Abstract
We propose and demonstrate a new method of an all-optical, contactless, one-step injection of single gold nanoparticles through phospholipid membranes. The method is based on the combination of strong optical forces acting on and simultaneous optical heating of a gold nanoparticle exposed to laser light tuned to the plasmon resonance of the nanoparticle. A focused laser beam captures single nanoparticles from the colloidal suspension, guides them towards a phospholipid vesicle and propels them through the gel-phase membrane, resulting in the nanoparticle internalization into the vesicle. Efficient resonant optical heating of the gold nanoparticle causes a pore to form in the gel-phase membrane, a few-hundred nanometers in size, which remains open for several minutes.
Keywords: gold, nanoparticle, phospholipid, membrane, vesicle, injection, drug-delivery
The delivery of hydrophilic biological molecules, such as proteins, DNA and RNA, to the interior of living cells is of extreme importance for biocellular research, gene therapy and drug development.1 The cellular membrane, which is impermeable to most hydrophilic substances, is a barrier shielding the interior of a cell from its surrounding. To pass through this barrier a number of advanced biological, chemical and physical methodologies have been developed in the last three decades, including lipoplex2 and polyplex3 injection, a “gene gun”,4 electroporation,5 photoporation6 and liposomal release.7,8,9,10 Each of these methods has its advantages and disadvantages, which must be considered carefully taking into account the delivery purpose and the cell type.11
Among others the photoporation or optical injection has increasingly attracted attention as it is a contactless, all-optical and therefore aseptic technique. It relies on the transient increase of a phospholipid membrane permeability induced by a laser beam, allowing hydrophilic substances to diffuse across the membrane. Although the effect of photoporation has been extensively studied and employed for DNA and RNA delivery, the mechanism of the membrane permeability increase upon illumination by light has still not been completely understood. Often the photoporation mechanism is discussed as a combination of several processes, depending on the laser source used: heating, thermoelastic stress, multi-photon absorption and generation of a free electron plasma.12 Occurrence of these processes requires extremely high peak laser powers13 and therefore poses a danger of inducing photochemical damage to cell regions illuminated by out-of-focus light.14,15 In this regard, novel, less harmful approaches to the optical poration of phospholipid membranes and injection of hydrophilic substances through membranes are in strong demand.
Here we introduce a novel strategy for active all-optical photoporation of phospholipid membranes with gold nanoparticles which relies on the combination of optical forces acting on and plasmonic heating of gold nanoparticles exposed to laser light. In a previous work, we successfully used gold nanoparticles as local nanoscopic heat sources on phospholipid membranes.16 We have shown that it is possible to induce reversible gel-fluid phase transitions in gel phase giant unilamellar vesicles (GUVs) by irradiating gold nanoparticles, bound to the vesicle membranes, at their plasmon resonance. GUVs have served as model cells for many years and enable the study of processes occurring at the membrane that are difficult or even not possible to investigate in real cells.17 Fluid phase membranes are known to be more flexible than gel phase membranes18 and can be deformed, bent and stretched much easier, i.e. by a smaller force. Recently19 we explored the optical forces acting on a gold nanoparticle under laser irradiation at the plasmon resonance. We have investigated in detail how strong axial optical forces can be used to propel the gold nanoparticles in the direction of the propagation of a laser beam. In the work presented here, we exploit the strong optical forces acting on and efficient plasmonic heating of gold nanoparticles for photoporation of phospholipid membranes.
RESULTS AND DISCUSSION
Injection of nanoparticles immobilized on vesicle membranes
The vesicles used here were grown via the electroformation process20, 21 from a phospholipid 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) with a main transition temperature of 41°C, ensuring that the lipid membrane is in the gel phase at room temperature. An upright dark-field microscope is used for imaging of individual gold nanoparticles and the vesicles (Figure 1a). The injection laser (cw @ 532 nm) is coupled into the microscope and defocused, resulting in a spot size of 6 μm (FWHM) in the focal plane of the microscope objective (Figure 1b). The vesicles are immobilized by a polyelectrolyte coating on the glass cover slide and 80 nm CTAB-coated gold nanospheres are attached to the vesicle membranes prior to injection, as described elsewhere.16
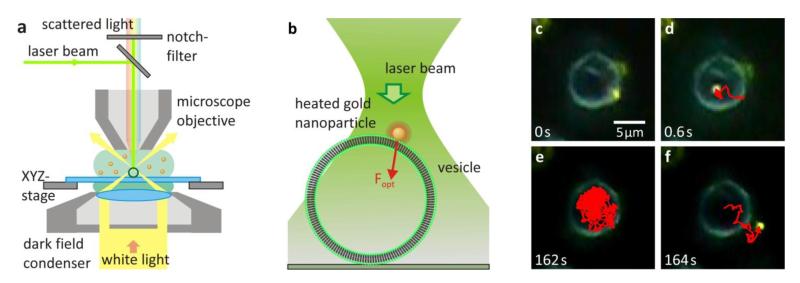
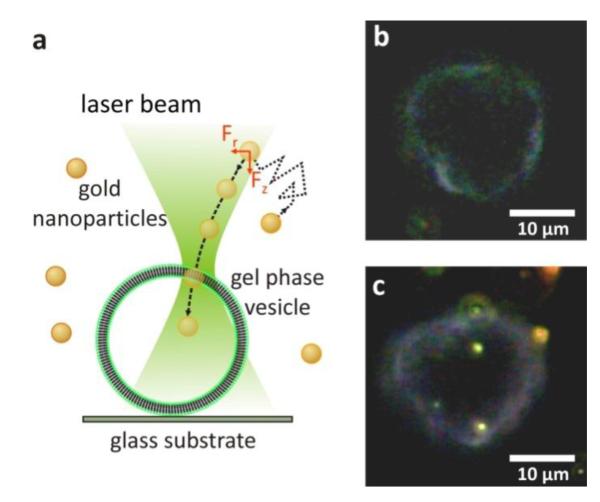
Figure 1. Injecting Nanoparticles from the Membrane.
(a) Sketch of the experimental setup used for optical injection and imaging. (b) Gold nanoparticles are attached to the membrane of giant unilamellar vesicles prior to injection. The laser is defocused, resulting in a spot size of 6μm at the focal plane of the microscope objective. (c) A DPPC vesicle before injection of a gold nanoparticle attached to the membrane. (d-e) The gold nanoparticle movement is tracked (red trace) to show it is confined to the inside of the vesicle. (f) Often, after a certain time the nanoparticle was observed leaving the vesicle at the same position it was injected. This suggests that the injection process forms a pore in the gel phase membrane. (See movie in Supporting Information.)
To perform optical injection of immobilized gold nanoparticles, the nanoparticles are first irradiated by the laser at low laser power densities (P = 50 kW/cm2). Then the laser power density is gradually increased. For power densities P > 80 kW/cm2 the nanoparticles begin to diffuse on the vesicle membrane, indicative of a lipid phase transition to the fluid phase around the nanoparticle. Increasing the laser power further, the nanoparticles are pushed through the vesicle membrane, upon which they begin diffusing inside the vesicle (Figures 1c, d). The injection process was recorded by a digital CCD camera (50 fps) for the single nanoparticle tracking. The tracking confirms that the nanoparticle movement is confined laterally to the inside of the vesicle after internalization (Figure 1e) and the axial confinement is confirmed by comparing the focal planes of the vesicle top and the nanoparticle position. The injection was repeated several times (>20) and was achieved for laser power densities at the gold nanoparticles between 84 and 206 kW/cm2. The internalization of nanoparticles did not occur every time; especially at lower laser powers some of the nanoparticles detach from the membrane and diffuse off through the solution.
Surprisingly, in some cases the nanoparticles were observed to escape the vesicle at the exact position at which they were injected (Figure 1f). This suggests that during the injection a pore in the membrane is formed that can remain open at least for several minutes (See movie 1 in Supporting Information). This stands in contrast to work by previous groups on transient pores in fluid-phase vesicles.22, 23 Here, they find that pores in the membranes reseal automatically within a matter of seconds. The main difference between this previous work and the results shown here lies in the different phases of the phospholipid vesicles, suggesting that the membrane phase plays a critical role in pore formation and temporal evolution.
Injection mechanism
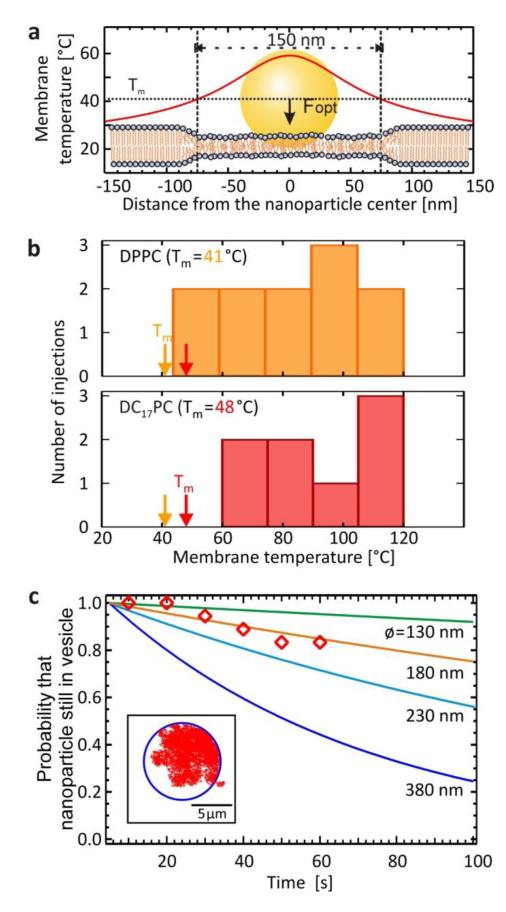
To study the injection process in more detail we repeated the experiments with two additional phospholipids with main transition temperatures below (33°C – DC15PC) and above (48°C – DC17PC) that of DPPC. Injection was also achieved for vesicles comprised of these two phospholipids. To compare the experimental situations for all three phospholipids we have simulated the heat transfer in the system by finite element calculations. The temperature profile of a DPPC membrane underneath an irradiated gold nanoparticle is shown in Figure 2a. During injection a phase transition is induced in a region of the membrane, ranging in size between 100 nm and 230 nm for the power densities of the laser at which injection occurred through DPPC membranes. The maximum temperature inside the membrane for each injection event in the DPPC and DC17PC vesicles is plotted in Figure 2b. Here it is clearly seen that injection only occurs when the maximum temperature in the membrane is optically increased above the main transition temperature of the respective phospholipid. The transition from the gel to the fluid phase is important for the injection process. However due to the high speed of the injection process, the internalization could not be temporally resolved with our video camera (max 50 fps). The observation that injected nanoparticles could leave the vesicle after a certain time suggests a stable pore remains in the membrane after injection. As this happens for vesicles comprised of three different phospholipids, which are all in the gel-phase under experimental conditions, the state of the membrane must be critical for pore formation and temporal evolution. To gather more information about the pore parameters, we observed the inclusion time of 18 nanoparticles inside the vesicles and recorded the times after which they escaped. We performed Monte-Carlo simulations of diffusing gold nanoparticles inside vesicles (Figure 3c, inset) in order to estimate the size of the pore necessary to explain the time needed for a nanoparticle to diffuse out of a vesicle. This time is strongly dependent on the pore size (Figure 3c, solid lines). When we compare the simulations with the actual inclusion times of the measurements (diamonds), we obtain the best fit for a pore size of 180 nm. With a probability of P>0.95 we further exclude values smaller than 130 nm and larger than 380 nm. This value of the pore size not only corresponds well to the size of the fluid phase diameter induced in the membrane by the gold nanoparticle. It is also very close to the diameter of the area needed to completely wrap a 80 nm gold nanoparticle with membrane (160 nm), similar to endocytotic uptake occurring in living cells.
Figure 2. Injection Is Preceded by a Phospholipid Phase Transition.
(a) An illuminated gold nanoparticle heats up the gel phase membrane and can induce a phase transition to the fluid phase in a region 100-200 nm in diameter. (b) Maximum membrane temperatures (Tmax) during optical injection. Two phospholipids with different main transition temperatures (Tm) were used to form the vesicles. Injection only occurred when Tmax > Tm. (c) Monte-Carlo simulations were carried out to estimate the pore size of the vesicle (inset). Here the probability that the nanoparticle is still inside a vesicle of 10 μm diameter (inset) at a given time is plotted for various pore sizes (solid lines). Experiments were recorded on video. 18 videos were analyzed and the relative number of nanoparticles remaining in the vesicles is plotted against time after first entry (diamonds). The data suggest a pore size of 180 nm.
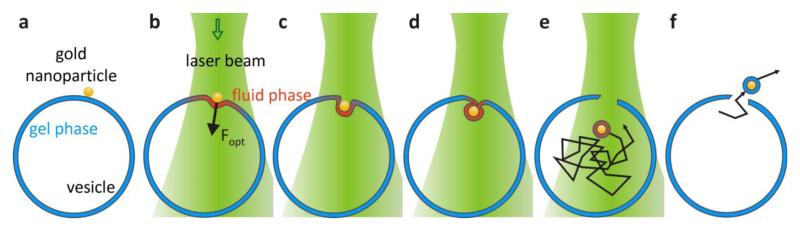
Figure 3. Mechanism of Optical Injection.
(a) The gold nanoparticles are attached to a GUV in the gel phase. (b-d) The gold nanoparticle is irradiated by the laser, inducing a phase change in a membrane region around the nanoparticle into the fluid phase. The optical forces push the gold nanoparticle in to the vesicle and the elastic fluid phase membrane bends inwards until the membrane pinches off and (e) the nanoparticle diffuses through the vesicle. The vesicle membrane cools down quickly after the injection and the phospholipids retract, forming a pore in the membrane (f) enabling the nanoparticle to leave the vesicle again.
Combining the above described observations, we propose a mechanism for the injection of the gold nanoparticles into gel phase phospholipid vesicles (Figure 3). The resonant laser beam heats up the gold nanoparticle which in turn melts the vesicle membrane in its close proximity (Figure 3b). Simultaneously, the strong optical forces acting on the gold nanoparticle push it inwards. The fluid phase membrane is much more flexible18 and bends inwards (Figures 3b, c) until the membrane fully wraps around the nanoparticle and is pinched off (Figure 3d). The nanoparticle can then diffuse inside the vesicle (Figure 3e). The remaining membrane, which has been stretched considerably during the injection, cools down below the main transition temperature and the phospholipids retract creating a pore in the membrane, out of which the nanoparticle can escape (Figure 3f).
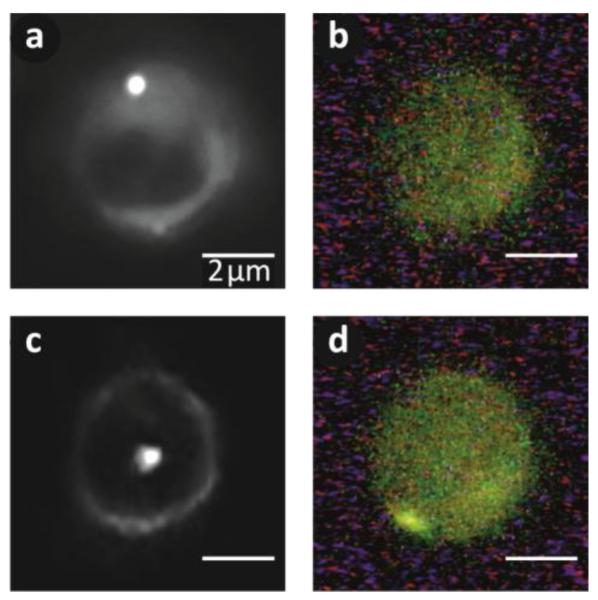
To corroborate this hypothesis, we created GUVs using a 1:500 mixture of DPPC and a dye-tagged phospholipid (1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-(7-nitro-2-1,3-benzoxadiazol-4-yl)) and repeated the experiment. After allowing the gold nanoparticles to bind to the GUVs we viewed the GUVs both in dark-field and in fluorescence mode (Figure 4). In the dark-field image, the gold nanoparticle can be clearly seen bound to the lipid membrane (Figure 4a); however the nanoparticle is invisible in the fluorescence image (Figure 4b). The nanoparticle was then injected into the vesicle and could be seen in dark-field diffusing within the vesicle, as in the previous experiments (Figure 4c). However, after injection the gold nanoparticle is also visible in the fluorescence images (Figure 4d). Since the only source for this fluorescence is the dye-tagged phospholipids, there must be a significant portion of the membrane bound to the gold nanoparticle. This supports our hypothesized injection mechanism in which the membrane wraps around the gold nanoparticle during injection.
Figure 4. Fluorescent Dye Reveals Membrane Wrapping around Injected Gold Nanoparticle.
A fluorescent dye-tagged phospholipid is incorporated into the vesicle membrane to visualize the injection. (a) Before injection the gold nanoparticle can be clearly seen in a dark-field image. (b) By inserting a 500 nm short pass filter in the illumination path and a 532 nm long pass emission filter, only fluorescence is collected. The gold nanoparticle cannot be seen bound to the vesicle. (c) The nanoparticle is injected and diffuses within the confines of the vesicle, which can be seen in dark-field. (d) The same nanoparticle is also visible after injection in the fluorescence image (lower left corner), indicating the membrane actually wraps around the gold nanoparticle during injection.
Injection of freely diffusing nanoparticles into vesicles
As discussed above, to achieve injection the nanoparticles are passively immobilized on the vesicle membranes. This approach is not very efficient due to the necessity of binding the nanoparticles prior to injection. To eliminate this drawback we propose to use optical forces for active delivery of nanoparticles onto the vesicle surface, followed by the injection process (Figure 5a).
Figure 5. Injecting Nanoparticles from the Solution.
(a) Schematic depicting the injection process. The optical forces of the laser catch the nanoparticles from the solution, guide them towards the beam center and the vesicle surface and push them through the membrane. (b) A DPPC vesicle before injection. (c) The same vesicle two minutes later. Four nanoparticles can be seen diffusing inside the vesicle. (See movie in Supporting Information.)
In the proof-of-principle experiment the vesicles are immobilized on the glass substrates (Figure 4b). The gold nanoparticles are added to the solution and the laser is focused onto the vesicle membrane (380 nm FWHM). The laser beam exerts strong optical forces on the gold nanoparticles. For laser powers <60mW nanoparticles are attached optically to the vesicle membrane. The process occurs in the following way: the forces exerted by the laser beam catch a gold nanoparticle out of the solution, guide it towards the center of the beam, and propel it downwards, where it can bind to the vesicle membrane. At higher laser powers (70-90mW) the nanoparticles not only adhere to the membrane, but perforate the latter. Upon entry the nanoparticles also begin to diffuse inside the vesicle. In the experiments we successfully photoporated over 30 vesicles, some up to five or six times (Figure 5c). Every vesicle that we experimented on was photoporated, however the rate of injection varied considerably. This rate is controlled by two parameters, the concentration of gold nanoparticles in the solution and the laser power. While the first parameter controls the probability that a nanoparticle will diffuse into the volume from which it can be injected, the laser power controls the size of this volume. Nanoparticles were also observed exiting the vesicles, an indication that the internalization mechanism is the same as in the experiments in which the nanoparticles were randomly attached to the vesicles prior to injection.
The technique presented here is a novel method for injecting gold nanoparticles through phospholipid membranes and has several advantages. The nanoparticles can be injected into specific vesicles; moreover they can be injected at specific sites of the vesicle. In contrast to previous reports,13 only a single laser beam is required to perform the optical injection in a single step. Additionally the process is highly efficient; 100% of the vesicles we experimented on were perforated. This technique should be applicable to living cells and should also exhibit high cell viability. Since the created pore is only 130-250 nm in size, only a small area of the cell is affected. This size is on the order of or smaller than pores formed in conventional photoporation methods.9 Also, due to the low absorption of the laser light by the membrane, unwanted damage to other parts of the cells can be prevented.
The method presented here, shooting gold nanoparticles through phospholipid membranes by light, can be used for optical transfection. This can be achieved via one of two methods. First, optically driven gold nanoparticles perforate phospholipid membrane allowing substances to diffuse through the membrane as it is realized during conventional photoporation process. Second, light accelerated gold nanoparticles can serve as carries to actively deliver a cargo linked to them, similar to the approach used by the “gene gun”.4
The single cell approach that is demonstrated can easily be extended to large cell-arrays and be fine-tuned by controlling the concentration of gold nanoparticles in the cell medium. A further improvement to this technique can be achieved by using gold nanoparticles presenting a plasmon resonance in the near-infrared window, e.g. nanoshells,24 further reducing the damage to cells or tissue.
Another application for this technique is controlled release from liposomes, another method for drug-delivery. While remotely triggered release from liposomes has been shown previously,25, 26 the here presented technique enables a high control of the release rate, as each injected nanoparticle opens a single pore of well-defined size.
In conclusion, we have optically injected gold nanoparticles into phospholipid vesicles in the gel phase. This novel mechanism relies solely on a single laser tuned to the plasmon resonance of the gold nanoparticles to induce a phase transition of the membrane into the fluid phase and inject the nanoparticle into the vesicle by means of optical forces. During injection a pore is formed the membrane, under 250 nm large and is stable for at least several minutes. The technique is easy to apply, requiring only one laser to achieve injection in a single step. Due to the low laser powers required and the small area of the membrane that is affected this new technique should be much less harmful to cells. It can be easily improved even further by using nanoparticles with plasmon resonances in the near-infrared. The method presented here not only has promise as a single-cell drug-delivery technique, but also for controlled injection in large-scale cell assays, in-vivo experiments and for targeted release the liposomal content.
METHODS
Experimental setup
A Zeiss Axiotech 100 upright microscope with a water-immersion microscope objective (Zeiss, 100x NA 1.0) was used as the basis for the experiments. The injection laser is a Nd:YAG solid state laser emitting cw-light at 532 nm (Spectra-Physics, Millennia VS). Imaging was done with a digital camera (Canon 550D).
Surface modification of gold nanoparticles
80 nm large gold nanoparticles, coated with citrate, were purchased from BBInternational. The surface molecules are replaced with cetyltrimethyl-ammoniumbromide (CTAB), providing the nanoparticles with a positive surface charge. To exchange the surface molecules the gold is mixed with deionised water and a 10 mM CTAB solution in a volume ratio of (5:10:1) and shaken vigorously for one minute. Successful exchange is monitored by zeta potential and extinction spectrum measurements of the solution.
Polymer coating of the glass slides
The slides were covered with a polyelectrolyte layer of polydiallyldimethylammonium chloride (PDADMAC, MW ~ 400,000 – 500,000), providing them with a positive surface charge, following a published procedure:27 Briefly, a PDADMAC layer is deposited onto the freshly cleaned slides by immersion into a PDADMAC solution (1 mg/ ml in 0.5 M NaCl) for 30 minutes followed by thorough rinsing with deionised water.
Growth of giant unilamellar vesicles (GUVs)
GUVs were grown via the electroformation method17, 18 using three different lipids: 1,2-dipentadecanoyl-sn-glycero-3-phosphocholine (DC15PC); 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC); and 1,2-diheptadecanoyl-sn-glycero-3-phosphocholine (DC17PC). The lipids are identical except in the number of methyl-group in the chains (15, 16, and 17 respectively). The temperature was kept at 75°C, so above the main transition temperature of the three phospholipids. A dye-tagged lipid (1,2-dipalmitoylsn-glycero-3-phosphoethanolamine-N-(7-nitro-2-1,3-benzoxadiazol-4-yl)) was added in a ratio of 1:500 to DPPC lipids to create fluorescent vesicles for some of the experiments.
Supplementary Material
Two videos demonstrating the optical injection into vesicles of both immobilized and freely diffusing gold nanoparticles. This material is also available free of charge via the Internet at http://pubs.acs.org.
Acknowledgements
The authors thank J. Rädler for helpful discussions. Financial support by the DFG through the Nanosystems Initiative Munich (NIM) and by the ERC through the Advanced Investigator Grant HYMEM is gratefully acknowledged.
References
- (1).Luo D, Saltzman WM. Synthetic DNA delivery systems. Nat Biotechnol. 2000;18:33–37. doi: 10.1038/71889. [DOI] [PubMed] [Google Scholar]
- (2).Felgner PL, Gadek TR, Holm M, Roman R, Chan HW, Wenz M, Northrop JP, Ringold GM, Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc. Natl. Acad. Sci. U. S. A. 1987;84:7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).WU G, WU C. Receptor-mediated in vitro gene transformation by a soluble DNA carrier system. JOURNAL OF BIOLOGICAL CHEMISTRY. 1987;262:4429–4432. [PubMed] [Google Scholar]
- (4).Klein TM, Wolf ED, Wu R, Sanford JC. High-velocity microprojectiles for delivering nucleic acids into living cells. Nature. 1987;327:70–73. [PubMed] [Google Scholar]
- (5).Neumann E, Schaefer-Ridder M, Wang Y, Hofschneider PH. Gene transfer into mouse lyoma cells by electroporation in high electric fields. EMBO J. 1982;1:841–845. doi: 10.1002/j.1460-2075.1982.tb01257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Tsukakoshi M, Kurata S, Nomiya Y, Ikawa Y, Kasuya T. A novel method of DNA transfection by laser microbeam cell surgery. Appl. Phys. B. 1984;35:135–140. [Google Scholar]
- (7).Wu G, Mikhailovsky A, Khant HA, Fu C, Chiu W, Zasadzinski JA. Remotely Triggered Liposome Release by Near-Infrared Light Absorption via Hollow Gold Nanoshells. J. Am. Chem. Soc. 2008;130:8175–8177. doi: 10.1021/ja802656d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Paasonen L, Laaksonen T, Johans C, Yliperttula M, Kontturi K, Urtti A. Gold nanoparticles enable selective light-induced contents release from liposomes. Journal of Controlled Release. 2007;122:86–93. doi: 10.1016/j.jconrel.2007.06.009. [DOI] [PubMed] [Google Scholar]
- (9).Troutman TS, Leung SJ, Romanowski M. Light-Induced Content Release from Plasmon-Resonant Liposomes. Adv. Mater. 2009;21:2334–2338. doi: 10.1002/adma.200900018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Volodkin DV, Skirtach AG, Möhwald H. Near-IR Remote Release from Assemblies of Liposomes and Nanoparticles. Angew. Chem. Int. Ed. Engl. 2009;48:1807–1809. doi: 10.1002/anie.200805572. [DOI] [PubMed] [Google Scholar]
- (11).Kim TK, Eberwine JH. Mammalian cell transfection: the present and the future. Anal Bioanal Chem. 2010;397:3173–3178. doi: 10.1007/s00216-010-3821-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Stevenson DJ, Gunn-Moore FJ, Campbell P, Dholakia K. Single cell optical transfection. Journal of The Royal Society Interface. 2010;7:863–871. doi: 10.1098/rsif.2009.0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).McDougall C, Stevenson DJ, Brown CTA, Gunn-Moore F, Dholakia K. Targeted optical injection of gold nanoparticles into single mammalian cells. Journal of Biophotonics. 2009;2:736–743. doi: 10.1002/jbio.200910030. [DOI] [PubMed] [Google Scholar]
- (14).Godley BF, Shamsi FA, Liang F-Q, Jarrett SG, Davies S, Boulton M. Blue Light Induces Mitochondrial DNA Damage and Free Radical Production in Epithelial Cells. Journal of Biological Chemistry. 2005;280:21061–21066. doi: 10.1074/jbc.M502194200. [DOI] [PubMed] [Google Scholar]
- (15).Hockberger PE, Skimina TA, Centonze VE, Lavin C, Chu S, Dadras S, Reddy JK, White JG. Activation of flavin-containing oxidases underlies light-induced production of H2O2 in mammalian cells. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:6255–6260. doi: 10.1073/pnas.96.11.6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Urban AS, Fedoruk M, Horton MR, Rädler JO, Stefani FD, Feldmann J. Controlled Nanometric Phase Transitions of Phospholipid Membranes by Plasmonic Heating of Single Gold Nanoparticles. Nano Letters. 2009;9:2903–2908. doi: 10.1021/nl901201h. [DOI] [PubMed] [Google Scholar]
- (17).Bagatolli LA. To see or not to see: Lateral organization of biological membranes and fluorescence microscopy. Biochimica et Biophysica Acta. 2006;1758:1541–1556. doi: 10.1016/j.bbamem.2006.05.019. [DOI] [PubMed] [Google Scholar]
- (18).Heimburg T. Thermal Biophysics of Membranes. WILEY VCH; 2007. [Google Scholar]
- (19).Urban AS, Lutich AA, Stefani FD, Feldmann J. Laser Printing Single Gold Nanoparticles. Nano Letters. 2010;10:4794–4798. doi: 10.1021/nl1030425. [DOI] [PubMed] [Google Scholar]
- (20).Bagatolli LA, Gratton E. Two-photon fluorescence microscopy observation of shape changes at the phase transition in phospholipid giant unilamellar vesicles. Biophys J. 1999;77:2090–2101. doi: 10.1016/S0006-3495(99)77050-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Angelova MI, Dimitrov DS. Liposome electroformation. Faraday Discuss. Chem. Soc. 1986;81:303. [Google Scholar]
- (22).Sandre O, Moreaux L, Brochard-Wyart F. Dynamics of transient pores in stretched vesicles. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:10591–10596. doi: 10.1073/pnas.96.19.10591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Karatekin E, Sandre O, Brochard-Wyart F. Transient pores in vesicles. Polym. Int. 2003;52:486–493. [Google Scholar]
- (24).Averitt RD, Westcott SL, Halas NJ. Linear optical properties of gold nanoshells. J. Opt. Soc. Am. B. 1999;16:1824–1832. [Google Scholar]
- (25).Seol Y, Carpenter AE, Perkins TT. Gold nanoparticles: enhanced optical trapping and sensitivity coupled with significant heating. Opt. Lett. 2006;31:2429–2431. doi: 10.1364/ol.31.002429. [DOI] [PubMed] [Google Scholar]
- (26).Chen Y, Bose A, Bothun GD. Controlled Release from Bilayer-Decorated Magnetoliposomes via Electromagnetic Heating. ACS Nano. 2010;4:3215–3221. doi: 10.1021/nn100274v. [DOI] [PubMed] [Google Scholar]
- (27).Caruso F, Lichtenfeld H, Donath E, Möhwald H. Investigation of Electrostatic Interactions in Polyelectrolyte Multilayer Films: Binding of Anionic Fluorescent Probes to Layers Assembled onto Colloids. Macromolecules. 1999;32:2317–2328. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.