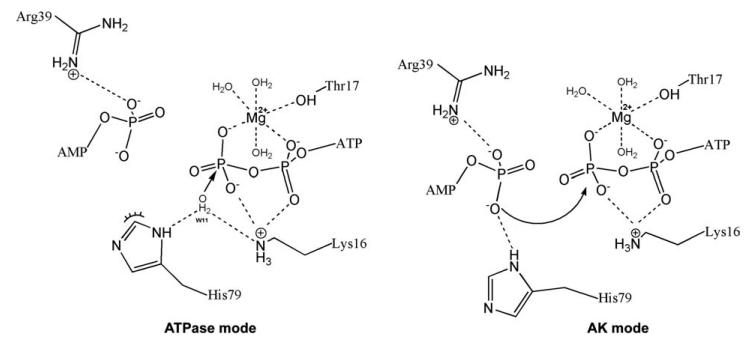
Figure 6.
A model of the role of His79 as a structural switch for the selectivity of enzymatic activity: Juxtaposition of “ATPase mode” and “AK mode.” According to the proposed ring-flip hypothesis, based on the structural analysis, the imidazole nitrogen ND1 of His79 can coordinate a lytic water molecule (together with the NZ of Lys16) during the ATPase reaction (ATP + H2O → ADP + Pi; ATPase-mode) or coordinate the α-phosphate of AMP (together with the NH1 of Arg39) during the reverse AK reaction (ATP + AMP → 2ADP; AK-mode; further details in the “Discussion” section).