Abstract
Low-frequency alleles with large phenotypic effects may guide the development of therapeutics and treatments.
The cost of bringing a drug to market is staggering (estimated at more than 1 billion dollars) and the failure rate is daunting: Only one in three drugs that reach phase 3 clinical trials ultimately reach the marketplace. Accordingly, there has been considerable interest in improving strategies to predict the effects of new therapeutic agents. The outcome of several recent trials of a new class of cholesterol-lowering agents—antibodies against an enzyme called pro-protein convertase subtilisin kexin type 9 (PCSK9) (1) — illustrates the potential role of human genetics not only in identifying drug targets, but also in predicting the likely consequences of specific interventions.
PCSK9 was first implicated in the metabolism of low-density lipoprotein (LDL)—the so-called “bad” cholesterol—when mutations in the PCSK9 gene were shown to cause a rare form of hypercholesterolemia (2). Subsequently, both normal and mutant forms of PCSK9 were found to promote degradation of hepatic LDL receptors (LDLRs), the primary conduit for clearance of circulating LDL (3). This suggested that the PCSK9 mutations that cause hypercholesterolemia confer a gain-of-function. Loss-of-function mutations in PCSK9 would be expected to increase the number of cell surface LDLRs and decrease circulating LDL concentrations. Confirming this prediction, nonsense mutations in PCSK9 are associated with a 30 to 40% reduction in LDL concentrations and confer an 88% reduction in incident coronary heart disease (CHD) with no apparent adverse effects (3). Two women with no PCSK9 and extremely low LDL concentrations (14 to 16 mg/dl) have no apparent health problems (4, 5). These data pointed to PCSK9 inhibition as a potentially effective and safe strategy to prevent CHD.
Despite much effort, no viable small-molecule inhibitors of PCSK9 have been identified. An alternative approach based on the observation that PCSK9 acts from outside of the cell in mice and in cultured cells (3) has proved to be highly effective. In six clinical trials, monoclonal antibodies against PCSK9 that block its interaction with the LDLR reduced plasma concentrations of LDL (up to 70%) with few side effects, even in patients already treated with cholesterol-lowering statins (small molecules that inhibit cholesterol synthesis in the liver) (1). Moreover, anti-PCSK9 therapy was highly effective in patients who did not reach optimal LDL amounts on statins, or who are unable to tolerate statins (1). Phase 3 clinical trials are currently under way to determine the safety of prolonged anti-PCSK9 therapy and whether the additional LDL lowering achieved by inhibiting PCSK9 confers improved protection against CHD.
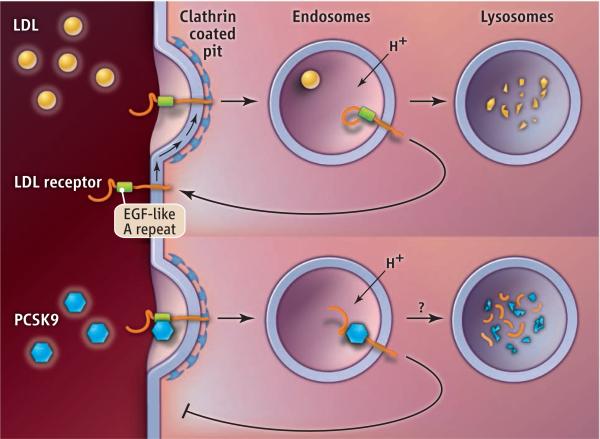
Another approach would be to target pathways required for PCSK9 secretion (6) or degradation. PCSK9 acts by rerouting internalized LDLRs from a pathway that returns them to the cell surface to a pathway that directs them to lysosomes, where they are degraded (see the figure). The structural features of both PCSK9 and LDLR that are required for lysosomal targeting have been defined (3), but the biochemical mechanism by which PCSK9 interdicts receptor recycling, and the pathway(s) by which LDLR-PCSK9 complexes reach the lysosome, are not known. Inhibition of established pathways to degradation in lysosomes, including the endosomal sorting complex required for traffi cking (ESCRT) pathway, does not block PCSK9-mediated degradation of LDLRs (7).
Rerouting. The intracellular pathways of the LDL receptor after binding LDL or PCSK9 are shown. Antibodies that target PCSK9 prevent the rerouting and degradation of LDL receptors, thereby reducing plasma concentrations of LDL. EGF, epidermal growth factor.
The identification of low-frequency PCSK9 alleles with large phenotypic effects facilitated the rapid development of PCSK9-based therapy. This approach is now being advocated as a model for other complex diseases. However, it is not yet clear whether PCSK9 will be paradigmatic or exceptional. The large effects of relatively common PCSK9 alleles on CHD reflect a remarkably linear causal cascade: LDL is the dominant determinant of CHD (8). Other risk factors such as diabetes and smoking only become prominent at permissive amounts of LDL. Circulating concentrations of LDL are profoundly influenced by LDLR activity (9), which in turn is strongly determined by the activity of PCSK9. Differences in PCSK9 activity are thus transduced through the direct coupling of LDLR-LDL to CHD risk. Such tight couplings to single genes may be incompatible with the stringent homeostatic constraints on pathways central to many other complex diseases (e.g., blood glucose, blood pressure, and inflammation).
The PCSK9 alleles that provided a direct path to clinical translation confer protection from, rather than susceptibility to, CHD. Identification of protective alleles is limited by several factors: Alleles that cause disease are likely to be more frequent in clinical populations, whereas protective alleles are not and must be ascertained from the general population. Proving genetic association with protection from disease is more difficult than is proving association with increased risk of disease, and is only feasible for alleles present at appreciable frequencies in the study population. Because purifying selection is a powerful barrier to accumulation of deleterious alleles, few mutations with large phenotypic effects are likely to reach the requisite allele frequencies (10).
The effectiveness of statin therapy for CHD has been firmly established, yet substantial residual risk of disease remains in treated individuals (>50% in most studies). By contrast, comparable reductions in LDL associated with PCSK9 mutations result in very low rates of incident CHD. This suggests that the residual risk of CHD in statin-treated individuals is not due to inadequate cholesterol lowering, or to other risk factors independent of LDL, but rather to delayed initiation of cholesterol-lowering intervention. Early intervention that ensures modest but lifelong reduction of plasma LDL may be the best approach to prevent CHD. Decreasing LDL by the requisite magnitude (~30%) can be easily achieved in most individuals by currently available agents. What then is the role of PCSK9 inhibitors?
Cost and convenience are considered the two major obstacles to large-scale use of antibodies against PCSK9. The importance of inconvenience as a barrier to antibody therapy may be overstated. Clinical experience with immunotherapy for allergy relief indicates that many patients will tolerate regular injections for years. The high cost of monoclonal antibodies (typically more than $1000/month) is likely to be a more substantial obstacle, particularly given that one of the most powerful and widely used statins is available in generic form for ~$10/month. Thus, antibody-based anti-PCSK9 therapy is likely to be targeted at individuals at high risk for CHD in the near term, and to those who do not achieve satisfactory LDL concentrations with, or are unable to tolerate, conventional cholesterol-lowering agents. A broader role for PCSK9-based therapy may have to await the development of small-molecule inhibitors.
Acknowledgments
J.C.C. is a speaker for Merck, Regeneron, and Eli Lilly and Co. J.C.C. and H.H.H. collaborate with Merck and Regeneron on projects unrelated to PCSK9. H.H.H. is on the board of directors of Pfizer.
References and Notes
- 1.Stein EA, Swergold GD. Curr. Atheroscler. Rep. 2013;15:310. doi: 10.1007/s11883-013-0310-3. [DOI] [PubMed] [Google Scholar]
- 2.Abifadel M, et al. Nat. Genet. 2003;34:154. doi: 10.1038/ng1161. [DOI] [PubMed] [Google Scholar]
- 3.Horton JD, et al. J. Lipid Res. 2009;50(suppl.):S172. doi: 10.1194/jlr.R800091-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao Z, et al. Am. J. Hum. Genet. 2006;79:514. doi: 10.1086/507488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hooper AJ, et al. Atherosclerosis. 2007;193:445. doi: 10.1016/j.atherosclerosis.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 6.Chen XW, et al. Elife. 2013;2:e00444. doi: 10.7554/eLife.00444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y, et al. J. Lipid Res. 2012;53:1932. doi: 10.1194/jlr.M028563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen JC, et al. N. Engl. J. Med. 2006;354:1264. doi: 10.1056/NEJMoa054013. [DOI] [PubMed] [Google Scholar]
- 9.Brown MS, Goldstein JL. Science. 1986;232:34. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- 10.Pritchard JK. Am. J. Hum. Genet. 2001;69:124. doi: 10.1086/321272. [DOI] [PMC free article] [PubMed] [Google Scholar]