Abstract
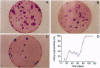
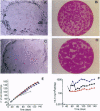
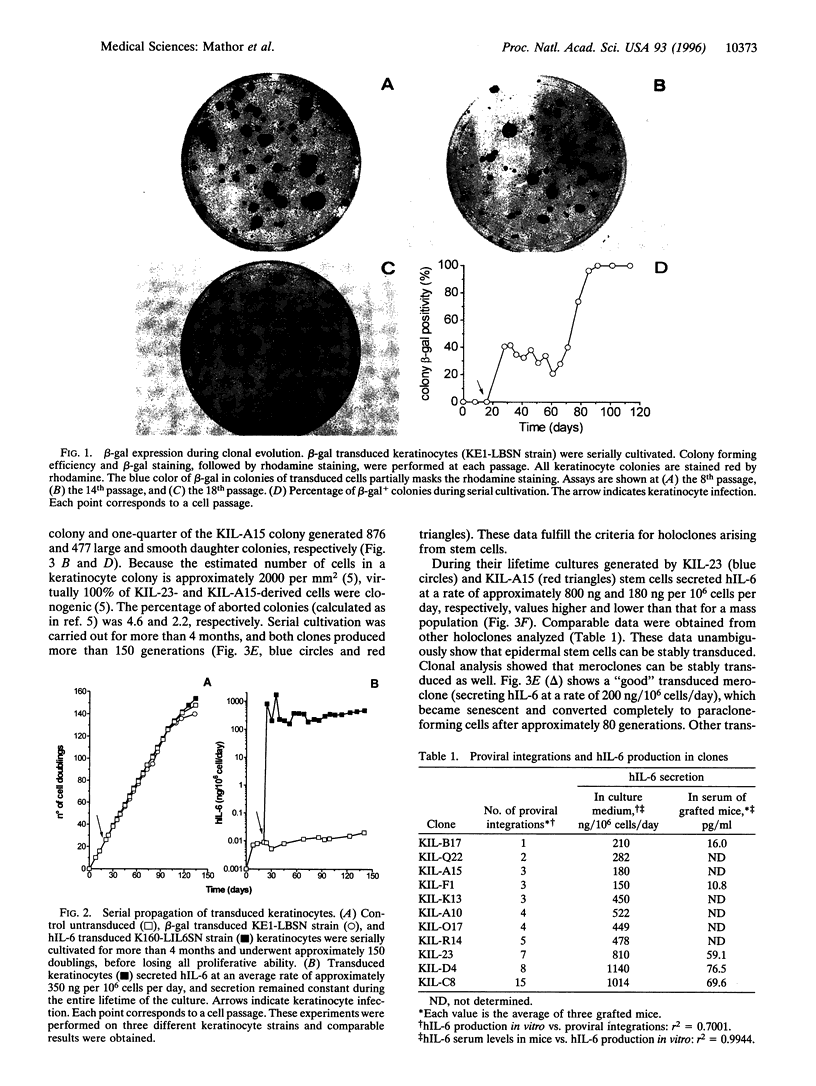
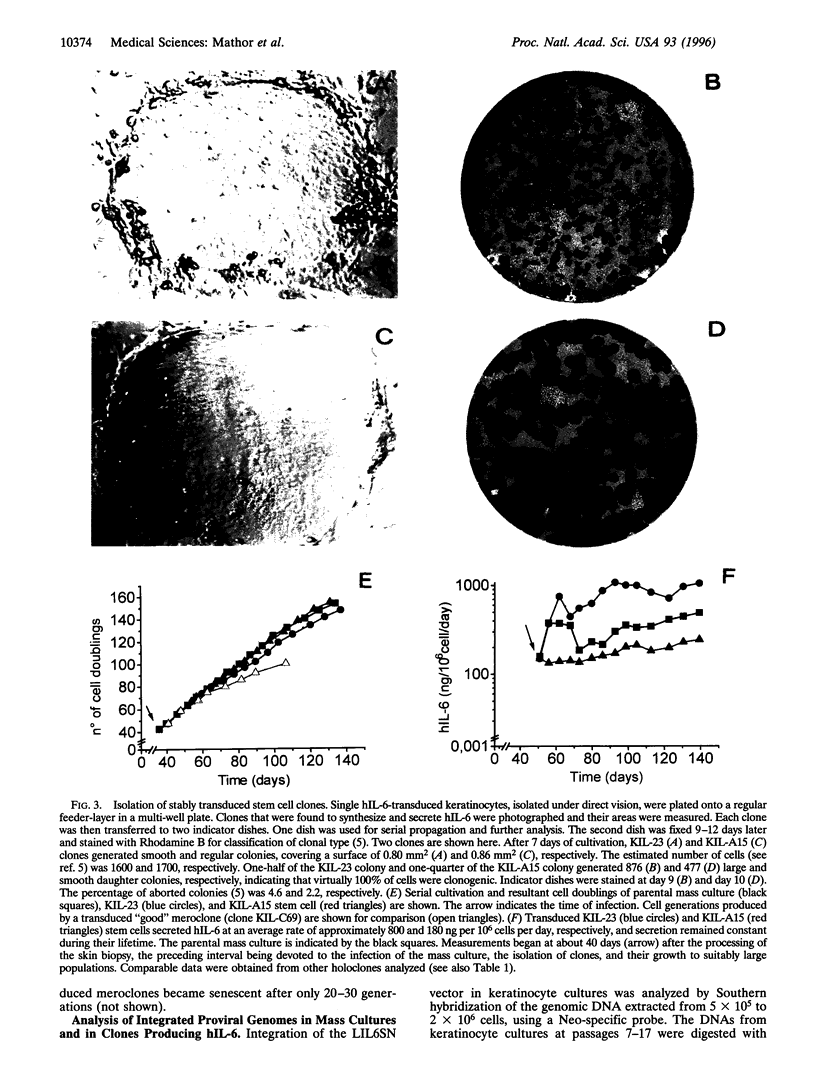
We have transduced normal human keratinocytes with retroviral constructs expressing a bacterial beta-galactosidase (beta-gal) gene or a human interleukin-6 (hIL-6) cDNA under control of a long terminal repeat. Efficiency of gene transfer averaged approximately 50% and 95% of clonogenic keratinocytes for beta-gal and hIL-6, respectively. Both genes were stably integrated and expressed for more than 150 generations. Clonal analysis showed that both holoclones and their transient amplifying progeny expressed the transgene permanently. Southern blot analysis on isolated clones showed that many keratinocyte stem cells integrated multiple proviral copies in their genome and that the synthesis of the exogenous gene product in vitro was proportional to the number of proviral integrations. When cohesive epidermal sheets prepared from stem cells transduced with hIL-6 were grafted on athymic animals, the serum levels of hIL-6 were strictly proportional to the rate of secretion in vitro and therefore to the number of proviral integrations. The possibility of specifying the level of transgene expression and its permanence in a homogeneous clone of stem cell origin opens new perspectives in the long-term treatment of genetic disorders.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander M. Y., Bidichandani S. I., Cousins F. M., Robinson C. J., Duffie E., Akhurst R. J. Circulating human factor IX produced in keratin-promoter transgenic mice: a feasibility study for gene therapy of haemophilia B. Hum Mol Genet. 1995 Jun;4(6):993–999. doi: 10.1093/hmg/4.6.993. [DOI] [PubMed] [Google Scholar]
- Barrandon Y., Green H. Three clonal types of keratinocyte with different capacities for multiplication. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2302–2306. doi: 10.1073/pnas.84.8.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrandon Y., Li V., Green H. New techniques for the grafting of cultured human epidermal cells onto athymic animals. J Invest Dermatol. 1988 Oct;91(4):315–318. doi: 10.1111/1523-1747.ep12475646. [DOI] [PubMed] [Google Scholar]
- Boyce S. T. Epidermis as a secretory tissue. J Invest Dermatol. 1994 Jan;102(1):8–10. doi: 10.1111/1523-1747.ep12371721. [DOI] [PubMed] [Google Scholar]
- Compton C. C., Gill J. M., Bradford D. A., Regauer S., Gallico G. G., O'Connor N. E. Skin regenerated from cultured epithelial autografts on full-thickness burn wounds from 6 days to 5 years after grafting. A light, electron microscopic and immunohistochemical study. Lab Invest. 1989 May;60(5):600–612. [PubMed] [Google Scholar]
- De Luca M., Albanese E., Bondanza S., Megna M., Ugozzoli L., Molina F., Cancedda R., Santi P. L., Bormioli M., Stella M. Multicentre experience in the treatment of burns with autologous and allogenic cultured epithelium, fresh or preserved in a frozen state. Burns. 1989 Oct;15(5):303–309. doi: 10.1016/0305-4179(89)90007-7. [DOI] [PubMed] [Google Scholar]
- Eady R. A., Dunnill M. G. Epidermolysis bullosa: hereditary skin fragility diseases as paradigms in cell biology. Arch Dermatol Res. 1994;287(1):2–9. doi: 10.1007/BF00370710. [DOI] [PubMed] [Google Scholar]
- Fenjves E. S. Approaches to gene transfer in keratinocytes. J Invest Dermatol. 1994 Nov;103(5 Suppl):70S–75S. doi: 10.1111/1523-1747.ep12399089. [DOI] [PubMed] [Google Scholar]
- Fenjves E. S., Gordon D. A., Pershing L. K., Williams D. L., Taichman L. B. Systemic distribution of apolipoprotein E secreted by grafts of epidermal keratinocytes: implications for epidermal function and gene therapy. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8803–8807. doi: 10.1073/pnas.86.22.8803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenjves E. S., Yao S. N., Kurachi K., Taichman L. B. Loss of expression of a retrovirus-transduced gene in human keratinocytes. J Invest Dermatol. 1996 Mar;106(3):576–578. doi: 10.1111/1523-1747.ep12344976. [DOI] [PubMed] [Google Scholar]
- Ferrari G., Rossini S., Giavazzi R., Maggioni D., Nobili N., Soldati M., Ungers G., Mavilio F., Gilboa E., Bordignon C. An in vivo model of somatic cell gene therapy for human severe combined immunodeficiency. Science. 1991 Mar 15;251(4999):1363–1366. doi: 10.1126/science.1848369. [DOI] [PubMed] [Google Scholar]
- Flowers M. E., Stockschlaeder M. A., Schuening F. G., Niederwieser D., Hackman R., Miller A. D., Storb R. Long-term transplantation of canine keratinocytes made resistant to G418 through retrovirus-mediated gene transfer. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2349–2353. doi: 10.1073/pnas.87.6.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallico G. G., 3rd, O'Connor N. E., Compton C. C., Kehinde O., Green H. Permanent coverage of large burn wounds with autologous cultured human epithelium. N Engl J Med. 1984 Aug 16;311(7):448–451. doi: 10.1056/NEJM198408163110706. [DOI] [PubMed] [Google Scholar]
- Garlick J. A., Katz A. B., Fenjves E. S., Taichman L. B. Retrovirus-mediated transduction of cultured epidermal keratinocytes. J Invest Dermatol. 1991 Nov;97(5):824–829. doi: 10.1111/1523-1747.ep12489019. [DOI] [PubMed] [Google Scholar]
- Gerrard A. J., Hudson D. L., Brownlee G. G., Watt F. M. Towards gene therapy for haemophilia B using primary human keratinocytes. Nat Genet. 1993 Feb;3(2):180–183. doi: 10.1038/ng0293-180. [DOI] [PubMed] [Google Scholar]
- Green H., Kehinde O., Thomas J. Growth of cultured human epidermal cells into multiple epithelia suitable for grafting. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5665–5668. doi: 10.1073/pnas.76.11.5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhalgh D. A., Rothnagel J. A., Roop D. R. Epidermis: an attractive target tissue for gene therapy. J Invest Dermatol. 1994 Nov;103(5 Suppl):63S–69S. doi: 10.1111/1523-1747.ep12399070. [DOI] [PubMed] [Google Scholar]
- Jensen T. G., Jensen U. B., Jensen P. K., Ibsen H. H., Brandrup F., Ballabio A., Bolund L. Correction of steroid sulfatase deficiency by gene transfer into basal cells of tissue-cultured epidermis from patients with recessive X-linked ichthyosis. Exp Cell Res. 1993 Dec;209(2):392–397. doi: 10.1006/excr.1993.1326. [DOI] [PubMed] [Google Scholar]
- Jensen U. B., Jensen T. G., Jensen P. K., Rygaard J., Hansen B. S., Fogh J., Kølvraa S., Bolund L. Gene transfer into cultured human epidermis and its transplantation onto immunodeficient mice: an experimental model for somatic gene therapy. J Invest Dermatol. 1994 Sep;103(3):391–394. doi: 10.1111/1523-1747.ep12395402. [DOI] [PubMed] [Google Scholar]
- Krueger G. G., Morgan J. R., Jorgensen C. M., Schmidt L., Li H. L., Kwan M. K., Boyce S. T., Wiley H. S., Kaplan J., Petersen M. J. Genetically modified skin to treat disease: potential and limitations. J Invest Dermatol. 1994 Nov;103(5 Suppl):76S–84S. doi: 10.1111/1523-1747.ep12399100. [DOI] [PubMed] [Google Scholar]
- Lajtha L. G. Stem cell concepts. Differentiation. 1979;14(1-2):23–34. doi: 10.1111/j.1432-0436.1979.tb01007.x. [DOI] [PubMed] [Google Scholar]
- Markowitz D., Goff S., Bank A. A safe packaging line for gene transfer: separating viral genes on two different plasmids. J Virol. 1988 Apr;62(4):1120–1124. doi: 10.1128/jvi.62.4.1120-1124.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz D., Goff S., Bank A. Construction and use of a safe and efficient amphotropic packaging cell line. Virology. 1988 Dec;167(2):400–406. [PubMed] [Google Scholar]
- Miller A. D., Miller D. G., Garcia J. V., Lynch C. M. Use of retroviral vectors for gene transfer and expression. Methods Enzymol. 1993;217:581–599. doi: 10.1016/0076-6879(93)17090-r. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Rosman G. J. Improved retroviral vectors for gene transfer and expression. Biotechniques. 1989 Oct;7(9):980-2, 984-6, 989-90. [PMC free article] [PubMed] [Google Scholar]
- Morgan J. R., Barrandon Y., Green H., Mulligan R. C. Expression of an exogenous growth hormone gene by transplantable human epidermal cells. Science. 1987 Sep 18;237(4821):1476–1479. doi: 10.1126/science.3629250. [DOI] [PubMed] [Google Scholar]
- Rochat A., Kobayashi K., Barrandon Y. Location of stem cells of human hair follicles by clonal analysis. Cell. 1994 Mar 25;76(6):1063–1073. doi: 10.1016/0092-8674(94)90383-2. [DOI] [PubMed] [Google Scholar]
- Romagnoli G., De Luca M., Faranda F., Bandelloni R., Franzi A. T., Cataliotti F., Cancedda R. Treatment of posterior hypospadias by the autologous graft of cultured urethral epithelium. N Engl J Med. 1990 Aug 23;323(8):527–530. doi: 10.1056/NEJM199008233230806. [DOI] [PubMed] [Google Scholar]
- Salvatori G., Ferrari G., Mezzogiorno A., Servidei S., Coletta M., Tonali P., Giavazzi R., Cossu G., Mavilio F. Retroviral vector-mediated gene transfer into human primary myogenic cells leads to expression in muscle fibers in vivo. Hum Gene Ther. 1993 Dec;4(6):713–723. doi: 10.1089/hum.1993.4.6-713. [DOI] [PubMed] [Google Scholar]
- Setoguchi Y., Jaffe H. A., Danel C., Crystal R. G. Ex vivo and in vivo gene transfer to the skin using replication-deficient recombinant adenovirus vectors. J Invest Dermatol. 1994 Apr;102(4):415–421. doi: 10.1111/1523-1747.ep12372181. [DOI] [PubMed] [Google Scholar]
- Stockschlaeder M. A., Storb R., Osborne W. R., Miller A. D. L-histidinol provides effective selection of retrovirus-vector-transduced keratinocytes without impairing their proliferative potential. Hum Gene Ther. 1991 Spring;2(1):33–39. doi: 10.1089/hum.1991.2.1-33. [DOI] [PubMed] [Google Scholar]
- Stockschläder M. A., Schuening F. G., Graham T. C., Storb R. Transplantation of retrovirus-transduced canine keratinocytes expressing the beta-galactosidase gene. Gene Ther. 1994 Sep;1(5):317–322. [PubMed] [Google Scholar]
- Teumer J., Lindahl A., Green H. Human growth hormone in the blood of athymic mice grafted with cultures of hormone-secreting human keratinocytes. FASEB J. 1990 Nov;4(14):3245–3250. doi: 10.1096/fasebj.4.14.2227214. [DOI] [PubMed] [Google Scholar]
- Vogt P. M., Thompson S., Andree C., Liu P., Breuing K., Hatzis D., Brown H., Mulligan R. C., Eriksson E. Genetically modified keratinocytes transplanted to wounds reconstitute the epidermis. Proc Natl Acad Sci U S A. 1994 Sep 27;91(20):9307–9311. doi: 10.1073/pnas.91.20.9307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambruno G., Marchisio P. C., Marconi A., Vaschieri C., Melchiori A., Giannetti A., De Luca M. Transforming growth factor-beta 1 modulates beta 1 and beta 5 integrin receptors and induces the de novo expression of the alpha v beta 6 heterodimer in normal human keratinocytes: implications for wound healing. J Cell Biol. 1995 May;129(3):853–865. doi: 10.1083/jcb.129.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Luca M., Albanese E., Megna M., Cancedda R., Mangiante P. E., Cadoni A., Franzi A. T. Evidence that human oral epithelium reconstituted in vitro and transplanted onto patients with defects in the oral mucosa retains properties of the original donor site. Transplantation. 1990 Sep;50(3):454–459. doi: 10.1097/00007890-199009000-00019. [DOI] [PubMed] [Google Scholar]