Abstract
BACKGROUND
Visit-to-visit BP variability (BPV) has been shown to be a prognostic indicator in hypertensive patients. We designed this study to clarify the impacts of clinic and ambulatory BPV in predicting cardiovascular disease (CVD).
METHODS
We performed ambulatory BP monitoring in 457 hypertensive patients. Visit-to-visit BPV and ambulatory BPV were calculated as the standard deviations (SDs) of clinic BP, awake BP and sleep BP. The mean age of the subjects was 67.0±9.2 years, and they were followed for 67±26 months. Stroke, myocardial infarction, and sudden cardiac death were defined as Hard CVD events, and these plus angina, heart failure, and other CVDs were defined as All CVD events. Multivariable Cox hazard regression models predicting CVD events were used to estimate the adjusted hazard ratio (HR) and 95% confidence interval (CI) for different measures of BPV with adjustment for significant covariates.
RESULTS
In multivariable analyses, the BPV of clinic SBP was an independent predictor for All CVD events [Hazard ratio (HR), 2.20; 95% CI, 1.25-3.88; P<0.01], but not for Hard CVD events (P=0.20). On the other hand, the BPV of sleep SBP was an independent predictor for Hard CVD events (HR, 2.21; 95% CI, 1.08-4.53; P=0.03), but not for All CVD events (P=0.88). Diastolic BPV exhibited the same pattern.
CONCLUSIONS
These findings suggest that visit-to-visit BPV and ambulatory BPV are separately useful in predicting cardiovascular outcomes.
Keywords: ambulatory blood pressure monitoring, clinic blood pressure variability, ambulatory blood pressure variability, cardiovascular disease
Introduction
It has been more than a decade since blood pressure (BP) variability has been recognized as both a marker and risk factor for cardiovascular disease.1-5 BP variability (BPV) reflects stiffening of the blood vessels,6 sympathetic nerve activation,7 impaired baroreflex sensitivity8 and other intrinsic and social factors.9 In recent years, along with the development of 24-h ambulatory BP monitoring (ABPM) and home BP monitoring (HBP), various types of BPV have been shown to be associated with cardiovascular risk. Variability in ambulatory BP has been shown to be associated with cardiovascular events in subjects with hypertension1, 2, 5, 10 and diabetes.11 Home BP variability, especially day-to-day BPV, has been shown to be associated with adverse cardiovascular prognosis in hypertensive patients.12 Diurnal BP variations, such as morning BP surge13 and a riser pattern,14-16 have been shown to be associated with future stroke events in hypertensive patients.
Short-term BPV, such as episodic hypertension, has not been regarded as important for the assessment of hypertension.17 When clinic BP has been compared with ABPM or HBP, it has been found inferior to both these measures for predicting target organ damage and cardiovascular outcomes.16, 18-22 ABPM and HBP are useful methods to assess BPV because they can provide large quantities of BP data, but one disadvantage of these methods is that there is currently no large database of ABPM and HBP data, such as a large-scale clinical trial. In contrast, visit-to-visit BPV in large scale clinical trials has been shown to be associated with cardiovascular risk. Rothwell et al. have demonstrated that visit-to-visit BPV may be an even stronger predictor than ambulatory BPV in hypertensive subjects.23 However, there is still no appropriate dataset for comparing visit-to-visit clinic BPV and ambulatory BPV as predictors of cardiovascular events. In the present study, we tested the hypothesis that clinic BPV would be superior to ambulatory BPV in predicting CVD.
Methods
This study was performed in a sample of 457 asymptomatic subjects who were seen for the evaluation and management of hypertension in general internal medicine clinics at 3 institutes in Japan: 1 clinic and 2 hospitals that participated in the Karatsu-Nishiarita Study.24
Subjects and definitions
During the period of recruitment, from 1996 to 2002, hypertensive or possibly hypertensive patients who agreed to undergo ABPM were enrolled consecutively in the clinics. The mean age was 67.0 ± 9.2 years (range 33-88 years) and there were 172 men and 285 women. Hypertension was diagnosed when the clinic systolic BP (SBP) was ≥140 mmHg and/or the diastolic BP (DBP) was ≥90 mmHg on at least two occasions according to current guidelines,25 or when there was a previous diagnosis of hypertension with current antihypertensive medication use. Subjects took no antihypertensive medication for a minimum of 7 days before the ABPM, and more than 95% took no medications during the 14 days preceding the ABPM study. Type 2 diabetes was diagnosed according to the guidelines of the American Diabetes Association26 or when there was a previous diagnosis with current use of anti-diabetic medication. We excluded patients with type 1 or secondary diabetes, renal dysfunction (serum creatinine >1.9 mg/dl), hepatic damage (AST, ALT >twice their upper limits), ischemic heart disease or other cardiac diseases, congestive heart failure, arrhythmias (including atrial fibrillation), stroke (including transient ischemic attacks), or other major concomitant non-cardiovascular diseases. Ischemic heart disease and stroke were checked by attending doctors with medical records, physical examinations, and laboratory and radiological findings, and those were reviewed by the investigators. Body mass index (BMI) was calculated as weight (kg)/height2 (m2). Current smoking status was defined as smoking within the past year. This study was approved by the Institutional Review Board of each participating hospital or clinic. All the subjects studied were ambulatory and gave informed consent for the study.
Clinic BP measurement
At baseline, 3 clinic BP readings were taken on each of at least two visits (6 readings in all) after at least 5 min of rest in the sitting position, which included before or after being fitted with an ABPM in subjects who stopped medication for ABPM. “Baseline clinic BP,” was defined as the average BP from two different visits in untreated subjects. For treated subjects, the antihypertensive medications were stopped for 14 days and the BP readings during the untreated period were used. After the baseline assessment, clinic BP was measured with a mercury sphygmomanometer every month. Clinic BP was measured three times after a 5-min rest and the average of the 2nd and 3rd readings was recorded. All available clinic BP assessments between the baseline and the end of follow up were entered into the database for each subject, and their average (“mean clinic BP”) was used in subsequent analyses. The number of post-baseline clinic BP assessments ranged from 1 to 78 (average±SD, 36.5±22.6 assessments per subject), and the 5 subjects who had only 1 assessment were excluded from the analysis.
Ambulatory BP monitoring
Noninvasive ABPM was performed on a weekday with an automatic system (either TM2421 or TM2425; A&D, Tokyo) which records BP, using the oscillometric method and pulse rate every 30 min for 24 h. These devices have been previously validated.27 Awake and sleep times were defined based on patients’ written diaries recorded during ABPM. Mean awake and sleep levels of SBP and DBP were computed and the nocturnal BP fall (%) was calculated as (awake SBP–sleep SBP)/awake SBP.
Follow-up and events
During the follow-up period, standard medical therapy was performed based on current guidelines.28, 29 The subjects’ medical records were reviewed annually for the purpose of identifying incident CVD. When annual contact was not sufficient, a research assistant made phone calls for missing subjects. Attending doctors reviewed all the medical records, blinded to ABPM data, and the authors evaluated the endpoints based on the following criteria. Strokes and cardiac events were diagnosed by the physician caring for the patient at the time of the event, and independent neurologists or cardiologists reviewed the cases and confirmed the diagnosis by referrals or medical records including brain CT or MRI. MI was diagnosed based on the American Heart Association criterion of “definite” MI.30 Stroke was diagnosed on the basis of sudden onset of a neurological deficit that persisted for >24 h in the absence of any other disease process that could explain the symptoms.15 Stroke events included ischemic stroke (cerebral infarction and cerebral embolism), hemorrhagic stroke (cerebral hemorrhage and subarachnoid hemorrhage), and undefined types of stroke. Sudden cardiac death was defined as sudden unexpected death due to cardiac causes within one hour after the onset of symptom.
A follow-up examination was performed in all participants in the Karatsu-Nishiarita Study from March 2004 to October 2007. The mean follow-up period was 66±27 months. We defined three outcomes as Hard CVD events: stroke (n=26), fatal or non-fatal myocardial infarction (MI; n=5), and sudden cardiac death (n=3). Participants who became dependent in their daily living (n=6), those who died or suffered from non-cardiovascular causes such as malignant disease, accident, or neurologic disorders (n=16), and those who moved or changed their telephone number (n=4) were censored as of the time such events took place (n=26 subjects in total). In this study, angina confirmed by a significant stenosis by coronary angiography (n=7), congestive heart failure requiring hospitalization (n=9), end-stage renal disease requiring hemodialysis (n=2), peripheral artery disease confirmed by objective tests such as ankle-brachial index <0.9 (n=3)31 and transient ischemic attacks requiring hospitalization (n=3), in which the neurological deficit was completely cleared within 24 h,15 were treated as Soft CVD events. These CVD events were combined with Hard CVD events to define All CVD events. When subjects did not visit the clinics, we interviewed them by telephone.
Statistical analyses
All statistical analyses were carried out with IBM SPSS Statistics, version 19 (IBM Inc., Armonk, New York). The data are expressed as the mean (± SD) or percentage. BPV was measured as the standard deviation (SD) of the clinic BP assessments, the awake ABP readings and the sleep ABP readings. The chi-square test was used to compare proportions. The independent samples t-test was performed to test group differences in means. In the survival analyses for Hard CVD, duration of follow up was defined as the months from the baseline to the first occurrence of a Hard CVD event, or last follow-up date of subjects who had soft CVD or no CVD event; for All CVD events, the duration of follow up was defined as the months from the baseline to the first occurrence of a Hard or Soft CVD event, or last follow-up date of subjects without any CVD event. All inferential statistics are based on the Cox regression analyses (see below) where the BPV measures are treated as continuous predictors. BP readings after the onset of the first CVD event were excluded from the computation of mean clinic BP and clinic BPV measures. Adjusted hazard ratios (HRs) with 95% confidence intervals were based on univariate and multivariable Cox regression analyses. HRs for each BPV measure were expressed as HR per 5mmg increase in BPV and per 1 SD increase in BPV. In preliminary analyses, we performed Cox regressions using all potential predictors except the BPV parameters. We included age, sex, BMI, smoking status, the presence of diabetes, serum creatinine, cholesterol, and clinic BP at baseline. Those variables with P<0.10 in the preliminary analysis were included as covariates in the primary analysis: the selected variables were age, diabetes, creatinine, smoking, and clinic SBP for All CVD, and age, diabetes, and creatinine for Hard CVD events. The null hypothesis concerning the effect of BPV on incident CVD was rejected when two-tailed P<0.05. The results of a post-hoc power analysis for the Cox regression analyses are reported. In order to illustrate the univariate associations of BPV with incident CVD, we dichotomized the continuous predictors at their medians (the cutoff values were 13.3 mmHg for clinic BPV and 12.2 mmHg for sleep BPV) and present the separate Kaplan-Meier survival curves for those in the top and bottom halves of the BPV distribution.
Results
Table 1 shows the baseline characteristics of subjects. The mean age was 67.0±9.2 years; females outnumbered males; 44% had diabetes; and 56% of the subjects were on antihypertensive treatment.
Table 1.
Baseline Characteristics of Subjects, N=457
| Mean ± SD or % |
Range Min, Max |
|
|---|---|---|
| Age, years | 67.0 ± 9.2 | 33, 88 |
| Sex, male % | 37.6 | |
| Body mass index, kg/m2 | 23.9 ± 3.5 | 15.4, 46.3 |
| Current smoking, % | 24.9 | |
| Type 2 diabetes, % | 44.2 | |
| Duration of hypertension, years | 6.2 ± 7.4 | 0, 54 |
| Antihypertensive medications, % | 55.6 | |
| Total cholesterol, mg/dl | 203 ± 35 | 101, 306 |
| Triglycerides, mg/dl | 124 ± 65 | 36, 400 |
| Creatinine, mg/dl | 0.77 ± 0.21 | 0.30, 1.79 |
| Clinic systolic BP, mmHg | 154 ± 20 | 100, 210 |
| Clinic diastolic BP, mmHg | 84 ± 12 | 47, 116 |
| 24hr systolic BP, mmHg | 140 ± 17 | 99, 199 |
| 24hr diastolic BP, mmHg | 79 ± 10 | 55, 106 |
| 24hr pulse rate, bpm | 68 ± 9 | 42, 98 |
| Awake systolic BP, mmHg | 146 ± 18 | 102, 213 |
| Awake diastolic BP, mmHg | 83 ± 10 | 56, 110 |
| Awake pulse rate, bpm | 72 ± 9 | 43, 103 |
| Sleep systolic BP, mmHg | 129 ± 19 | 85, 187 |
| Sleep diastolic BP, mmHg | 73 ± 10 | 47, 103 |
| Sleep pulse rate, bpm | 61 ± 9 | 38, 93 |
| Night/day ratio of SBP | 0.89 ± 0.09 | 0.60, 1.26 |
| BPV (sd) of clinic systolic BP, mmHg | 13.7 ± 4.6 | 2.9, 40.9 |
| BPV (sd) of clinic diastolic BP, mmHg | 8.2 ± 2.9 | 0, 33.2 |
| BPV (sd) of Awake systolic BP, mmHg | 17.7 ± 5.2 | 7.9, 36.4 |
| BPV (sd) of Awake diastolic BP, mmHg | 10.5 ± 2.5 | 5.1, 19.8 |
| BPV (sd) of Sleep systolic BP, mmHg | 12.9 ± 4.5 | 4.2, 30.5 |
| BPV (sd) of Sleep diastolic BP, mmHg | 8.6 ± 2.6 | 0, 21.1 |
BP, blood pressure; BPV, blood pressure variability; SD, standard deviation
During the follow-up period, 34 Hard CVD events and 58 All CVD events occurred. In univariate analyses of systolic BPV measures, BPV of sleep SBP was significantly associated with Hard CVD events, while BPV of clinic and both ambulatory BPV measures were significantly associated with All CVD events (Table 2). While the results were similar for diastolic BPV when predicting Hard CVD events, only the BPV of clinic DBP was a significant predictor of All CVD events (Table 2). The results of the multivariable Cox regression analyses for BPV that controlled for significant covariates are shown in Table 3. Ambulatory awake systolic BPV and diastolic BPV were not significantly associated with either outcome after adjustment for covariates. Therefore, for ambulatory BPV, only sleep systolic BPV and clinic systolic BPV were included in the models. For Hard CVD events, sleep systolic BPV was a significant predictor independent of clinic systolic BPV and the covariates; on the other hand, for All CVD events, clinic systolic BPV was a significant predictor independent of sleep systolic BPV and the other covariates (Table 3). The hazard ratio (95% CI) for clinic systolic BPV when predicting All CVD events did not change even when the use of antihypertensive medication(s) at baseline was entered into the model (HR, 1.48 per 5 mmHg, 95%CI=1.12-1.97, P=0.007). Diastolic BPV exhibited a similar pattern of results to systolic BPV (data not shown).
Table 2.
Univariate Analyses of Systolic and Diastolic BP Variability for Cardiovascular Events
| Hazard ratio | Hard CVD 95% CI |
P | Hazard ratio | All CVD 95% CI |
P | |
|---|---|---|---|---|---|---|
| Systolic BP measures | ||||||
| BPV (sd) of Clinic SBP per 5mmHg (per 1SD) | 0.95 (0.96) | 0.61-1.48 | 0.82 | 1.58 (1.52) | 1.25-2.00 | <0.01 |
| BPV (sd) of Awake SBP per 5mmHg (per 1SD) | 1.28 (1.30) | 0.96-1.72 | 0.09 | 1.27 (1.28) | 1.02-1.59 | 0.04 |
| BPV (sd) of Sleep SBP per 5mmHg (per 1SD) | 1.58 (1.51) | 1.15-2.17 | <0.01 | 1.37 (1.33) | 1.07-1.78 | 0.02 |
| Diastolic BP measures | ||||||
| BPV (sd) of Clinic DBP per 5mmHg (per 1SD) | 1.10 (1.06) | 0.58-2.08 | 0.77 | 1.55 (1.29) | 1.12-2.14 | <0.01 |
| BPV (sd) of Awake DBP per 5mmHg (per 1SD) | 1.42 (1.19) | 0.75-2.68 | 0.28 | 1.29 (1.14) | 0.79-2.11 | 0.31 |
| BPV (sd) of Sleep DBP per 5mmHg (per 1SD) | 2.04 (1.45) | 1.14-3.65 | 0.02 | 1.59 (1.27) | 0.99-2.57 | 0.06 |
These variables were entered one-by-one.
CVD, cardiovascular disease; CI, confidence interval; BPV, blood pressure variability; SBP, systolic blood pressure; DBP, diastolic blood pressure; SD, standard deviation.
Table 3.
Multivariable Cox regression analyses of Systolic BP variability for Cardiovascular Events
| Hard CVD | All CVD | |||||
|---|---|---|---|---|---|---|
| Hazard ratio |
95% CI | P | Hazard ratio | 95% CI | P | |
| Age, mean per 10y | 2.27 | 1.37-3.76 | <0.01 | 1.63 | 1.16-2.31 | <0.01 |
| Diabetes (yes or no) | 2.99 | 1.35-6.63 | <0.01 | 2.97 | 1.61-5.45 | <0.01 |
| Creatinine level per 0.1 mg/dl | 1.24 | 1.05-1.46 | 0.01 | 1.12 | 0.98-1.28 | 0.09 |
| Current smoking (yes or no) | - | - | - | 1.61 | 0.88-2.94 | 0.12 |
| Clinic SBP, mean per 10 mmHg | - | - | - | 1.18 | 1.03-1.35 | 0.02 |
| BPV (sd) of clinic SBP, per 5 mmHg (per 1sd) | 0.75 (0.76) | 0.48-1.17 | 0.20 | 1.48 (1.44) | 1.12-1.97 | <0.01 |
| BPV (sd) of sleep SBP, per 5 mmHg (per 1sd) | 1.49 (1.43) | 1.04-2.13 | 0.03 | 1.03 (1.02) | 0.74-1.43 | 0.88 |
These variables were entered together for each endpoint.
CVD, cardiovascular disease; CI, confidence interval; BPV, blood pressure variability; SBP, systolic blood pressure; SD, standard deviation.
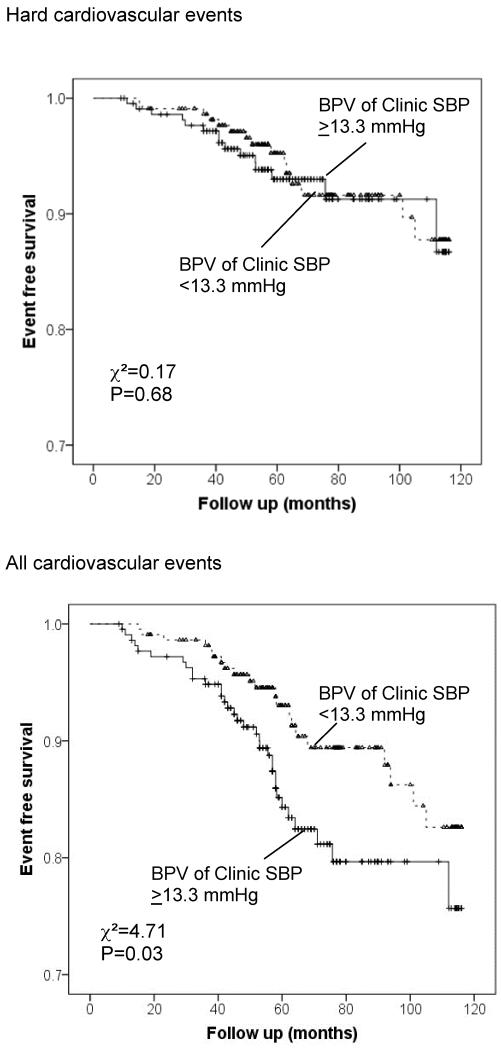
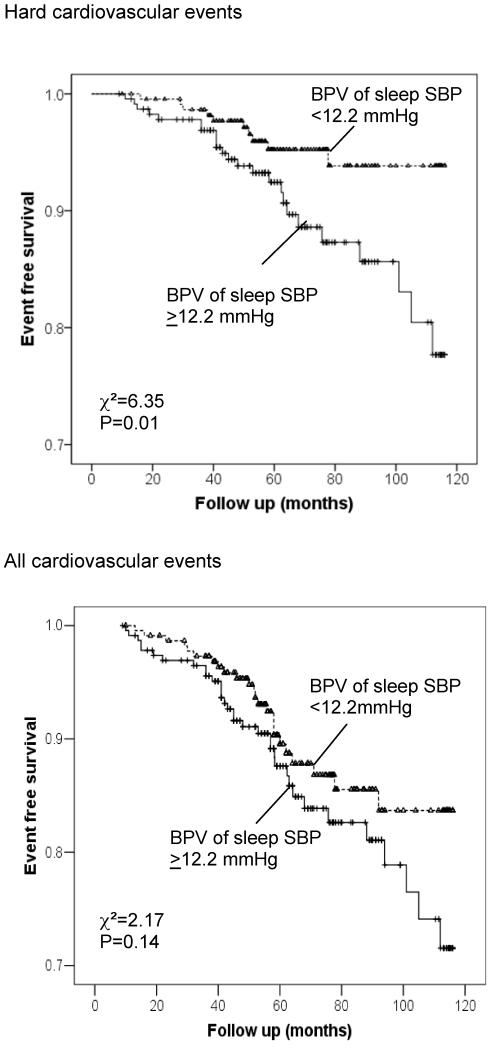
The Kaplan-Meier survival curves for those above and below the median of clinic systolic BPV (≥ 13.3 mmHg or <13.3 mmHg for both Hard CVD and All CVD) are shown in Figure 1. For Hard CVD events, the two survival curves are similar, whereas for All CVD, those with BPV of Clinic SBP >13.3mmHg had a significantly higher event rate than those with BPV <13.3mmHg. In terms of sleep systolic BPV, the survival curves were similar between the group with BPV of sleep systolic BPV >12.2 mmHg and the group with BPV<12.2mmHg for All CVD events, but was significantly different for Hard CVD events (Figure 2).
Figure1.
Kaplan-Meier curves of event-free survival for two categories of clinic systolic BPV (SD ≥13.3 vs. <13.3 mmHg) for Hard cardiovascular events (upper panel) and All cardiovascular events (lower panel).
Figure 2.
Kaplan-Meier curves of event-free survival for two categories of sleep systolic BPV (SD ≥12.2 vs. <12.2 mmHg) by ABPM for Hard cardiovascular events (upper panel) and All cardiovascular events (lower panel).
When the number of clinic BP assessments were divided into tertiles, those in the lowest tertile (1-22 readings), and middle tertile (23-50 readings) were at greater risk for Hard CVD than those in the highest tertile (51-78 readings) (HR=3.91, 95%CI=1.44-10.62, P<0.01 and HR=2.87, 95%CI=1.07-7.73, P=0.04, respectively); for All CVD, HR=3.46, 95%CI=1.60-7.50, P<0.01 for lowest tertile and HR=2.75, 95%CI=1.35-5.59, P<0.01 for middle tertile than those in the highest tertile. These are largely tautological, since clinic BP assessments obtained after a CVD event were not used. When we further added the interaction of tertile with Clinic BPV to the Cox regression model, the interactions were not significant (all p-values >0.4), indicating that the effect of clinic BPV on CVD risk was not associated with the number of clinic BP assessments. Therefore, there was no relationship between the number of BP readings and the predictability of events. Parallel analyses predicting All CVD yielded equivalent results.
Although there were significant correlations of the clinic systolic BPV with the ambulatory sleep and awake systolic BPV measures (r=0.19, P<0.01; r=0.20, P<0.01; respectively), multicollinearity among these measures was clearly not an issue (variance inflation factor <2.0).
Post hoc power calculations for our study showed that the analyses predicting hard CVD events had 80% power to detect a hazard ratio of 1.62 (per 1 SD) and 90% power to detect a HR of 1.76 (per 1 SD). The analyses predicting All CVD events had 80% power to detect a HR of 1.45 (per 1 SD) and 90% power to detect a HR of 1.54 (per 1 SD).
Discussion
This is the first clinical study to compare the associations of clinic BPV and ambulatory BPV in the same database. The results showed that clinic BPV was a significant predictor for All CVD events, while sleep BPV was associated with Hard CVD events. Thus, each BPV measure is separately useful for the assessment of future CVD events.
Clinic BPV and CV events
In the present study, clinic BPV was shown to be associated with All CVD events, which included both hard and soft CVD events, but was not associated with Hard CVD events alone. In previous studies, clinic BP was recorded every 4 months,32 or only 2-3 times33 for calculating clinic BPV. In this study, clinic BP was recorded every month for most of the subjects. Because there are seasonal variations in BP, it would be natural to have a greater BPV if the measurement interval was very long (4 months or more). Our data are therefore more typical of real clinical practice than previous studies because BP medications are commonly titrated once or twice every month until the BP is stabilized. Variations in BP at clinic visits reflect many factors: the subject’s emotional state, position, respiratory cycle, diet, salt intake, alcohol ingestion, physical activity, and amount of rest, as well as the time of day and room temperature during the measurement, and the potential presence of other non-standardized conditions for BP measurements.34, 35 The association between clinic BPV and CVD prognosis may be especially relevant to high-risk populations. In this study, almost half of the subjects had type 2 diabetes, putting them at high risk for CVD. In the Framingham study, clinic BP lability was not associated with cardiovascular events.36 A more recent study also failed to show a positive relationship between ambulatory BPV and CV events.20 In our series, although the clinic BP was measured under standard conditions to the greatest extent possible, patients with advanced atherosclerosis could have had greater variability. We speculate that patients with greater clinic BPV are more likely to have cardiovascular target organ damage, which would make them more susceptible to all CVD events, including congestive heart failure, angina, and transient ischemic attacks.23, 32, 33
ABP variability and CV events
In this study, ABP variability, especially BPV during sleep, was associated with Hard CVD events. This provides further confirmation of the previously reported utility of ABPM in clinical practice.4, 5, 17, 21 This is in line with our previous subgroup analysis of diabetic subjects in this database11 and with another Japanese study.37 The clinical significance of ambulatory BPV has also been shown in previous studies.4, 5, 10 Increased nighttime systolic BPV has been shown to be an independent risk factor for stroke in subjects with isolated systolic hypertension,2 and untreated essential hypertension,10 both of which are consistent with the present study. Ambulatory BP variability is influenced by various daily activities, such as diet, exercise, rest, change in temperature, sleep, and mental stress, and it reflects dynamic changes of BP during daily life. On the other hand, because clinic BP is measured under relatively controlled conditions, the mechanism of fluctuation is likely to be completely different. It can be speculated that hemodynamic instability under ambulatory conditions reflects more advanced atherosclerosis and further fluctuations of BP than those in clinic BPV. Therefore, we conclude that ABPM is an important tool for the prevention of hard CVD events.
There are several limitations in this study. The sample is a heterogeneous mixture of newly-diagnosed hypertension patients, hypertension patients receiving non-pharmacological treatments, and white-coat hypertension patients. However, this type of heterogeneity is the norm for general internal medicine clinics. Second, the sample was only intermediate in size. The other limitations are the relatively large number of covariates for the event number. Clinic BPV during follow up, which is a marker of longitudinal BP change, and ambulatory BPV at baseline, which is a marker of BP fluctuations during one day of daily life, are completely different in nature; therefore while one may be a better predictor of a specific outcome, it would be problematic to conclude that one is inherently superior to the other based on this or any other study. Finally, given that the present study only had 95% power to detect standardized hazard ratios of about 1.9 (or larger), and associations of less than this magnitude would certainly be of interest, readers should not interpret the non-statistically significant associations in this study as evidence that those associations are not present in the larger population.
Conclusions
In hypertensive patients, increased visit-to-visit clinic BPV was associated with increased incidence of All CVD events, and increased sleep BPV was associated with increased incidence of Hard CVD events. Clinic BPV has the advantage of providing data without any special device, but requires time (multiple visits) to collect sufficient readings to calculate BPV, even though the number of clinic BP readings was not predictive of CVD risk. On the other hand, the numerous BP readings obtained from a single 24-h ABPM allow the calculation of ambulatory BPV in daily life, but the generalizability of the result beyond the single day being measured is unclear, and ABPM obviously requires special equipment. Each BPV measure has advantages and disadvantages, and can be separately used as a marker of future cardiovascular outcomes.
Acknowledgments
This work was supported by grants-in-aid (1998–1999, 2001–2002, and 2004–2005) from the Foundation for the Development of the Community, Tochigi, Japan, and in part by NHLBI (P01 HL 47540 and R24 HL76857).
Footnotes
There is no conflict of interest in this paper.
References
- 1.Frattola A, Parati G, Cuspidi C, Albini F, Mancia G. Prognostic value of 24-hour blood pressure variability. J Hypertens. 1993;11:1133–1137. doi: 10.1097/00004872-199310000-00019. [DOI] [PubMed] [Google Scholar]
- 2.Pringle E, Phillips C, Thijs L, Davidson C, Staessen JA, de Leeuw PW, Jaaskivi M, Nachev C, Parati G, O’Brien ET, Tuomilehto J, Webster J, Bulpitt CJ, Fagard RH, Syst-Eur investigators Systolic blood pressure variability as a risk factor for stroke and cardiovascular mortality in the elderly hypertensive population. J Hypertens. 2003;21:2251–2257. doi: 10.1097/00004872-200312000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Kikuya M, Hozawa A, Ohokubo T, Tsuji I, Michimata M, Matsubara M, Ota M, Nagai K, Araki T, Satoh H, Ito S, Hisamichi S, Imai Y. Prognostic significance of blood pressure and heart rate variabilities: the Ohasama study. Hypertension. 2000;36:901–906. doi: 10.1161/01.hyp.36.5.901. [DOI] [PubMed] [Google Scholar]
- 4.Tatasciore A, Renda G, Zimarino M, Soccio M, Bilo G, Parati G, Schillaci G, De Caterina R. Awake systolic blood pressure variability correlates with target-organ damage in hypertensive subjects. Hypertension. 2007;50:325–332. doi: 10.1161/HYPERTENSIONAHA.107.090084. [DOI] [PubMed] [Google Scholar]
- 5.Mancia G, Bombelli M, Facchetti R, Madotto F, Corrao G, Trevano FQ, Grassi G, Sega R. Long-Term Prognostic Value of Blood Pressure Variability in the General Population: Results of the Pressioni Arteriose Monitorate e Loro Associazioni Study. Hypertension. 2007;49:1265–1270. doi: 10.1161/HYPERTENSIONAHA.107.088708. [DOI] [PubMed] [Google Scholar]
- 6.Mancia G. Prognostic value of long-term blood pressure variability: the evidence is growing. Hypertension. 2011;57:141–143. doi: 10.1161/HYPERTENSIONAHA.110.165852. [DOI] [PubMed] [Google Scholar]
- 7.Grassi G, Turri C, Vailati S, Dell’Oro R, Mancia G. Muscle and Skin Sympathetic Nerve Traffic During the “White-Coat” Effect. Circulation. 1999;100:222–225. doi: 10.1161/01.cir.100.3.222. [DOI] [PubMed] [Google Scholar]
- 8.Conway J, Boon N, Vann Jones J, Sleight P. Mechanisms concerned with blood pressure variability throughout the day. Clin Exp Hypertens. 1985;7:153–157. doi: 10.3109/10641968509073534. [DOI] [PubMed] [Google Scholar]
- 9.Mancia G, Grassi G. Mechanisms and clinical implications of blood pressure variability. J Cardiovasc Pharmacol. 2000;35:S15–S19. doi: 10.1097/00005344-200000004-00003. [DOI] [PubMed] [Google Scholar]
- 10.Verdecchia P, Angeli F, Gattobigio R, Rapicetta C, Reboldi G. Impact of blood pressure variability on cardiac and cerebrovascular complications in hypertension. Am J Hypertens. 2007;20:154–161. doi: 10.1016/j.amjhyper.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 11.Eguchi K, Ishikawa J, Hoshide S, Pickering TG, Schwartz JE, Shimada K, Kario K. Night time blood pressure variability is a strong predictor for cardiovascular events in patients with type 2 diabetes. Am J Hypertens. 2009;22:46–51. doi: 10.1038/ajh.2008.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kikuya M, Ohkubo T, Metoki H, Asayama K, Hara A, Obara T, Inoue R, Hoshi H, Hashimoto J, Totsune K, Satoh H, Imai Y. Day-by-day variability of blood pressure and heart rate at home as a novel predictor of prognosis: the Ohasama study. Hypertension. 2008;52:1045–1050. doi: 10.1161/HYPERTENSIONAHA.107.104620. [DOI] [PubMed] [Google Scholar]
- 13.Kario K, Pickering TG, Umeda Y, Hoshide S, Hoshide Y, Morinari M, Murata M, Kuroda T, Schwartz JE, Shimada K. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: a prospective study. Circulation. 2003;107:1401–1406. doi: 10.1161/01.cir.0000056521.67546.aa. [DOI] [PubMed] [Google Scholar]
- 14.Ohkubo T, Imai Y, Tsuji I, Nagai K, Watanabe N, Minami N, Kato J, Kikuchi N, Nishiyama A, Aihara A. Relation between nocturnal decline in blood pressure and mortality. The Ohasama study. Am J Hypertens. 1997;10:1201–1207. doi: 10.1016/s0895-7061(97)00274-4. [DOI] [PubMed] [Google Scholar]
- 15.Kario K, Pickering TG, Matsuo T, Hoshide S, Schwartz JE, Shimada K. Stroke prognosis and abnormal nocturnal blood pressure falls in older hypertensives. Hypertension. 2001;38:852–857. doi: 10.1161/hy1001.092640. [DOI] [PubMed] [Google Scholar]
- 16.Eguchi K, Pickering TG, Hoshide S, Ishikawa J, Ishikawa S, Schwartz JE, Shimada K, Kario K. Ambulatory blood pressure is a better marker than clinic blood pressure in predicting cardiovascular events in patients with/without type 2 diabetes. Am J Hypertens. 2008;21:443–450. doi: 10.1038/ajh.2008.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pickering TG, Shimbo D, Haas D. Ambulatory blood-pressure monitoring. N Engl J Med. 2006;354:2368–2374. doi: 10.1056/NEJMra060433. [DOI] [PubMed] [Google Scholar]
- 18.Mancia G, Zanchetti A, Agebiti-Rosei E, Benemio G, De Cesaris R, Fogari R, Pessino A, Porcellati C, Salvetti A, Trimarco B. Ambulatory blood pressure is superior to clinic blood pressure in predicting treatment-induced regression of left ventricular hypertrophy. Circulation. 1997;95:1464–1470. doi: 10.1161/01.cir.95.6.1464. [DOI] [PubMed] [Google Scholar]
- 19.Ohkubo T, Imai Y, Tsuji I, Nagai K, Kato J, Kikuchi N, Nishiyama A, Aihara A, Sekino M, Kikuya M, Ito S, Satoh H, Hisamichi S. Home blood pressure measurement has a stronger predictive power for mortality than does screening blood pressure measurement: a population-based observation in Ohasama, Japan. J Hypertens. 1998;16:971–975. doi: 10.1097/00004872-199816070-00010. [DOI] [PubMed] [Google Scholar]
- 20.Khattar RS, Swales JD, Banfield A, Dore C, Senior R, Lahiri A. Prediction of coronary and cerebrovascular morbidity and mortality by direct continuous ambulatory blood pressure monitoring in essential hypertension. Circulation. 1999;100:1071–1076. doi: 10.1161/01.cir.100.10.1071. [DOI] [PubMed] [Google Scholar]
- 21.Dolan E, Stanton A, Thijs L, Hinedi K, Atkins N, McClory S, Hond ED, McCormack P, Staessen JA, O’Brien E. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin outcome study. Hypertension. 2005;46:156–161. doi: 10.1161/01.HYP.0000170138.56903.7a. [DOI] [PubMed] [Google Scholar]
- 22.Sega R, Facchetti R, Bombelli M, Cesana G, Corrao G, Grassi G, Mancia G. Prognostic value of ambulatory and home blood pressures compared with office blood pressure in the general population: follow-up results from the Pressioni Arteriose Monitorate e Loro Associazioni (PAMELA) study. Circulation. 2005;111:1777–1783. doi: 10.1161/01.CIR.0000160923.04524.5B. [DOI] [PubMed] [Google Scholar]
- 23.Rothwell PM. Limitations of the usual blood-pressure hypothesis and importance of variability, instability, and episodic hypertension. Lancet. 2010;375:938–948. doi: 10.1016/S0140-6736(10)60309-1. [DOI] [PubMed] [Google Scholar]
- 24.Eguchi K, Kario K, Shimada K. Greater impact of coexistence of hypertension and diabetes on silent cerebral infarcts. Stroke. 2003;34:2471–2474. doi: 10.1161/01.STR.0000089684.41902.CD. [DOI] [PubMed] [Google Scholar]
- 25.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111:697–716. doi: 10.1161/01.CIR.0000154900.76284.F6. [DOI] [PubMed] [Google Scholar]
- 26.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2004;27:S5–S10. doi: 10.2337/diacare.27.2007.s5. [DOI] [PubMed] [Google Scholar]
- 27.Imai Y, Sasaki S, Minami N, Munakata M, Hashimoto J, Sakuma H, Sakuma M, Watanabe N, Imai K, Sekino H, Abe K. The accuracy and performance of the A&D TM 2421, a new ambulatory blood pressure monitoring device based on the cuff-oscillometric method and the Korotkoff sound technique. Am J Hypertens. 1992;5:719–726. doi: 10.1093/ajh/5.10.719. [DOI] [PubMed] [Google Scholar]
- 28.1999 World Health Organization/International Society of Hypertension Guidelines for the management of hypertension J Hypertens. 1999;17:151–183. [PubMed] [Google Scholar]
- 29.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr., Jones DW, Materson BJ, Oparil S, Wright JT, Jr., Roccella EJ, the National High Blood Pressure Education Program Coordinating Committee Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 30.Luepker RV, Apple FS, Christenson RH, Crow RS, Fortmann SP, Goff D, Goldberg RJ, Hand MM, Jaffe AS, Julian DG, Levy D, Manolio T, Mendis S, Mensah G, Pajak A, Prineas RJ, Reddy KS, Roger VL, Rosamond WD, Shahar E, Sharrett AR, Sorlie P, Tunstall-Pedoe H, AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention. Circulation. 2003;108:2543–2549. doi: 10.1161/01.CIR.0000100560.46946.EA. [DOI] [PubMed] [Google Scholar]
- 31.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WRC, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM, Jr., White CJ, White J, White RA, National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation. Circulation. 2006;113:e463–e465. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 32.Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JE, Dahlöf B, Sever PS, Poulter NR. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375:895–905. doi: 10.1016/S0140-6736(10)60308-X. [DOI] [PubMed] [Google Scholar]
- 33.Muntner P, Shimbo D, Tonelli M, Reynolds K, Arnett DK, Oparil S. The relationship between visit-to-visit variability in systolic blood pressure and all-cause mortality in the general population: findings from NHANES III, 1988 to 1994. Hypertension. 2011;57:160–166. doi: 10.1161/HYPERTENSIONAHA.110.162255. [DOI] [PubMed] [Google Scholar]
- 34.Mancia G, Omboni S, Parati G. The importance of blood pressure variability in hypertension. Blood Press Monit. 2000;5:S9–S15. doi: 10.1097/00126097-200005001-00003. [DOI] [PubMed] [Google Scholar]
- 35.Nadour W, Biederman RW. Is left ventricular hypertrophy regression important? Does the tool used to detect it matter? J Clin Hypertens (Greenwich) 2009;11:441–447. doi: 10.1111/j.1751-7176.2009.00137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kannel WB, Sorlie P, Gordon T. Labile hypertension: a faulty concept? The Framingham study. Circulation. 1980;61:1183–1187. doi: 10.1161/01.cir.61.6.1183. [DOI] [PubMed] [Google Scholar]
- 37.Tamura K, Tsurumi Y, Sakai M, Tanaka Y, Okano Y, Yamauchi J, Ishigami T, Kihara M, Hirawa N, Toya Y, Yabana M, Tokita Y, Ohnishi T, Umemura S. A possible relationship of nocturnal blood pressure variability with coronary artery disease in diabetic nephropathy. Clin Exp Hypertens. 2007;29:31–42. doi: 10.1080/10641960601096760. [DOI] [PubMed] [Google Scholar]