Abstract
Background
It is not clear whether cognitive decline progresses more quickly in long sleepers than in short sleepers or than in participants with usual sleep duration. We assessed cognitive decline as a function of self-reported sleep duration in a prospective population-based cohort (NEDICES).
Methods
Participants were evaluated at baseline and 3 years later. Baseline demographic variables were recorded and participants indicated their daily sleep usual duration as the sum of nighttime sleep and daytime napping. The average daily total usual sleep duration was grouped into three categories: ≤5 hours (short sleepers), 6 to 8 hours (reference category), and ≥9 hours (long sleepers). At baseline and at follow-up, a 37-item version of the Mini-Mental State Examination (37-MMSE) was administered.
Results
The final sample, 2,715 participants (72.9±6.1 years), comprised 298 (11%) short sleepers, 1,086 (40%) long sleepers, and 1,331 (49%) in the reference group (6 to 8 hours). During the three year follow-up period, the 37-MMSE declined by 0.5±4.0 points in short sleepers, 0.6±4.3 points in long sleepers, and 0.2±3.8 points in the reference group (p=0.08). The difference between short sleepers and the reference group was not significant (p=0.142); however, the difference between long sleepers and the reference group was significant (p=0.040). In analyses adjusted for baseline age and other potential confounders, this difference remained robust.
Conclusions
In this study, cognitive test scores among long sleepers declined more rapidly than observed in a reference group. Additional studies are needed to confirm these results.
Keywords: Cognitive function, elderly, epidemiology, sleep duration, population-based study
INTRODUCTION
Dementia and cognitive disorders are among the major public health challenges of aging societies today. It is not surprising therefore, that scientific and clinical research in the area of cognitive disorders has shifted during the last decade to focus on the possible predictors of these disorders in an effort to prevent the consequences of dementia. Thus, there is clearly a need to understand the possible predictors of cognitive disorders and to develop effective prevention strategies. Understanding the link between these disorders and sleep may represent one important part of that effort. Since sleep duration is potentially modifiable, the relation between sleep duration and cognitive decline might well have practical implications for the primary prevention of these disorders.
Sleep problems are common conditions in modern society, especially in elderly people.(Myers & Badia, 1995) Chronic insomnia or prolonged daytime sleepiness has been associated with poorer cognitive function in the elderly.(Bastien et al., 2003; Cricco, Simonsick, & Foley, 2001; Ohayon & Vecchierini, 2002) In addition, subjects with daytime sleepiness or prolonged sleep duration are at increased risk for incident dementia.(Benito-León, Bermejo-Pareja, Vega, & Louis, 2009; Foley et al., 2001) However, the few prospective population-based studies that have assessed whether sleep duration predicted cognitive decline have yielded conflicting results.(Ferrie et al., 2011; Keage et al., 2012; Potvin et al., 2012; Tworoger, Lee, Schernhammer, & Grodstein, 2006) Unmeasured confounders, including medications that potentially affect cognitive function (e.g., anxiolytics, stimulants, antipsychotics, antidepressants, antihistamines, or antiepileptics drugs) may have influenced the results of community or population-based surveys outcomes. It is not clear whether cognitive decline progresses in long sleepers more rapidly than in short sleepers or than in participants with usual sleep duration. We hypothesized that the cognitive deficits in long sleepers would worsen more than in short sleepers and in elderly participants reporting usual sleep duration (6-8 hours) (i.e., controls). To address this question, we utilized data from the Neurological Disorders in Central Spain (NEDICES) study, in which participants were prospectively evaluated at two times points separated by three years. We aimed to adjust for confounders such as medications with central nervous system effects.
MATERIAL AND METHODS
Study population
Data for these analyses were derived from the NEDICES study, a longitudinal, population-based survey of the prevalence, incidence, and determinants of major age-associated conditions of the elderly, including Parkinson's disease (PD), essential tremor, stroke, and dementia.(Benito-León, Bermejo-Pareja, Louis, & Neurological Disorders in Central Spain Study, 2005; Benito-León et al., 2004; Benito-León, Bermejo-Pareja, Morales, Vega, & Molina, 2003a; Benito-León et al., 2003b; Bermejo-Pareja et al., 2008a; Bermejo-Pareja, Benito-León, Vega, Medrano, & Román, 2008b; Bermejo-Pareja et al., 2009; Diaz-Guzman et al., 2008; Martínez-Salio, Benito-León, Diaz-Guzman, & Bermejo-Pareja, 2010; Morales et al., 2004; Vega et al., 2010) Detailed accounts of the study population and sampling methods have been published.(Bermejo-Pareja et al., 2008a; Morales et al., 2004; Vega et al., 2010) The survey area consisted of three communities: Margaritas (approximately 14,800 inhabitants), a working-class neighborhood in Getafe (Greater Madrid); Lista (approximately 150,000 inhabitants), a professional-class neighborhood in Salamanca (Central Madrid), and Arévalo (approximately 9,000 inhabitants), the agricultural zone of Arévalo County (125 km northwest of Madrid). Up-to-date lists of residents were generated from population registers. In each community, survey eligibility was restricted to residents aged 65 years or older who were present there on December 31, 1993, or during 6 or more months of 1993. Eligible persons who had moved away from the survey area were not traced. In Margaritas and Arévalo, every eligible subject was to be screened. However, in Lista, proportionate stratified random sampling was used to select subjects for screening because of the large number of elderly residents. All procedures were approved by the ethical standards committees on human experimentation at the University Hospitals “12 de Octubre” (Madrid) and “La Princesa” (Madrid). Written (signed) informed consent was obtained from all enrollees.
Study evaluation
Briefly, at the time of their baseline assessment (1994–1995), 5,278 elderly subjects were interviewed using a 500-item screening questionnaire that assessed demographic factors and medical conditions. The face-to-face interview included data collection on demographics, current medications (including drugs that affect the central nervous system), and medical conditions. Subjects were asked to bring all medications taken in the past one week to the clinic where the interviewer viewed and recorded the name and the dose of each one. We assessed depressive symptoms by self-report, using a single screening question (‘Do you suffer from depression?’). This same approach has similarly been utilized in previous population studies of depression.(Benito-León, Louis, Bermejo-Pareja, & Neurological Disorders in Central Spain Study, 2009; Benito-León et al., 2010a; Louis, Benito-León, Bermejo-Pareja, & Neurological Disorders in Central Spain Study, 2007b) We also assessed the use of antidepressant medications, a marker that may be less prone to biases than a simple screening question.(Louis, Benito-León, & Bermejo-Pareja, 2007a) Participants indicated their total daily usual sleep duration as the sum of nighttime sleep and daytime napping.
A short form of the questionnaire was mailed to subjects who refused, or were unavailable for face-to-face or telephone screening. This form assessed demographic characteristics, several neurological disorders (essential tremor, stroke, dementia, and parkinsonism), current medications, and the name of their family doctor. During the second (i.e., follow-up) evaluation (1997–1998), the same methods were used.
As described,(Bermejo-Pareja et al., 2008a; Morales et al., 2004; Vega et al., 2010) a 37-item Mini-Mental State Examination (37-MMSE) was administered in both baseline assessment (1994–1995) and follow-up evaluation (1997–1998).(Benito-León, Louis, & Bermejo-Pareja, 2006a; b; Benito-León et al., 2011; Benito-León, Louis, Vega, & Bermejo-Pareja, 2010b; Benito-León, Mitchell, Vega, & Bermejo-Pareja, 2010c; Bermejo-Pareja et al., 2008b; Bermejo-Pareja et al., 2009; Prieto, Contador,Tapias-Merino, Mitchell, & Bermejo-Pareja, 2012) This was a Spanish adaptation of the standard MMSE. (Benito-León et al., 2006a; b; Benito-León et al., 2011; Benito-León et al., 2010b; Benito-León et al., 2010c; Bermejo-Pareja et al., 2008b; Bermejo-Pareja et al., 2009; Prieto et al., 2012) It included all of the standard MMSE items as well as three additional items: (1) an attention task, i.e., “say 1, 3, 5, 7, 9 backwards”, (2) a visual order, i.e., a man raising his arms, and (3) a simple construction task, i.e., copying two overlapping circles. (Benito-León et al., 2006a; b; Benito-León et al., 2011; Benito-León et al., 2010b; Benito-León et al., 2010c; Bermejo-Pareja et al., 2008b; Bermejo-Pareja et al., 2009; Prieto et al., 2012)
Ten percent of our sample was illiterate, although only a small proportion was completely illiterate and many could read or write a simple phrase. If the participant was completely illiterate, then the one 37-MMSE reading item and the one 37-MMSE writing item were assigned the value 0. Diagnosis of dementia (Bermejo-Pareja et al., 2008b; Bermejo-Pareja et al., 2009) fulfilled the criteria of the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (American Psychiatric Association. & American Psychiatric Association. Task Force on DSM-IV., 1994) and required evidence of cognitive impairment (based on a neuropsychological test battery and a clinical mental status examination) as well as impairment in social or occupational function.
Final selection of participants
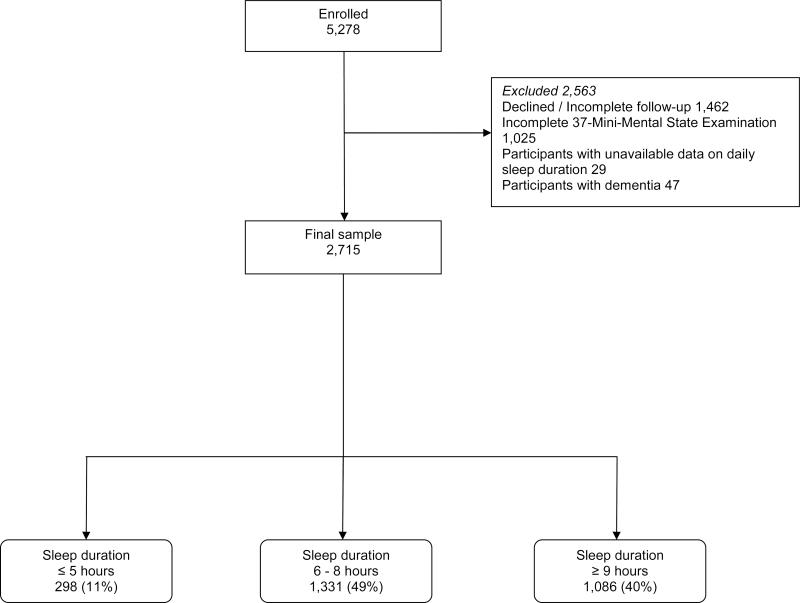
Of the 5,278 participants evaluated at baseline, we excluded 1,462 participants who were evaluated at baseline because they declined a follow-up assessment or had incomplete follow-up assessments, had died or were unreachable (Figure 1). We further excluded 1,025 participants with incomplete 37-MMSE examinations, 29 without available data on daily sleep duration, and 47 with prevalent dementia, which left 2,715 remaining participants who were included in our analyses (Figure 1). The final sample of 2,715 was similar to the base sample of 5,278 participants in terms of gender (1,545 [56.9%] vs. 3,040 [57.6%] women, chi-square = 0.35, p = 0.55). However, they were more educated (298 [11.0%] vs. 711 [13.6%] were illiterate, chi-square = 11.15, p = 0.011) and, on average, 1.4 years younger (72.9 ± 6.1 vs. 74.3 ± 7.0 years, t = 9.23, p < 0.001).
Figure 1.
Flow-Chart of the Study
Statistical analyses
Analyses were performed in SPSS (version 20.0). All tests were two sided, and significance was accepted at the 5% level (α = 0.05). Using a one-sample Kolmogorov–Smirnov test, we determined that age and sleep duration were not normally distributed. Therefore, although mean and median values were reported, differences were compared using nonparametric tests (Mann-Whitney and Kruskal–Wallis tests). The X2 test was used to analyze categorical variables. The change in 37-MMSE score = baseline score – follow-up score. The 37-MMSE scores (baseline, follow-up, and change in 37-MMSE) were not normally distributed, even after transformations were attempted. Therefore, scores were compared using the same non-parametric approach (Mann-Whitney and Kruskal–Wallis tests). Linear regression analyses were not possible because the change in 37-MMSE was not normally distributed. Therefore, to initially assess the effects of possible confounders (age, depressive symptoms and medications with central nervous system effects), stratified analyses were performed. The aim of these analyses was to determine whether the magnitude of the case–control difference persisted after stratification. Due to the loss of power in these stratified analyses, p values were not reported; rather, the aim of these analyses was to determine whether the magnitude of the case-control difference persisted after stratification.
In additional analyses, we divided change in 37-MMSE into two groups. Those who declined 4 or more points were considered as the “decline group” vs. those who declined by 3 or fewer points between the two evaluations (“no decline group”). This was based on previous reports of MMSE change in healthy elderly subjects.(Aevarsson & Skoog, 2000) In a longitudinal population-based Swedish study, a decrease of 4 or more points in MMSE during a 3-years follow-up had a sensitivity of 83% and a specificity of 80% for a diagnosis of dementia.(Aevarsson et al., 2000) Therefore, we decided to use this 4 point change score as a clinically relevant threshold.(Aevarsson et al., 2000) Logistic regression analyses were performed, thereby allowing us to assess, for a second time, potential confounders. In these models, the dependent variable was “Decline”/”No Decline” and the independent variable was sleep duration category. Participants were divided in short sleepers (≤ 5 hours daily) and long sleepers (≥ 9 hours daily), according to the categories used in previous studies.(Potvin et al., 2012; Tworoger et al., 2006) Short and long sleep duration categories were compared with the reference category (6 to 8 hours daily).
RESULTS
The final sample, 2,715 participants (mean ± standard deviation age = 72.9 ± 6.1 years), comprised 298 (11%) short sleepers (≤ 5 hours daily), 1,086 (40%) long sleepers (≥ 9 hours daily), and 1,331 (49%) who reported 6 to 8 hours of daily sleep (reference category) (Figure 1). The mean follow-up was 3.4 ± 0.5 years.
Baseline characteristics of the participants in the three sleep duration categories are shown (Table 1). At baseline, short sleepers were more frequently women, older and less educated. In addition, they were more likely to have diabetes mellitus, chronic obstructive pulmonary disease, and depressive symptoms/or antidepressant use; a higher proportion was taking medications with central nervous system effects (Table 1).
Table 1.
Baseline characteristics of the study participants, according to habitual sleep duration.
| Sleep duration (hours per day) | ||||
|---|---|---|---|---|
| ≤5 (n = 298) | 6 to 8 (n = 1,331) | ≥9 (n = 1,086) | p value | |
| Age in years | 74.2 ± 6.3 (73) | 72.6 ± 5.9 (71) | 73.0 ± 6.1 (72) | < 0.001a |
| Gender (women) | 205 (68.8%) | 774 (58.2%) | 566 (52.1%) | < 0.001b |
| Educational level | ||||
| Illiterate | 41 (13.8%) | 129 (9.7%) | 128 (11.8%) | 0.014b |
| Can read and write | 123 (41.3%) | 532 (40.0%) | 469 (43.2%) | |
| Primary studies | 104 (34.9%) | 455 (34.2%) | 351 (32.3%) | |
| Secondary and higher studies | 30 (10.1%) | 215 (16.2%) | 138 (12.7%) | |
| Geographical area | ||||
| Lista | 52 (17.4%) | 435 (32.7%) | 369 (34.0%) | < 0.001b |
| Arévalo | 123 (41.3%) | 504 (37.9%) | 468 (43.1%) | |
| Margaritas | 123 (41.3%) | 392 (29.5%) | 249 (22.9%) | |
| Hypertension | 156 (52.3%) | 677 (50.9%) | 545 (50.3%) | 0.815b |
| Diabetes mellitus | 62 (20.8%) | 187 (14.2%) | 187 (17.3%) | 0.008b |
| Chronic obstructive pulmonary disease | 58 (19.7%) | 168 (12.7%) | 162 (15.0%) | 0.006b |
| Depressive symptoms or antidepressant use | 113 (38.3%) | 323 (24.5%) | 236 (21.8%) | < 0.001b |
| On medication with central nervous system effects | 72 (24.2%) | 202 (15.2%) | 156 (14.4%) | < 0.001b |
Mean ± SD (median) and frequency (%) are reported.
Kruskal Wallis test was used for continuous variables
chi-square test for categorical variables.
At baseline, the mean 37-MMSE in short sleepers was 28.3 ± 5.4 (median = 28.5) vs. 29.7 ± 5.0 (median = 30) in long sleepers vs. 30.1 ± 4.8 (median = 31) in the reference category (Kruskal-Wallis, p < 0.001), with a significant difference in short sleepers and the reference group (Mann-Whitney test, p < 0.001); but no difference between the long sleepers and the reference group (Mann-Whitney test, p = 0.113). During the three year follow-up period, the 37-MMSE declined by 0.5 ± 4.0 points (median = 0 point) in short sleepers vs. 0.6 ± 4.3 points in long sleepers (median = 0 points) vs. 0.2 ± 3.8 points in the reference group (median = 0 points) (Kruskal-Wallis, p = 0.08) (Table 2). The difference between short sleepers and the reference group was not significant (Mann-Whitney test, p = 0.142), similarly that between short and long sleepers was not significant (Mann-Whitney test, p = 0.908); however, the difference between long sleepers and the reference group was significant (Mann-Whitney test, p = 0.040).
Table 2.
Mean decline in 37-MMSE score during follow-up (points).
| Sleep duration (hours per day) | |||
|---|---|---|---|
| ≤5 | 6 to 8 | ≥9 | |
| All participants | 0.5 [0.0] ± 4.0 | 0.2 [0.0] ± 3.8 | 0.6 [0.0] ± 4.3 |
| Age Strata | |||
| Tertile 1 (≤ 69) | 0.6 [0.0] ± 3.5 (67.1 ± 1.4) | −0.1 [0.0] ± 3.5 (67.1 ± 1.3) | 0.5 [0.0] ± 4.3 (67.1 ± 1.4) |
| Tertile 2 (70-74) | 0.4 [0.0] ± 3.7 (71.9 ± 1.4) | 0.0 [0.0] ± 3.7 (71.8 ± 1.4) | 0.5 [0.0] ± 3.8 (72.0 ± 1.3) |
| Tertile 3 (≥ 75) | 0.6 [0.0] ± 4.6 (80.2 ± 4.0) | 0.6 [0.0] ± 4.2 (79.9 ± 4.0) | 0.9 [1.0] ± 4.7 (80.3 ± 4.3) |
| Depressive symptoms or antidepressant use strata | |||
| Yes | 0.3 [0.0] ± 4.5 | 0.2 [0.0] ± 3.6 | 0.8 [0.0] ± 4.4 |
| No | 0.6 [0.0] ± 3.7 | 0.2 [0.0] ± 3.9 | 0.6 [0.0] ± 4.3 |
| Medications with central nervous system effects strata | |||
| Yes | 0.2 [0.0] ± 4.1 | 0.6 [0.0] ± 3.6 | 1.0 [0.5] ± 4.1 |
| No | 0.7 [0.0] ± 4.0 | 0.1 [0.0] ± 3.8 | 0.5 [0.0] ± 4.3 |
| Geographical área | |||
| Las Margaritas | 0.2 [0.0] ± 3.9 | 0.3 [0.0] ± 3.7 | 0.7 [0.0] ± 4.5 |
| Lista | 0.1 [0.0] ± 4.4 | 0.1 [0.0] ± 3.9 | 0.3 [0.0] ± 4.3 |
| Arévalo | 1.1 [1.0] ± 4.0 | 0.1 [0.0] ± 3.8 | 0.8 [1.0] ± 4.2 |
Mean [Median] ± standard deviation and frequency (%) are reported. Negative value indicates that the baseline 37-MMSE score was lower than the 37-MMSE score at follow-up (i.e., improvement in score). All positive values indicate a decline in score (i.e., baseline 37-MMSE ± follow-up 37-MMSE). In each age stratum, the numbers in parentheses indicate the mean ± standard deviation age of participants in that stratum; these values demonstrate that cases and controls had similar ages within the three age strata.
In the reference group, we examined whether baseline 37-MMSE scores were associated with potential confounding variables. The 37-MMSE was correlated with age (rS = −0.277, p<0.001), educational category (rS = 0.387, p<0.001), geographical area (mean ± SD [median] = 28.8 ± 4.9 [29] in Las Margaritas vs. 32.3 ± 4.0 [33] in Lista and 29.1 ± 4.8 [29] in Arévalo, Kruskal-Wallis test, p <0.001), subjective depressive symptoms or antidepressant use (29.1 ± 4.9 [29] in those who responded “yes” vs. 30.4 ± 4.8 [31] in those who responded “no”, Mann-Whitney test, p <0.001) and gender (mean ± SD [median] = 31.8 ± 4.2 [33] in men vs. 28.8 ± 4.9 [29] in women, Mann-Whitney test, p <0.001). However, baseline 37-MMSE was not correlated with medications that could affect cognition (mean ± SD [median] = 29.8 ± 5.1 [30] in those taking a medication vs. 30.1 ± 4.8 [31] in those who do not take a medication, Mann-Whitney test, p = 0.437).
In stratified analyses, in nearly all strata, except for medications with central nervous system effects and Las Margaritas geographical area for short sleepers, the decline in 37-MMSE score in both short and long sleepers was higher than the decline in score in the reference group (Table 2), indicating that these variables were not likely to be a source of confounding.
In 14 (0.5%) participants, the 37-MMSE changed by greater than 15 points; when these outliers were excluded, the results were similar (data not shown).
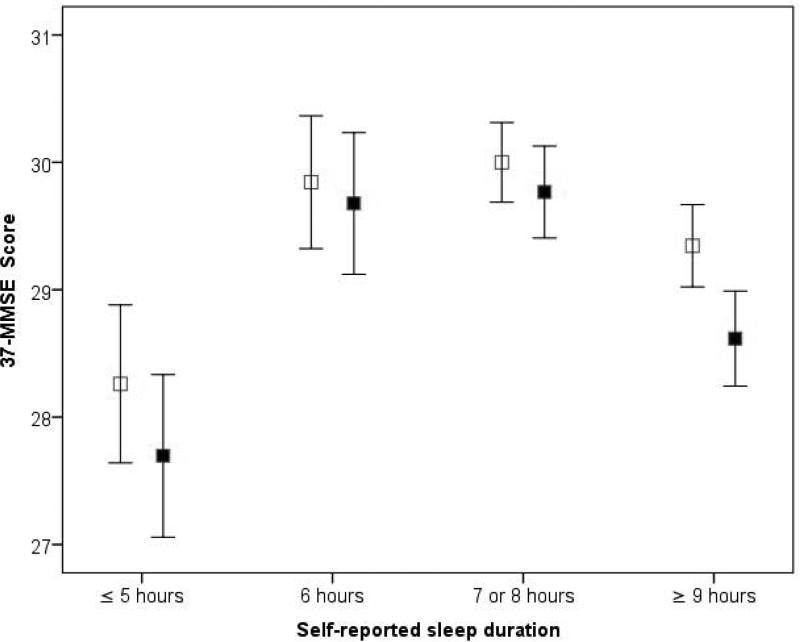
We also assessed the cognitive decline per unit time (i.e., the rate of cognitive decline). The rate of cognitive decline was 0.0 ± 1.2 (median = 0.0) points/year for the reference group, 0.2 ± 1.2 (median = 0.0) points/year for short sleepers, and 0.2 ± 1.3 (median = 0.0) points/year for long duration sleepers (Kruskal-Wallis test, p = 0.07). The difference between short sleepers and controls was not significant (Mann-Whitney test, p = 0.106); however, there was a statistically significant difference between long sleepers and the reference group (Mann-Whitney test, p = 0.043) (figure 2). On the other hand, the difference between short and long sleepers was not significant (Mann-Whitney test, p = 0.762).
Figure 2. MMSE decline from baseline to follow up at the NEDICES Cohort.
Mean baseline 37-MMSE scores are shown by open squares. Follow-up 37-MMSE scores are shown by closed squares. Mean ± 1 standard deviation are shown. Baseline scores were lower in shor and long sleepers than the reference group (those who reported to sleep 7 to 8 hours); moreover, during the three year follow-up period, these scores declined to a greater extent in long sleepers cases than in the reference group.
In a logistic regression model, long sleepers were 1.3 times more likely than the reference group to have a “decline” in 37-MMSE (OR= 1.3, 95% CI= 1.0-1.6, p = 0.020). However, the odds of “decline” in 37-MMSE was similar in short sleepers vs. controls (OR = 1.2, 95% CI= 0.9-1.7, p = 0.178). To further assess the potential confounding effect of age, gender, geographical area, educational level, diabetes mellitus, chronic obstructive pulmonary disease, depressive symptoms or antidepressant use, and medications with central nervous system effects, we adjusted for these in a logistic regression model, and long sleepers were 1.3 times more likely to decline than the reference group (OR = 1.3, 95%, CI= 1.1-1.6 p = 0.012) yet the odds of “decline” in 37-MMSE was similar in short sleepers than controls (OR = 1.1, 95%, CI= 0.8-1.5, p = 0.630). In another analyses, we included rather than excluded all participants with prevalent dementia. In these analyses, long sleepers cases were 1.3 times more likely to decline than the reference group (adjusted OR = 1.3, 95%, CI= 1.0-1.6, p = 0.025). Likewise, the odds of “decline” in 37-MMSE was similar in short sleepers vs. controls (adjusted OR = 1.1, 95%, CI= 0.7-1.5, p = 0.735).
DISCUSSION
In the current prospective study of non-demented community-dwelling elders, we further demonstrated that baseline cognitive test scores were lower in longer sleepers than the reference group; moreover, during the three year follow-up period, these scores declined at a rate in long sleepers than the reference group. Long sleepers on average experienced a 0.6 point reduction in the 37-MMSE over 3 years. Although this reduction was significantly greater than that seen in controls, in absolute terms, it was a modest change.
There has been several studies addressing cognitive function according to sleep duration; however, the majority of them have been performed using a cross-sectional design,(Auyeung et al., 2013; Faubel et al., 2009; Kronholm et al., 2009; Saint Martin, Sforza, Barthelemy, Thomas-Anterion, & Roche, 2012; Xu et al., 2011) making it impossible to determine the causal relationship between sleep duration and the risk of cognitive decline, or have been performed using clinical series, potentially biasing the results due to case ascertainment.(Miyata et al., 2013; Vetter, Juda, & Roenneberg, 2012). We could only identify four previous prospective community or population-based studies that analyzed the risk of cognitive decline according to sleep duration.(Ferrie et al., 2011; Keage et al., 2012; Potvin et al., 2012; Tworoger et al., 2006). In one study in older women using a 2-year follow-up, no association was found between sleep variables (subjective sleep duration, subjective sleep difficulties, and snoring) and the decline in cognitive function measured by the Telephone Interview for Cognitive Status.(Tworoger et al., 2006) In the Whitehall II study, 1459 women and 3972 men aged 45-69 at baseline were assessed at two times (baseline and between 2002-2004, average follow-up 5.4 years) using a complete neuropsychological battery.(Ferrie et al., 2011) In analyses adjusted for age, gender, and education, and corrected for multiple testing, adverse changes in sleep between baseline and follow-up (decrease from 6, 7, or 8 hours, increase from 7 or 8 hours) were associated with lower scores on most cognitive function tests.(Ferrie et al., 2011) In a sample of 2,012 elderly cognitively unimpaired participants from the MRC Cognitive Function and Ageing Study, it was found that daytime napping at baseline was associated with a lower risk of cognitive decline at two and 10 years, and that obtaining ≤6.5 hours of night-time sleep and excessive daytime sleepiness at baseline were associated with an increased risk at 10 years. (Keage et al., 2012) Finally, in a sample of 1,664 cognitively intact individuals age 65 to 96 years, cognitive functioning was assessed at baseline and 12 months later using the MMSE.(Potvin et al., 2012) Incident general cognitive impairment was defined according to a follow-up MMSE score below the 15(th) percentile according to normative data and of at least 2 points below baseline.(Potvin et al., 2012) In women, long sleep duration (≥ 9 hours) was associated with amnestic incident cognitive impairment. In men, short sleep duration (≤ 5 hours) was associated with amnestic cognitive impairment.(Potvin et al., 2012)
Although the current findings suggest that longer sleeping may predict a significant cognitive decline, the mechanisms underlying this association remain unknown. One possibility is that an unrecognized confounder (e.g., sleep apnea) could lead to both cognitive decline and an increased need for sleep.[29] Second, long sleep duration may be an early symptom of cognitive decline. Third, excessive sleep per se could directly lead to an increased risk of cognitive decline. Currently, however, we know of no plausible physiologic explanation for such a cause-and-effect relationship.
This study had several limitations. First, the 37-MMSE is a relatively abbreviated screening tool for dementia. The use of more detailed neuropsychological test batteries would enable future investigators to study these changes in greater detail. Nevertheless, even with this relatively simple, abbreviated tool, we were able to establish clear case-control differences. Second, the 37-MMSE was administered at two time points; use of additional time points would allow one to assess the extent to which the case-control difference continued beyond the three-year time window. Third, there were some baseline differences with potential confounding effects between the cases and the controls. However, we controlled this source of confounding with stratified analysis and logistic regression. Finally, although we performed analyses in which we adjusted for depressive symptoms, our evaluation of depression was limited and we may have under-ascertained depression, resulting in residual confounding. Nevertheless, a validation study showed a high level of agreement between the data generated from the screening question we used and a more detailed in-person psychiatric assessment, suggesting that such residual confounding is likely to have been low.(Louis et al., 2007a)
This study also had several strengths. First, short and long sleepers were compared to a large sample size of several thousand participants who reported sleeping between 6 and 8 hours. Second, the assessments were conducted prospectively in a standardized manner. Finally, we were able to adjust for the potential confounding effects of a number of important factors.
Using a prospective, population-based design, we demonstrated that cognitive test scores in long sleepers declined more rapidly compared to those who reported sleeping 7 to 8 hours per day. This study provides further evidence that cognitive deficits in sleep disturbances are not static.
Acknowledgments and Funding
Additional information about collaborators and detailed funding of the NEDICES Study can be found on the web (http://www.ciberned.es/estudionedices). The Spanish Health Research Agency and the Spanish Office of Science and Technology supported NEDICES. Dr. Benito-León is supported by the National Institutes of Health, Bethesda, MD, USA (R01 NS039422), the Commission of the European Union (grant ICT-2011-287739, NeuroTREMOR), and the Spanish Health Research Agency (grant FIS PI12/01602). Dr. Bermejo-Pareja is supported by NIH R01 NS039422 from the National Institutes of Health, Bethesda, MD, USA and from the Commission of the European Union (grant ICT-2011-287739, NeuroTREMOR). Dr. Elan D. Louis has received research support from the National Institutes of Health, Bethesda, MD, USA: NINDS #R01 NS042859 (principal investigator), NINDS #R01 NS39422 (principal investigator), NINDS #T32 NS07153-24 (principal investigator), NINDS #R01 NS073872 (principal investigator), NINDS #R21 NS077094 (co-Investigator), and NINDS #R01 NS36630 (co-Investigator), as well as the Parkinson's disease Foundation (principal investigator).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
Dr. Benito-León reports no disclosures.
Dr. Louis reports no disclosures.
Dr. Bermejo-Pareja reports no disclosures.
Authors Roles:
Dr. Benito-León (jbenitol@meditex.es) collaborated in: 1) the conception, organization and execution of the research project; 2) the statistical analysis design; and 3) the writing of the manuscript first draft and the review and critique of the manuscript.
Dr. Louis (edl2@columbia.edu) collaborated in: 1) the conception, organization and execution of the research project; 2) the statistical analysis design; and 3) the review and critique of the manuscript.
Dr. Bermejo-Pareja (fbermejop@meditex.es) collaborated in: 1) the conception, organization and execution of the research project; and 2) the review and critique of the manuscript.
References
- Aevarsson O, Skoog I. A longitudinal population study of the mini-mental state examination in the very old: relation to dementia and education. Dement Geriatr Cogn Disord. 2000;11:166–75. doi: 10.1159/000017231. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association., & American Psychiatric Association . American Psychiatric Association; Washington, DC: 1994. Task Force on DSM-IV. Diagnostic and statistical manual of mental disorders : DSM-IV. [Google Scholar]
- Auyeung TW, Lee JS, Leung J, Kwok T, Leung PC, Woo J, Wing YK. Cognitive deficit is associated with phase advance of sleep-wake rhythm, daily napping, and prolonged sleep duration--a cross-sectional study in 2,947 community-dwelling older adults. Age (Dordr) 2013;35:479–86. doi: 10.1007/s11357-011-9366-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastien CH, Fortier-Brochu E, Rioux I, LeBlanc M, Daley M, Morin CM. Cognitive performance and sleep quality in the elderly suffering from chronic insomnia. Relationship between objective and subjective measures. J Psychosom Res. 2003;54:39–49. doi: 10.1016/s0022-3999(02)00544-5. [DOI] [PubMed] [Google Scholar]
- Benito-León J, Bermejo-Pareja F, Louis ED. Neurological Disorders in Central Spain Study, G. Incidence of essential tremor in three elderly populations of central Spain. Neurology. 2005;64:1721–5. doi: 10.1212/01.WNL.0000161852.70374.01. [DOI] [PubMed] [Google Scholar]
- Benito-León J, Bermejo-Pareja F, Morales-González JM, Porta-Etessam J, Trincado R, Vega S, Louis ED. Neurological Disorders in Central Spain Study, G. Incidence of Parkinson disease and parkinsonism in three elderly populations of central Spain. Neurology. 2004;62:734–41. doi: 10.1212/01.wnl.0000113727.73153.68. [DOI] [PubMed] [Google Scholar]
- Benito-León J, Bermejo-Pareja F, Morales JM, Vega S, Molina JA. Prevalence of essential tremor in three elderly populations of central Spain. Mov Disord. 2003a;18:389–94. doi: 10.1002/mds.10376. [DOI] [PubMed] [Google Scholar]
- Benito-León J, Bermejo-Pareja F, Rodriguez J, Molina JA, Gabriel R, Morales JM. Prevalence of PD and other types of parkinsonism in three elderly populations of central Spain. Mov Disord. 2003b;18:267–74. doi: 10.1002/mds.10362. [DOI] [PubMed] [Google Scholar]
- Benito-León J, Bermejo-Pareja F, Vega S, Louis ED. Total daily sleep duration and the risk of dementia: a prospective population-based study. Eur J Neurol. 2009;16:990–7. doi: 10.1111/j.1468-1331.2009.02618.x. [DOI] [PubMed] [Google Scholar]
- Benito-León J, Louis ED, Bermejo-Pareja F. Elderly-onset essential tremor is associated with dementia. Neurology. 2006a;66:1500–5. doi: 10.1212/01.wnl.0000216134.88617.de. [DOI] [PubMed] [Google Scholar]
- Benito-León J, Louis ED, Bermejo-Pareja F. Population-based case-control study of cognitive function in essential tremor. Neurology. 2006b;66:69–74. doi: 10.1212/01.wnl.0000192393.05850.ec. [DOI] [PubMed] [Google Scholar]
- Benito-León J, Louis ED, Bermejo-Pareja F. Neurological Disorders in Central Spain Study, G. Population-based case-control study of morale in Parkinson's disease. Eur J Neurol. 2009;16:330–6. doi: 10.1111/j.1468-1331.2008.02428.x. [DOI] [PubMed] [Google Scholar]
- Benito-León J, Louis ED, Posada IJ, Sánchez-Ferro A, Trincado R, Villarejo A, Mitchell AJ. Bermejo-Pareja, F. Population-based case-control study of cognitive function in early Parkinson's disease (NEDICES). J Neurol Sci. 2011;310:176–82. doi: 10.1016/j.jns.2011.06.054. [DOI] [PubMed] [Google Scholar]
- Benito-León J, Louis ED, Rivera-Navarro J, Medrano MJ, Vega S. Bermejo-Pareja, F. Low morale is associated with increased risk of mortality in the elderly: a population-based prospective study (NEDICES). Age Ageing. 2010a;39:366–73. doi: 10.1093/ageing/afq028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito-León J, Louis ED, Vega S, Bermejo-Pareja F. Statins and cognitive functioning in the elderly: a population-based study. J Alzheimers Dis. 2010b;21:95–102. doi: 10.3233/JAD-2010-100180. [DOI] [PubMed] [Google Scholar]
- Benito-León J, Mitchell AJ, Vega S, Bermejo-Pareja F. A population-based study of cognitive function in older people with subjective memory complaints. J Alzheimers Dis. 2010c;22:159–70. doi: 10.3233/JAD-2010-100972. [DOI] [PubMed] [Google Scholar]
- Bermejo-Pareja F, Benito-León J, Vega QS, Díaz-Guzmán J, Rivera-Navarro J, Molina JA, Olazarán-Rodriguez J, Morales-González JM. [The NEDICES cohort of the elderly. Methodology and main neurological findings]. Rev Neurol. 2008a;46:416–23. [PubMed] [Google Scholar]
- Bermejo-Pareja F, Benito-León J, Vega S, Medrano MJ, Román GC. Incidence and subtypes of dementia in three elderly populations of central Spain. J Neurol Sci. 2008b;264:63–72. doi: 10.1016/j.jns.2007.07.021. [DOI] [PubMed] [Google Scholar]
- Bermejo-Pareja F, Benito-León J, Vega S, Olazarán J, de Toledo M, Díaz-Guzmán J, Sánchez-Sánchez F, Morales-González JM, Trincado R, Portera-Sánchez A, Román GC. Consistency of clinical diagnosis of dementia in NEDICES: A population-based longitudinal study in Spain. J Geriatr Psychiatry Neurol. 2009;22:246–55. doi: 10.1177/0891988709335794. [DOI] [PubMed] [Google Scholar]
- Cricco M, Simonsick EM, Foley DJ. The impact of insomnia on cognitive functioning in older adults. J Am Geriatr Soc. 2001;49:1185–9. doi: 10.1046/j.1532-5415.2001.49235.x. [DOI] [PubMed] [Google Scholar]
- Díaz-Guzmán J, Bermejo-Pareja F, Benito-León J, Vega S, Gabriel R, Medrano MJ. Prevalence of stroke and transient ischemic attack in three elderly populations of central Spain. Neuroepidemiology. 2008;30:247–53. doi: 10.1159/000135643. [DOI] [PubMed] [Google Scholar]
- Faubel R, López-García E, Guallar-Castellón P, Graciani A, Banegas JR, Rodriguez-Artalejo F. Usual sleep duration and cognitive function in older adults in Spain. J Sleep Res. 2009;18:427–35. doi: 10.1111/j.1365-2869.2009.00759.x. [DOI] [PubMed] [Google Scholar]
- Ferrie JE, Shipley MJ, Akbaraly TN, Marmot MG, Kivimaki M, Singh-Manoux A. Change in sleep duration and cognitive function: findings from the Whitehall II Study. Sleep. 2011;34:565–73. doi: 10.1093/sleep/34.5.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley D, Monjan A, Masaki K, Ross W, Havlik R, White L, Launer L. Daytime sleepiness is associated with 3-year incident dementia and cognitive decline in older Japanese-American men. J Am Geriatr Soc. 2001;49:1628–32. doi: 10.1046/j.1532-5415.2001.t01-1-49271.x. [DOI] [PubMed] [Google Scholar]
- Keage HA, Banks S, Yang KL, Morgan K, Brayne C, Matthews FE. What sleep characteristics predict cognitive decline in the elderly? Sleep Med. 2012;13:886–92. doi: 10.1016/j.sleep.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Kronholm E, Sallinen M, Suutama T, Sulkava R, Era P, Partonen T. Self-reported sleep duration and cognitive functioning in the general population. J Sleep Res. 2009;18:436–46. doi: 10.1111/j.1365-2869.2009.00765.x. [DOI] [PubMed] [Google Scholar]
- Louis ED, Benito-León J, Bermejo-Pareja F. Self-reported depression and anti-depressant medication use in essential tremor: cross-sectional and prospective analyses in a population-based study. Eur J Neurol. 2007a;14:1138–46. doi: 10.1111/j.1468-1331.2007.01923.x. [DOI] [PubMed] [Google Scholar]
- Louis ED, Benito-León J, Bermejo-Pareja F. Neurological Disorders in Central Spain Study, G. Self-reported depression and anti-depressant medication use in essential tremor: cross-sectional and prospective analyses in a population-based study. Eur J Neurol. 2007b;14:1138–46. doi: 10.1111/j.1468-1331.2007.01923.x. [DOI] [PubMed] [Google Scholar]
- Martínez-Salio A, Benito-León J, Diaz-Guzman J, Bermejo-Pareja F. Cerebrovascular disease incidence in central Spain (NEDICES): a population-based prospective study. J Neurol Sci. 2010;298:85–90. doi: 10.1016/j.jns.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Miyata S, Noda A, Iwamoto K, Kawano N, Okuda M, Ozaki N. Poor sleep quality impairs cognitive performance in older adults. J Sleep Res. 2013 doi: 10.1111/jsr.12054. [DOI] [PubMed] [Google Scholar]
- Morales JM, Bermejo FP, Benito-León J, Rivera-Navarro J, Trincado R, Gabriel SR, Vega S, Group NS. Methods and demographic findings of the baseline survey of the NEDICES cohort: a door-to-door survey of neurological disorders in three communities from Central Spain. Public Health. 2004;118:426–33. doi: 10.1016/j.puhe.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Myers BL, Badia P. Changes in circadian rhythms and sleep quality with aging: mechanisms and interventions. Neurosci Biobehav Rev. 1995;19:553–71. doi: 10.1016/0149-7634(95)00018-6. [DOI] [PubMed] [Google Scholar]
- Ohayon MM, Vecchierini MF. Daytime sleepiness and cognitive impairment in the elderly population. Arch Intern Med. 2002;162:201–8. doi: 10.1001/archinte.162.2.201. [DOI] [PubMed] [Google Scholar]
- Potvin O, Lorrain D, Forget H, Dube M, Grenier S, Preville M, Hudon C. Sleep quality and 1-year incident cognitive impairment in community-dwelling older adults. Sleep. 2012;35:491–9. doi: 10.5665/sleep.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto G, Contador I, Tapias-Merino E, Mitchell AJ, Bermejo-Pareja F. The Mini-Mental-37 test for dementia screening in the Spanish population: an analysis using the Rasch Model. Clin Neuropsychol. 2012;26:1003–18. doi: 10.1080/13854046.2012.704945. [DOI] [PubMed] [Google Scholar]
- Saint Martin M, Sforza E, Barthelemy JC, Thomas-Anterion C, Roche F. Does subjective sleep affect cognitive function in healthy elderly subjects? The Proof cohort. Sleep Med. 2012;13:1146–52. doi: 10.1016/j.sleep.2012.06.021. [DOI] [PubMed] [Google Scholar]
- Tworoger SS, Lee S, Schernhammer ES, Grodstein F. The association of self-reported sleep duration, difficulty sleeping, and snoring with cognitive function in older women. Alzheimer Dis Assoc Disord. 2006;20:41–8. doi: 10.1097/01.wad.0000201850.52707.80. [DOI] [PubMed] [Google Scholar]
- Vega S, Benito-León J, Bermejo-Pareja F, Medrano MJ, Vega-Valderrama LM, Rodriguez C, Louis ED. Several factors influenced attrition in a population-based elderly cohort: neurological disorders in Central Spain Study. J Clin Epidemiol. 2010;63:215–22. doi: 10.1016/j.jclinepi.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Vetter C, Juda M, Roenneberg T. The influence of internal time, time awake, and sleep duration on cognitive performance in shiftworkers. Chronobiol Int. 2012;29:1127–38. doi: 10.3109/07420528.2012.707999. [DOI] [PubMed] [Google Scholar]
- Xu L, Jiang CQ, Lam TH, Liu B, Jin YL, Zhu T, Zhang WS, Cheng KK, Thomas GN. Short or long sleep duration is associated with memory impairment in older Chinese: the Guangzhou Biobank Cohort Study. Sleep. 2011;34:575–80. doi: 10.1093/sleep/34.5.575. [DOI] [PMC free article] [PubMed] [Google Scholar]