Abstract
Context
It is not known whether short duration of sleep is a predictor of future cardiovascular events in hypertensive patients.
Objective
To test the hypothesis that short duration of sleep is independently associated with incident cardiovascular diseases (CVD).
Design, Setting, and Participants
We performed ambulatory BP monitoring (ABPM) in 1255 subjects with hypertension (mean age: 70.4±9.9 years) and they were followed for an average of 50±23 months. Short sleep duration was defined as <7.5 hrs (20th percentile). Multivariable Cox hazard models predicting CVD events were used to estimate the adjusted hazard ratio (HR) and 95% CI for short sleep duration. A riser pattern was defined when average nighttime SBP exceeded daytime SBP.
Main Outcome Measures
The end point was cardiovascular events: stroke, fatal or non-fatal myocardial infarction (MI), and sudden cardiac death.
Results
In multivariable analyses, short duration of sleep (<7.5 hrs) was associated with incident CVD (HR=1.68; 1.06–2.66, P=.03). A synergistic interaction was observed between short sleep duration and the riser pattern (P=.089). When subjects were categorized on the basis of their sleep time and riser/non-riser patterns, the shorter sleep+riser group had a substantially and significantly higher incidence of CVD than the predominant normal sleep+non-riser group (HR=4.43;2.09–9.39, P<0.001), independent of covariates.
Conclusions
Short duration of sleep is associated with incident CVD risk, and the combination of riser pattern and short duration of sleep that is most strongly predictive of future CVD, independent of ambulatory BP levels. Physicians should inquire about sleep duration in the risk assessment of hypertensive patients.
INTRODUCTION
Reflecting changing lifestyles, people are sleeping less in modern societies.1 A good sleep of adequate duration is essential because sleep fragmentation and sleep deprivation, commonly seen in contemporary society, are associated with multiple health disorders2 including cardiovascular diseases (CVD).3 Several sleep-related phenomena, such as sleep disordered breathing,4 nocturnal hypertension,5, 6 and high variability of nocturnal BP7, 8 have been suggested to be independent risk factors for cardiovascular events. Short duration of sleep has been shown to be related to obesity,9, 10 diabetes,11 hypertension,12 and sleep apnea in the elderly.13 Sleep deprivation is a risk factor for all cause mortality14 and coronary heart disease15 in epidemiological studies, but these studies were performed in relatively young subjects14, 15 and women;15 this is the first such study to assess ambulatory blood pressure (ABP) and thus be able to examine the role of nocturnal BP pattern in this relationship.
Non-dipping of BP, especially a nocturnal rise of BP, often referred to as the “riser pattern,” has been reported to be associated with cardiovascular events.5, 6 However, it was reported that the higher night-time than daytime BP might not be a cause but a marker of total mortality.16 Verdecchia et al. have reported that the non-dipping nocturnal BP pattern was associated with increased cardiovascular risk, but this association became non-significant when perceived sleep deprivation was statistically controlled.17 Therefore, nocturnal BP could be closely associated with sleep status. Nevertheless, there have been no reports examining the impact on incident CVD of diary-based short sleep time and its possible interaction with nocturnal BP. Furthermore, these relationships have not been examined in the elderly. Thus we tested two hypotheses: first, that short duration of sleep is associated with future cardiovascular events in an elderly hypertensive population; and second, that this association may be moderated by the circadian BP pattern.
METHODS
Setting and Patient Recruitment
This prospective study was performed in a sample of 1268 asymptomatic patients referred for the evaluation of hypertension, who were seen in clinics at 9 participating medical institutions in Japan: 3 clinics, 2 hospitals and one outpatient clinic of a university hospital (the Jichi Medical School- JMS ABPM Study Wave 1); and 1 clinic and 2 hospitals (the Karatsu-Nishiarita Study).18–20 The study designs and assessment of the outcome of these two cohorts were essentially the same differing only with respect to the sites and the time performed. This study was approved by the Institutional Review Board of each participating hospital or clinic. All participants were ambulatory and gave informed consent for the study. During the period of recruitment, 1990–1998 for the JMS ABPM Study Wave 1 sample and 1996–2002 for the Karatsu-Nishiarita Study, all consecutive patients who were being treated or evaluated for hypertension in the clinic and agreed to undergo ABP monitoring (ABPM) were enrolled. Because 13 subjects did not have information of exact sleep time, a total of 1255 subjects were analyzed in this study. The mean age was 70.4 ± 9.9 years (range 33–97 years); there were 476 men and 779 women; 94% of subjects were hypertensive.
Hypertension was diagnosed, according to current guidelines,21 when the clinic systolic BP (SBP) was ≥140 and/or diastolic BP (DBP) was ≥90 mmHg on at least two occasions or when the patient had a previous diagnosis of hypertension and was currently using antihypertensive medication(s). Clinic BP was measured at least twice on two separate occasions after at least 5 min of rest in the sitting position. Subjects took no antihypertensive medications for a minimum of 7 days before the ABPM and most took no medication during the 14 days preceding the ABPM study. Type 2 diabetes was diagnosed according to the guidelines of the American Diabetes Association22 or if the patient had a previous diagnosis and was currently taking anti-diabetic medication. We excluded patients with a history of type 1 or secondary diabetes, renal dysfunction (serum creatinine >1.9 mg/dl), hepatic damage, ischemic heart disease or other cardiac diseases, congestive heart failure, arrhythmias (including atrial fibrillation), stroke (including transient ischemic attacks), or other major concomitant non-cardiovascular diseases. Body mass index (BMI) was calculated as weight/height2 (kg/m2). Smoking was defined as current smoking.
Ambulatory BP Monitoring
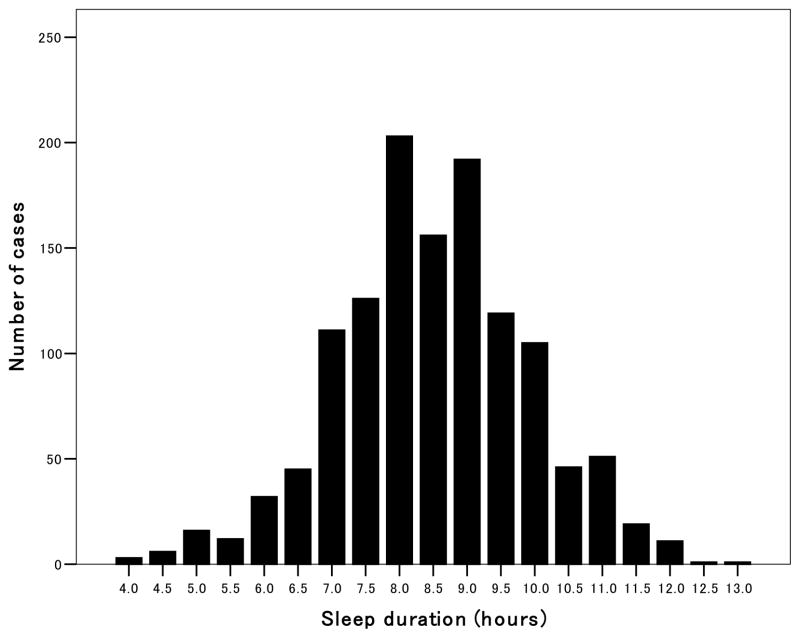
Noninvasive ABPM was performed on a weekday with an automatic system (either ABPM-630, Nippon Colin. Co, TM2421, or TM2425, A&D, Tokyo) which recorded BP by the oscillometric method and pulse rate every 30 minutes for 24 hours. These devices have been previously validated.23, 24 Awake and sleep time were defined based on patients’ diaries recorded during ABPM. The nocturnal BP fall (%) was calculated as (awake SBP–sleep SBP)/awake SBP. We classified subjects’ nocturnal BP fall as follows: riser if the nocturnal BP fall was <0%, non-dipping pattern if it was <10% (including risers).5, 6 Sleep duration was defined as the difference between sleep and wake times. Subjects who reported apparent sleep disturbance as a result of the ABPM procedure were excluded from all analyses. The subjects were divided into two groups according to self-reported sleep duration of more or less than 7.5 hours. This was based on 20th percentile of sleep time in the entire sample (Figure 1). The median (25th and 75th percentiles) of sleep time was 8.5 hours (7.5, 9.5). The cut-off value was based on previous papers12, 25, 26 showing that a sleep time of 7–8 hours had the lowest risk. Because older people tend to sleep (or stay in bed) longer than the young,12, 25–28 the overall sleep time of our population (the mean age 70 years) might have been longer than the other papers. The choice of 7.5 hours as a cutoff for identifying shortened sleep is somewhat arbitrary, reflecting a compromise between seeking to identify a group that was large enough to provide reasonable statistical power and not wanting to include individuals that previous studies12, 25, 26 have identified as being at low risk.
Figure 1.
Histogram of sleep duration in this population. The sleep time was categorized into 30-minute intervals (e.g. 6:01 to 6:30 as 6.5 hours, and 6:31 to 7:00 as 7 hours). The median sleep time was 8.5 hours and the five quintiles were 4.0–7.4, 7.5–7.8, 8.0–8.8, 9.0–9.4, and 9.4–13.0 hours, respectively.
Follow-up and Events
The subjects’ medical records were reviewed once a year after ABPM for the purpose of identifying any new onset of CVD: the 798 participants enrolled in 1990–1998 for the JMS ABPM Study Wave 1 were followed from 1996 to 1998 for up to 5.7 years or until they moved, changed their telephone number or died; the 457 participants enrolled in 1996–2002 for the Karatsu-Nishiarita Study were similarly followed from March 2004 to October 2006 for up to 9.7 years. Participants who died from non-cardiovascular causes were censored as of the time of their death. The average follow-up period was 41 ± 14 months (range: 1 to 68 months) in the JMS ABPM Study Wave 1 and 66 ± 27 months (range: 9 to 116 months) in Karatsu-Nishiarita Study. When subjects did not visit the clinics, we interviewed them by telephone. We assessed three outcomes: stroke, fatal or non-fatal myocardial infarction (MI), and sudden cardiac death. Strokes and cardiac events were diagnosed by the physician, caring for the patient at the time of the event, and independent neurologists or cardiologists reviewed the cases and confirmed the diagnosis. Stroke was diagnosed on the basis of sudden onset of a neurological deficit that persisted for >24 hours in the absence of any other disease process that could explain the symptoms. Stroke events were further classified as ischemic stroke (cerebral infarction and cerebral embolism), hemorrhagic stroke (cerebral hemorrhage and subarachnoid hemorrhage), and undefined types of stroke. We excluded transient ischemic attacks, in which the neurological deficit cleared completely in <24 hours.6 MI was diagnosed based on the AHA criterion of “definite” MI.29 We did not include angina or congestive heart failure as endpoints.
Statistical Analyses
All statistical analyses were carried out with SPSS/Windows, version 13.0 (SPSS Inc., Chicago, Illinois). The data are expressed as the mean (± SD) or percentage. The chi-square test was used to compare proportions. A one-way ANOVA was performed to test differences among group means (Table 1). Kaplan-Meier curves were used to compare the unadjusted survival functions of subgroups and the modified Bonferroni correction30 for multiple tests of significance was used to evaluate the significance of difference between groups (Figure 2). Adjusted hazard ratios (HRs) with 95% confidence intervals (CI) were based on multivariable Cox regression analyses predicting incident CVD with the primary independent variable being sleep time (dichotomized as <7.5 hrs or ≥7.5 hrs). Because the follow-up lengths of the two participant groups were different, the Cox regression models were stratified by site. Model 1 statistically controlled for age (years) and sex (male=1, female=0), with Model 2 further controlling for BMI (kg/m2), current smoking (yes=1, no=0), diabetes status (yes=1, no=0), total cholesterol (mg/dl), serum creatinine (mg/dl), log-transformed triglycerides, and 24-hour SBP; and Model 3 adding riser pattern (yes=1, no=0) as additional control variables. Sleep time was not treated as a continuous variable because a U-shaped relationship between sleep time and mortality has been reported.14 In order to examine the potential moderating effect of circadian BP pattern on the relationship of short sleep time to incident CVD, an interaction term (between sleep time <7.5hrs and the riser pattern) was tested (Table 3) as a post-hoc analysis. The null hypothesis was rejected when two-tailed P<0.05. Because the power to detect interaction effects is low, we used two tailed P<.10 as the criterion to judge statistical significance.
Table 1.
Baseline characteristics of subjects
| 1st (n=248) | 2nd (n=129) | 3rd (n=346) | 4th (n=196) | 5th (n=336) | P | |
|---|---|---|---|---|---|---|
| Age (years) | 67.4 ± 10.2 | 68.2 ± 8.9 | 68.7 ± 9.5 | 72.7 ± 9.5 | 73.8 ± 9.5 | <0.001 |
| Male sex (%) | 37.5 | 40.3 | 32.1 | 39.3 | 42.6 | 0.07 |
| Body mass index (kg/m2) | 24.4 ± 3.5 | 24.0 ± 3.7 | 24.1 ± 3.6 | 23.6 ± 3.2 | 23.5 ± 3.4 | 0.009 |
| Smoking (%) | 22.6 | 25.6 | 18.5 | 23.5 | 23.2 | 0.41 |
| Diabetes (%) | 26.6 | 32.6 | 25.4 | 23.0 | 17.3 | 0.005 |
| Cholesterol (mg/dl) | 204 ± 36 | 200 ± 34 | 204 ± 34 | 199 ± 34 | 198 ± 35 | 0.08 |
| Triglycerides (mg/dl) | 143 ± 82 | 144 ± 71 | 139 ± 76 | 128 ± 61 | 128 ± 76 | 0.02 |
| Serum creatinine (mg/dl) | 0.85 ± 0.22 | 0.83 ± 0.21 | 0.85 ± 0.23 | 0.88 ± 0.23 | 0.86 ± 0.22 | 0.22 |
| Clinic SBP (mmHg) | 160 ± 20 | 158 ± 21 | 160 ± 19 | 160 ± 18 | 163 ± 19 | 0.10 |
| Clinic DBP (mmHg) | 88 ± 13 | 87 ± 13 | 88 ± 14 | 88 ± 13 | 89 ± 14 | 0.72 |
| 24-hour SBP (mmHg) | 140 ± 17 | 140 ± 18 | 138 ± 17 | 137 ± 15 | 139 ± 17 | 0.30 |
| 24-hour DBP (mmHg) | 80 ± 10 | 80 ± 10 | 78 ± 10 | 78 ± 9 | 78 ± 10 | 0.05 |
| 24-hour PR (beats/min) | 72 ± 7 | 71 ± 8 | 69 ± 8 | 69 ± 8 | 69 ± 8 | <0.001 |
| Awake SBP (mmHg) | 146 ± 18 | 147 ± 20 | 145 ± 18 | 143 ± 15 | 146 ± 18 | 0.30 |
| Awake DBP (mmHg) | 83 ± 10 | 83 ± 11 | 82 ± 11 | 81 ± 9 | 82 ± 11 | 0.29 |
| Awake PR (beats/min) | 76 ± 8 | 75 ± 9 | 74 ± 9 | 74 ± 9 | 75 ± 9 | 0.04 |
| Sleep SBP (mmHg) | 128 ± 18 | 129 ± 18 | 126 ± 19 | 127 ± 18 | 129 ± 18 | 0.35 |
| Sleep DBP (mmHg) | 73 ± 10 | 73 ± 11 | 72 ± 11 | 72 ± 10 | 73 ± 10 | 0.38 |
| Sleep PR (beats/min) | 62 ± 9 | 62 ± 8 | 60 ± 8 | 60 ± 8 | 61 ± 8 | 0.03 |
| Night-day SBP ratio | 0.88 ± 0.09 | 0.88 ± 0.08 | 0.88 ± 0.09 | 0.89 ± 0.09 | 0.89 ± 0.09 | 0.54 |
| Non-dipping pattern (%) | 37.1 | 40.3 | 40.5 | 39.8 | 44.6 | 0.47 |
| Risers (%) | 8.1 | 4.7 | 6.9 | 9.2 | 8.0 | 0.61 |
Data are shown as percentage or mean±SD.
SBP, systolic blood pressure; DBP, diastolic blood pressure; PR, pulse rates.
1st: 4.0 – 7.4hrs; 2nd: 7.5–7.8 hrs; 3rd: 8.0–8.8 hrs; 4th: 9.0 – 9.4 hrs; 5th: 9.4–13.0hrs
P-values are based on a 1-way ANOVA for the continuous measures, and the chi-square test of independence for dichotomous measures.
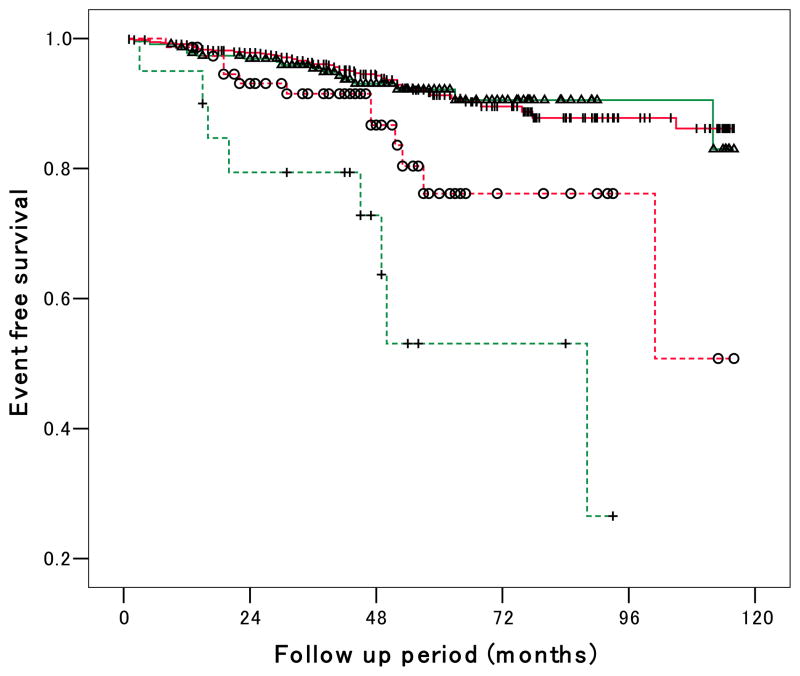
Figure 2.
Event-free survival Kaplan-Meier curves for four categories by shorter/longer sleep time and riser/non-riser pattern. Log-rank statistic between shorter- vs. longer sleep risers is 6.42 (P=0.03), shorter- vs. longer sleep non-risers 0.07 (P=.79). Log-rank statistic between longer sleep risers vs. longer sleep non-risers was 9.45 (P=0.008), and shorter sleep risers vs. shorter sleep non-risers was 23.8 (P<0.001). Log-rank statistic between shorter sleep risers vs. longer sleep non-risers is 35.0 (P<0.001), and longer sleep risers vs. shorter sleep non-risers is 5.66 (P=0.03).
Table 3.
Hazard Ratios Predicting Incident Cardiovascular Events for Subgroups Defined by Sleep Duration and Nocturnal Blood Pressure Pattern
| Non-risers | Risers | |
|---|---|---|
| ≥ 7.5 hrs sleep | 1 --- (n=932) |
1.37 (0.70 – 2.68) (n=75) |
| < 7.5 hrs sleep | 1.27 (0.73 – 2.18) (n=228) |
4.43* (2.09 – 9.39) (n=20) |
Hazard ratios (95% confidence intervals) are shown adjusting for the same variables of Model 2, Table 2.
The interaction term of riser BP pattern with short sleep duration was significant with p=0.089.
p<.001
RESULTS
When the subjects were divided into five groups according to sleep time, the groups with shorter sleep times were younger, and had higher BMI, pulse rates and diabetes rates as graded relationships; however other variables were similar among the five groups (Table 1).
During the follow-up of 50 ± 23 months, 99 cardiovascular events occurred. The incidence of CVD was 2.4/100 person-years in subjects with sleep less than 7.5 hrs and 1.8/100 person-years in subjects with longer sleep time. The Kaplan-Meier survival curves for the four categories of shorter/longer sleep time and riser/non-riser pattern are shown in Figure 2. The group with shorter sleep time who were also risers had the highest incidence of CVD among the 4 groups. Subjects with shorter sleep time had a higher incidence of CVD than those with longer sleep time within the risers, but the incidence of CVD between longer and shorter sleep time was similar in the non-risers. In multivariable Cox regression analyses, shorter sleep time was significantly associated with incident CVD independent of demographic variables (Model 1 in Table 2), plus traditional risk factors (Model 2), plus abnormal (riser) circadian BP pattern (Model 3), which was marginally significant.
Table 2.
Multivariable Cox Regression Analyses of Shortened Sleep Time as a Predictor of Incident Cardiovascular Events
| Covariates | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| P | P | P | ||||
| Sleep time <7.5 hrs (vs. ≥7.5 hrs) | 1.68 (1.06–2.66) | 0.026 | 1.59 (1.00–2.52) | 0.049 | 1.57 (0.99–2.49) | 0.055 |
| Age - mean (per 5 years) | 1.48 (1.32–1.65) | <0.001 | 1.43 (1.26–1.62) | <0.001 | 1.40 (1.23–1.59) | <0.001 |
| Male sex (%) | 1.71 (1.15–2.54) | 0.008 | 1.22 (0.76–1.96) | 0.40 | 1.25 (0.78–2.01) | 0.36 |
| Body mass index (kg/m2) | 1.00 (0.94–1.07) | 0.94 | 1.01 (0.95–1.08) | 0.77 | ||
| Current smoking (yes or no) | 1.80 (1.12–2.87) | 0.015 | 1.78 (1.11–2.85) | 0.016 | ||
| Diabetes (yes or no) | 1.48 (0.93–2.37) | 0.10 | 1.38 (0.86–2.22) | 0.19 | ||
| Cholesterol (per 20 mg/dl) | 1.01 (0.89–1.15) | 0.88 | 1.02 (0.89–1.16) | 0.79 | ||
| Creatinine (per 0.1mg/dl) | 1.09 (1.00–1.19) | 0.06 | 1.10 (1.00–1.20) | 0.039 | ||
| Log triglycerides | 0.87 (0.31–2.50) | 0.80 | 0.85 (0.30–2.47) | 0.77 | ||
| 24-hr SBP - mean (per 10 mmHg) | 1.36 (1.22–1.51) | <0.001 | 1.33 (1.19–1.48) | <0.001 | ||
| Riser (yes or no) | 1.86 (1.10–3.16) | 0.02 | ||||
Values are hazard ratios (95% CI). SBP indicates systolic blood pressure.
All covariates shown in each model were entered altogether for adjustment.
Mean values of age and 24-hour SBP were 70 years and 139mmHg.
Because higher ABP levels and the riser pattern are likely to reflect a failure of the restorative function of sleep, we decided to test whether there was a synergistic effect of ABP and/or the riser pattern on the relationship of short sleep to CVD risk. The interactions between shorter sleep time (<7.5 hrs) and 24h SBP and sleep SBP were not significant (P=.92, P=.97, respectively), but the interaction of shorter sleep time (<7.5hrs) with the riser pattern suggests that this combination of risk factors is associated with a marked increase in CVD risk (Table 3). It needs to be empirically replicated in an independent study. When the same analysis was performed using non-dipper (vs. dipper) pattern instead of riser (vs. non-riser), the interaction of non-dipper status with shortened sleep was not significant (P=0.51). We performed parallel analyses using 24-hour DBP, and the results were essentially the same.
Using the estimates for this model, Table 3 summarizes the relationship of the four groups, defined by the duration of sleep of more or less than 7.5 hours and the presence or absence of the riser pattern, to CVD. The group that had both shorter sleep time plus the riser pattern had a 4.4-fold increase of CVD compared to those with longer sleep time who were not risers. However, the other groups did not exhibit a significantly increased risk of CVD. Thus, although we cannot definitively conclude that there is a synergistic interaction of sleep duration with riser status (as opposed to these two factors having additive effects on the log hazard), it is clear that the group at greatest risk is those who are risers and have shortened sleep.
COMMENT
In this study, a shorter duration of sleep (<7.5 hours) evaluated by a diary during ABPM was an independent predictor for the future incidence of CVD. The riser pattern, a known predictor of CVD, showed a significant interaction with shorter sleep duration. This is the first report showing the prognostic significance of shorter sleep time in combination with the nocturnal BP dipping pattern as a risk for incident CVD.
Sleep time and CVD
In our study, shorter sleep time was associated with increased cardiovascular events independently of other covariates. There have been a few papers showing a significant relationship between short duration of sleep and cardiovascular events. Ayas et al. reported that short duration of sleep was associated with increased risk of coronary events in women.15 But their study was very different from ours: the outcome measure was only coronary events, the subjects were much younger (52 vs. 70 years), and long (as well as short) sleep time was also associated with an increased risk. Some other prospective studies examined the relationship of sleep time and all cause mortality in large epidemiological databases,14, 31, 32 but cardiovascular morbidity or mortality were not analyzed. Perhaps, these older people had their sleep duration shortened by other processes such as depression, 33–35 or nocturia 36, 37 Both these conditions may shorten sleep duration and adversely affect cardiovascular outcome. To our knowledge, ours is the first study showing the relationship between diary-based sleep duration and future cardiovascular events in a mostly elderly cohort. In contrast to previous studies,14, 15, 32 we did not observe a U-shaped relationship between sleep duration and CVD risk (details not shown); this may be because our study patients were relatively healthy, and we did not include cancer or other non-cardiovascular events as outcomes. Therefore, our study findings may generalize to middle aged and elderly patients with uncomplicated hypertension.
Nocturnal BP pattern and sleep time
In addition to BP levels, nocturnal BP dipping patterns, especially non-dipping, are established risk factors for cardiovascular events.5, 6, 38, 39–41 In a recent population study, subjects with higher night-to-day ratio of SBP 1.0 or more were older and at higher risk of death than those whose night-to-day ratio was normal (>0.80 to <0.90).16 One of the presumed causes of an abnormal nocturnal BP dipping pattern is disordered sleep. Greater physical activity during sleep,42, 43 poor sleep quality,44 and sleep apnea episodes45, 46 have been implicated as causes of abnormal BP dipping patterns. Sleep deprivation is related to poor sleep quality, and it worsens obstructive sleep apnea.47 Taking all these findings together, short sleep duration might be related to ABP levels and the non-dipping pattern. However, in our study, ABP levels and the percentage of non-dippers (which included risers) were similar between the shorter sleep time and the longer sleep time groups. There was also no significant interaction effect of shorter sleep time and ABP level on the risk of CVD. Verdecchia et al. reported that the prognostic impact of nocturnal BP disappeared when subjects reported that they slept at least 2 hours less than usual on the night of the ABPM.17 Our study does not have data on perceived sleep deprivation, and their focus was different from ours: they did not analyze the riser pattern and actual sleep time.17 We found a significant interaction of shorter sleep time (<7.5 hours) and the riser pattern on the risk of CVD which, in combination resulted in a 4.4-fold increased risk of CVD when compared with the subjects who slept longer than 7.5 hours and were non-risers. Lack of sleep in hypertensive patients 48 and higher night/day ratio of SBP 49 have both been shown to increase sympathetic nervous activity during the night, therefore short sleep time and the riser pattern might have an interactive effect to increase cardiovascular risk. It is a relatively small group of individuals who have this combination, and thus a group that could be easily identified and monitored more closely.
In our data, serum creatinine was a significant predictor of incident CVD. This is consistent with serum creatinine as a marker for hypertensive target organ damage and a strong risk factor for future CVD 50, 51 even within the normal range.52
Study limitations
There are some limitations in this study. We have no data relating to polysomnography, snoring, or the use of hypnotics. However, there are no data showing that the use of hypnotics affects cardiovascular outcomes. Because our sleep time data are from a diary filled out at the time of the ABPM, the sleep time could have been a little different from the subjects’ usual time. Because our data did not include the subjects who reported sleep disturbance, and our subjects reported a very wide range of sleep times (range 4–13 hours), it was unlikely that the possible minor difference of sleep time during the ABPM interfered with the results. Because of the limited number of risers, the study was underpowered to detect an interaction at the p<0.05 level. Nevertheless the group with both short sleep duration and riser BP pattern were at much higher risk of incident CVD than the other groups shown in Table 3. Finally, our subjects were mostly elderly, so our results may not be applicable to younger populations.
Conclusions
In conclusion, shorter duration of sleep is a predictor of incident cardiovascular disease in elderly hypertensive individuals, particularly when it co-occurs with a riser pattern of nocturnal BP. Its effect tended to be independent of ambulatory BP levels and other standard cardiovascular risk factors.
Acknowledgments
Supported in part by grants-in-aid (1998–1999, 2001–2002, 2004–2005) from the Foundation for the Development of the Community, Tochigi, Japan, the Banyu Fellowship Program sponsored by Banyu Life Science Foundation International, and NHLBI (R24 HL76857 and P01 HL 47540).
References
- 1.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 2.Bonnet MH, Arand DL. Clinical effects of sleep fragmentation versus sleep deprivation. Sleep Med Rev. 2003;7:297–310. doi: 10.1053/smrv.2001.0245. [DOI] [PubMed] [Google Scholar]
- 3.Pickering TG. Could hypertension be a consequence of the 24/7 society? The effects of sleep deprivation and shift work. J Clin Hypertens (Greenwich) 2006;8:819–822. doi: 10.1111/j.1524-6175.2006.05126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolk R, Kara T, Somers VK. Sleep-Disordered Breathing and Cardiovascular Disease. Circulation. 2003;108:9–12. doi: 10.1161/01.CIR.0000072346.56728.E4. [DOI] [PubMed] [Google Scholar]
- 5.Ohkubo T, Imai Y, Tsuji I, et al. Relation between nocturnal decline in blood pressure and mortality. The Ohasama Study. Am J Hypertens. 1997;10:1201–1207. doi: 10.1016/s0895-7061(97)00274-4. [DOI] [PubMed] [Google Scholar]
- 6.Kario K, Pickering TG, Matsuo T, Hoshide S, Schwartz JE, Shimada K. Stroke prognosis and abnormal nocturnal blood pressure falls in older hypertensives. Hypertension. 2001;38:852–857. doi: 10.1161/hy1001.092640. [DOI] [PubMed] [Google Scholar]
- 7.Pringle E, Phillips C, Thijs L, et al. Systolic blood pressure variability as a risk factor for stroke and cardiovascular mortality in the elderly hypertensive population. J Hypertens. 2003;21:2251–2257. doi: 10.1097/00004872-200312000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Verdecchia P, Angeli F, Gattobigio R, Rapicetta C, Reboldi G. Impact of blood pressure variability on cardiac and cerebrovascular complications in hypertension. Am J Hypertens. 2007;20:154–161. doi: 10.1016/j.amjhyper.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 9.Cizza G, Skarulis M, Mignot E. A link between short sleep and obesity: building the evidence for causation. Sleep. 2005;28:1217–1220. doi: 10.1093/sleep/28.10.1217. [DOI] [PubMed] [Google Scholar]
- 10.Kohatsu ND, Tsai R, Young T, et al. Sleep duration and body mass index in a rural population. Arch Intern Med. 2006;166:1701–1705. doi: 10.1001/archinte.166.16.1701. [DOI] [PubMed] [Google Scholar]
- 11.Yaggi HK, Araujo AB, McKinlay JB. Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes Care. 2006;29:657–661. doi: 10.2337/diacare.29.03.06.dc05-0879. [DOI] [PubMed] [Google Scholar]
- 12.Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006;47:833–839. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]
- 13.Neubauer DN. Sleep problems in the elderly. Am Fam Physician. 1999;59:2551–2558. [PubMed] [Google Scholar]
- 14.Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131–136. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 15.Ayas NT, White DP, Manson JE, et al. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. 2003;163:205–209. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- 16.Boggia J, Li Y, Thijs L, et al. Prognostic accuracy of day versus night ambulatory blood pressure: a cohort study. Lancet. 2007;370:1219–1229. doi: 10.1016/S0140-6736(07)61538-4. [DOI] [PubMed] [Google Scholar]
- 17.Verdecchia P, Angeli F, Borgioni C, Gattobigio R, Reboldi G. Ambulatory blood pressure and cardiovascular outcome in relation to perceived sleep deprivation. Hypertension. 2007;49:777–783. doi: 10.1161/01.HYP.0000258215.26755.20. [DOI] [PubMed] [Google Scholar]
- 18.Kario K, Matsuo T, Kobayashi H, Imiya M, Matsuo M, Shimada K. Nocturnal fall of blood pressure and silent cerebrovascular damage in elderly hypertensive patients : advanced silent cerebrovascular damage in extreme dippers. Hypertension. 1996;27:130–135. doi: 10.1161/01.hyp.27.1.130. [DOI] [PubMed] [Google Scholar]
- 19.Kario K, Shimada K, Schwartz JE, Matsuo T, Hoshide S, Pickering TG. Silent and clinically overt stroke in older Japanese subjects with white-coat and sustained hypertension. J Am Coll Cardiol. 2001;38:238–245. doi: 10.1016/s0735-1097(01)01325-0. [DOI] [PubMed] [Google Scholar]
- 20.Eguchi K, Kario K, Shimada K. Greater impact of coexistence of hypertension and diabetes on silent cerebral infarcts. Stroke. 2003;34:2471–2474. doi: 10.1161/01.STR.0000089684.41902.CD. [DOI] [PubMed] [Google Scholar]
- 21.Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the subcommittee of professional and public education of the american heart association council on high blood pressure research. Circulation. 2005;111:697–716. doi: 10.1161/01.CIR.0000154900.76284.F6. [DOI] [PubMed] [Google Scholar]
- 22.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 23.White WB, Lund-Johansen P, McCabe EJ. Clinical evaluation of the Colin ABPM 630 at rest and during exercise: an ambulatory blood pressure monitor with gas-powered cuff inflation. J Hypertens. 1989;7:477–483. doi: 10.1097/00004872-198906000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Imai Y, Sasaki S, Minami N, et al. The accuracy and performance of the A&D TM 2421, a new ambulatory blood pressure monitoring device based on the cuff-oscillometric method and the Korotkoff sound technique. Am J Hypertens. 1992;5:719–726. doi: 10.1093/ajh/5.10.719. [DOI] [PubMed] [Google Scholar]
- 25.Amagai Y, Ishikawa S, Gotoh T, et al. Sleep duration and mortality in Japan: the Jichi Medical School Cohort Study. J Epidemiol. 2004;14:124–128. doi: 10.2188/jea.14.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tamakoshi A, Ohno Y JACC Study Group. Self-reported sleep duration as a predictor of all-cause mortality: results from the JACC study, Japan. Sleep. 2004;27:51–54. [PubMed] [Google Scholar]
- 27.Foley DJ. An epidemiological perspective on one tale of a two-tailed hypothesis. Sleep Medicine Reviews. 2004;8:155–157. doi: 10.1016/j.smrv.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Cappuccio FP, Stranges S, Kandala N-B, et al. Gender-specific associations of short sleep duration with prevalent and incident hypertension: the Whitehall II study. Hypertension. 2007;50:693–700. doi: 10.1161/HYPERTENSIONAHA.107.095471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luepker RV, Apple FS, Christenson RH, et al. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation. 2003;108:2543–2549. doi: 10.1161/01.CIR.0000100560.46946.EA. [DOI] [PubMed] [Google Scholar]
- 30.Holm S. A simple sequentially rejective multiple test procedure. Scandinavian J of Statistics. 1979;6:65–70. [Google Scholar]
- 31.Wingard DL, Berkman LF. Mortality risk associated with sleeping patterns among adults. Sleep. 1983;6:102–107. doi: 10.1093/sleep/6.2.102. [DOI] [PubMed] [Google Scholar]
- 32.Patel SR, Ayas NT, Malhotra MR, et al. A prospective study of sleep duration and mortality risk in women. Sleep. 2004;27:440–444. doi: 10.1093/sleep/27.3.440. [DOI] [PubMed] [Google Scholar]
- 33.Ahto M, Isoaho R, Puolijoki H, Vahlberg T, Kivelä SL. Stronger symptoms of depression predict high coronary heart disease mortality in older men and women. Int J Geriatr Psychiatry. 2007;22:757–763. doi: 10.1002/gps.1735. [DOI] [PubMed] [Google Scholar]
- 34.Mendes de Leon CF, Krumholz HM, Seeman TS, et al. Depression and risk of coronary heart disease in elderly men and women: New Haven EPESE, 1982–1991. Arch Intern Med. 1998;158:2341–2348. doi: 10.1001/archinte.158.21.2341. [DOI] [PubMed] [Google Scholar]
- 35.Ariyo AA, Haan M, Tangen CM, et al. Depressive symptoms and risks of coronary heart disease and mortality in elderly Americans. Circulation. 2000;102:1773–1779. doi: 10.1161/01.cir.102.15.1773. [DOI] [PubMed] [Google Scholar]
- 36.Bursztyn M, Jacob J, Stessman J. Usefulness of nocturia as a mortality risk factor for coronary heart disease among persons born in 1920 or 1921. Am J Cardiol. 2006;98:1311–1315. doi: 10.1016/j.amjcard.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 37.Asplund R. Mortality in the elderly in relation to nocturnal micturition. BJU Int. 1999;84:297–301. doi: 10.1046/j.1464-410x.1999.00157.x. [DOI] [PubMed] [Google Scholar]
- 38.Verdecchia P. Prognostic value of ambulatory blood pressure : current evidence and clinical implications. Hypertension. 2000;35:844–851. doi: 10.1161/01.hyp.35.3.844. [DOI] [PubMed] [Google Scholar]
- 39.Dolan E, Stanton A, Thijs L, et al. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin outcome study. Hypertension. 2005;46:156–161. doi: 10.1161/01.HYP.0000170138.56903.7a. [DOI] [PubMed] [Google Scholar]
- 40.Sega R, Facchetti R, Bombelli M, et al. Prognostic value of ambulatory and home blood pressures compared with office blood pressure in the general population: follow-up results from the Pressioni Arteriose Monitorate e Loro Associazioni (PAMELA) study. Circulation. 2005;111:1777–1783. doi: 10.1161/01.CIR.0000160923.04524.5B. [DOI] [PubMed] [Google Scholar]
- 41.Pickering TG, Shimbo D, Haas D. Ambulatory blood-pressure monitoring. N Engl J Med. 2006;354:2368–2374. doi: 10.1056/NEJMra060433. [DOI] [PubMed] [Google Scholar]
- 42.Kario K, Schwartz JE, Pickering TG. Ambulatory physical activity as a determinant of diurnal blood pressure variation. Hypertension. 1999;34:685–691. doi: 10.1161/01.hyp.34.4.685. [DOI] [PubMed] [Google Scholar]
- 43.Mansoor GA. Sleep actigraphy in hypertensive patients with the ‘non-dipper’ blood pressure profile. J Hum Hypertens. 2002;16:237–242. doi: 10.1038/sj.jhh.1001383. [DOI] [PubMed] [Google Scholar]
- 44.Pedulla M, Silvestri R, Lasco A, et al. Sleep structure in essential hypertensive patients: differences between dippers and non-dippers. Blood Press. 1995;4:232–237. doi: 10.3109/08037059509077600. [DOI] [PubMed] [Google Scholar]
- 45.Portaluppi F, Provini F, Cortelli P, et al. Undiagnosed sleep-disordered breathing among male nondippers with essential hypertension. J Hypertens. 1997;15:1227–1233. doi: 10.1097/00004872-199715110-00006. [DOI] [PubMed] [Google Scholar]
- 46.Akashiba T, Minemura H, Yamamoto H, Kosaka N, Saito O, Horie T. Nasal continuous positive airway pressure changes blood pressure “non-dippers” to “dippers” in patients with obstructive sleep apnea. Sleep. 1999;22:849–853. doi: 10.1093/sleep/22.7.849. [DOI] [PubMed] [Google Scholar]
- 47.Persson HE, Svanborg E. Sleep deprivation worsens obstructive sleep apnea. Comparison between diurnal and nocturnal polysomnography. Chest. 1996;109:645–650. doi: 10.1378/chest.109.3.645. [DOI] [PubMed] [Google Scholar]
- 48.Lusardi P, Zoppi A, Preti P, Pesce RM, Piazza E, Fogari R. Effects of insufficient sleep on blood pressure in hypertensive patients: A 24-h study. Am J Hypertens. 1999;12:63–68. doi: 10.1016/s0895-7061(98)00200-3. [DOI] [PubMed] [Google Scholar]
- 49.Kario K, Motai K, Mitsuhashi T, et al. Autonomic nervous system dysfunction in elderly hypertensive patients with abnormal diurnal blood pressure variation : relation to silent cerebrovascular disease. Hypertension. 1997;30:1504–1510. doi: 10.1161/01.hyp.30.6.1504. [DOI] [PubMed] [Google Scholar]
- 50.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 51.Mancia G, De Backer G, et al. Authors/Task Force M. 2007 Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2007;28:1462–1536. doi: 10.1093/eurheartj/ehm236. [DOI] [PubMed] [Google Scholar]
- 52.Schillaci G, Reboldi G, Verdecchia P. High-normal serum creatinine concentration is a predictor of cardiovascular risk in essential hypertension. Arch Intern Med. 2001;161:886–891. doi: 10.1001/archinte.161.6.886. [DOI] [PubMed] [Google Scholar]