oxide in healthy subjects age 4 to 17 years. J Allergy Clin Immunol. 2005;115:1130–6. doi: 10.1016/j.jaci.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 41.Franklin PJ, Taplin R, Stick SM. A community study of exhaled nitric oxide in healthy children. Am J Respir Crit Care Med. 1999;159:69–73. doi: 10.1164/ajrccm.159.1.9804134. [DOI] [PubMed] [Google Scholar]
- 42.Kissoon N, Duckworth LJ, Blake KV, Murphy SP, Taylor CL, DeNicola LR, Silkoff PE. Exhaled nitric oxide concentrations: online versus offline values in healthy children. Pediatr Pulmonol. 2002;33:283–92. doi: 10.1002/ppul.10023. [DOI] [PubMed] [Google Scholar]
- 43.Shin HY, George SC. Microscopic modeling of NO and S-nitrosoglutathione kinetics and transport in human airways. J Appl Physiol. 2001;90:777–88. doi: 10.1152/jappl.2001.90.3.777. [DOI] [PubMed] [Google Scholar]
- 44.Kulig M, Bergmann R, Klettke U, Wahn V, Tacke U, Wahn U. Natural course of sensitization to food and inhalant allergens during the first 6 years of life. J Allergy Clin Immunol. 1999;103:1173–9. doi: 10.1016/s0091-6749(99)70195-8. [DOI] [PubMed] [Google Scholar]