Abstract
Multiple types of cell death exist including necrosis, apoptosis, and autophagic cell death. The Drosophila ovary provides a valuable model to study the diversity of cell death modalities, and we review recent progress to elucidate these pathways. At least five distinct types of cell death occur in the ovary, and we focus on two that have been studied extensively. Cell death of mid-stage egg chambers uses a novel caspase-dependent pathway that involves autophagy, and triggers phagocytosis by surrounding somatic epithelial cells. For every egg, fifteen germline nurse cells undergo developmental programmed cell death, which occurs independently of most known cell death genes. These forms of cell death are strikingly similar to cell death observed in the germline of other organisms.
Keywords: apoptosis, autophagy, Drosophila, oogenesis, ovary, programmed cell death
Introduction to cell death pathways
During development and homeostasis, programmed cell death (PCD) eliminates unnecessary or damaged cells. The intentional death of a cell is tightly regulated, as inappropriate cell death (or lack of cell death) could be disastrous for the organism. The three canonical types of PCD are apoptosis, autophagic cell death, and necrosis, but a variety of non-canonical types have been observed [1]. Studying diverse mechanisms of cell death is of central importance to understanding human disease. Here we review cell death in the Drosophila ovary, a powerful model system for investigating the genetic control and cell biological events of multiple types of cell death.
In apoptosis, a cell deliberately kills itself and orchestrates the dismantling of its corpse, usually without eliciting an inflammatory response [2]. Apoptosis can be activated by death signals or by stress, such as DNA damage or reactive oxygen species [3]. In apoptosis, caspases (cysteine aspartyl proteases) cleave proteins, DNA is condensed and fragmented, the cell membrane retracts, and packets of cytoplasm enclosed by plasma membrane (“blebs”) are released [4]. Molecularly, the decision to apoptose is the outcome of an equation which balances pro-apoptotic proteins, such as caspases, and anti-apoptotic proteins, such as the inhibitor of apoptosis proteins (IAPs) [4].
In Drosophila, IAPs are key regulators of apoptosis. The major cell death IAP in Drosophila melanogaster, DIAP1, suppresses caspases by ubiquitylation [5] until the cell commits to death, when DIAP1 is degraded to allow apoptosis to proceed. Other important apoptotic regulators are Ark (orthologous to mammalian Apaf-1), which forms a complex known as the apoptosome with the initiator caspase Dronc [6], and several proteins with ubiquitin ligase activity, such as dBruce [3, 7].
In most fly tissues, expression of the IAP antagonists reaper, grim, and head involution defective (hid), together often called the H99 or RHG genes, commits the cell to death by apoptosis [7, 8]. These proteins use their IBM (IAP binding motif) to bind to BIR domains on IAPs, thereby repressing them. Once upstream signals lead to DIAP1 degradation, activated initiator caspase Dronc then activates effector caspases Drice and Dcp-1 [7, 9]. Caspase targets include caspase-activated DNase (dCAD/Rep4), which cleaves DNA between nucleosomes [10], cytoskeletal components, such as actin and lamins [4], and proteasome subunits, resulting in reduced proteasome activity [11]. Concomitant with dismantling of the nucleus and cytoskeleton, mitochondrial networks are remodeled, requiring mitochondrial fission and fusion proteins [12–14].
Autophagy, the degradation of cytoplasmic components inside double-membrane vesicles, can be a pro-survival or pro-death mechanism [8, 15, 16]. Proteins and organelles are enclosed in a double-membraned vesicle (autophagosome) that fuses with an acidic lysosome, which degrades its contents [15, 17]. The presence of autophagosomes in dying cells may imply autophagic cell death, but could also indicate defective or stalled autophagy. Autophagic cell death can occur independently of caspases, in parallel with them, or one mechanism can be epistatic to the other [16]. The role of autophagy in cell death is highly context-dependent, but is known to be required for death in some cell types, and is sometimes upregulated when apoptosis is blocked [8]. In the Drosophila ovary, both apoptotic and autophagic mechanisms contribute to cell death [18–20].
Death by necrosis is now recognized as a bona fide form of PCD and not just the consequence of cellular injury [21, 22]. Necrosis is characterized by a swelling of cell volume and organelles (especially mitochondria) leading to eventual membrane rupture, as well as increases in cytosolic calcium and reactive oxygen species, release of pro-inflammatory signals, and lower pH and ATP levels [1] . Despite its disorderly reputation, several genes have been found to be required for necrotic death in C. elegans [23] and mammals [24], as well as flies [25].
The fly ovary as a model tissue
The Drosophila ovary is an outstanding model system for the study of cell death pathways. Both germline and somatic cells undergo cell death, and these cell death events use multiple PCD mechanisms. The large, easily dissected egg chambers are highly suitable for imaging analyses and can be cultured for short periods in vitro. Moreover, the rich repository of available Drosophila mutant lines and genetic tools allow quick generation of tissue-specific gene knockouts, knockdown or overexpression lines, mosaic egg chambers, and more. In this review, we focus on recent progress in uncovering the mechanisms of germline cell death.
The Drosophila female has two ovaries, which continuously produce eggs (Figure 1A). An ovary is a bundle of 15–20 ovarioles, sheaths of progressively developing egg chambers (Figure 1B), designated as stages 1–14 [26]. Each egg chamber contains a sixteen-cell germline-derived cyst, with one cell that differentiates into the oocyte. The other germline cells become polyploid nurse cells (NCs), which remain connected to the oocyte through intercellular bridges (ring canals) and stock the oocyte with organelles, protein and RNA. This germline cyst is surrounded by a layer of somatic follicle cells (FCs), which begin to produce yolk for the oocyte at stage 8. As the egg matures, the oocyte grows to fill the entire chamber as the NCs shrink and disappear, and a chorion coat and dorsal appendages develop by stage 14 [26, 27].
Figure 1. Structure of the fly ovary.
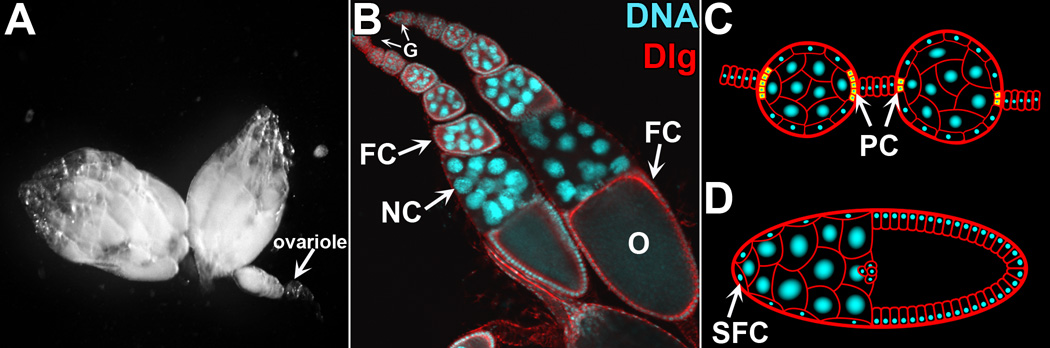
A) Fly ovaries contain strings of ovarioles (arrow). B) Two ovarioles stained with DAPI to label DNA (cyan) and anti-Discs large (Dlg, red) to label membranes. Each ovariole contains progressively developing egg chambers, which mature as they are pushed towards the posterior of the ovary. G=Germarium, NC=Nurse cell, FC=Follicle cell, O=oocyte. C) Schematic showing loss of polar cells (PC, yellow cytoplasm) between stage 3 (left) and stage 5 (right). D) Schematic showing stretch follicle cells (SFC) extending over the nurse cells in a stage 10 egg chamber.
Cell death in the germline of a wild-type (WT) fly occurs primarily at three stages of egg chamber development: in the germarium before the FC layer forms (stage 2b), in pre-vitellogenic stages 7–9 (“mid-stage death”), and as the egg nears maturation in stages 12–14 (“late-stage death”) [20, 28, 29]. Whereas late stage death occurs during the development of every egg, cell death in the germarium and stages 7–9 occurs sporadically in well-fed flies, and increases dramatically in response to developmental abnormalities or poor environmental conditions, such as protein starvation [19, 30, 31]. As part of normal development in late oogenesis, NCs transport their cytoplasm through the ring canals into the oocyte, and only their polyploid nuclei remain. By stage 14, all NC nuclei disappear, leaving a mature oocyte. These distinct forms of cell death in oogenesis involve multiple mechanisms, including apoptosis, autophagic cell death, and other pathways.
Somatic FCs can also die throughout oogenesis, but the mechanisms of FC death are generally not well understood. One exception is polar cell death, which is the only example of PCD in the ovary shown to require an RHG gene. The polar cells are derived from clusters of 3–6 specialized FCs located at the anterior and posterior ends of an egg chamber during early oogenesis (Figure 1C). By stage 5, the number of polar cells in each cluster is reduced to two, as supernumerary polar cells are eliminated by the canonical apoptotic pathway: Hid ⊣ DIAP1 ⊣ Dronc → Drice [32, 33]. Recently, it has been shown that the JAK/STAT pathway promotes this cascade [34]. Supernumerary polar cell death is developmentally important, as it is required for proper migration of border cells which produce the micropyle [32].
In addition to the selective death of polar cells, large numbers of FCs die when their support functions have been completed. During mid-stage germline cell death, the epithelial layer of about one thousand FCs coordinately engulfs dying germline cells [35–37]. As engulfment nears completion, the FCs lose membrane markers, display pyknotic nuclei, and eventually disappear. Overexpression of DIAP1 or the baculovirus caspase inhibitor p35 in the FCs fails to prevent FCs from becoming pyknotic, suggesting that they die via a caspase-independent mechanism [37]. Similarly, some FC nuclei in late stage egg chambers undergo chromatin condensation and DNA fragmentation [38]. Studies in other Diptera have demonstrated that FCs dying during late oogenesis lack caspase activity, and are most likely undergoing autophagic cell death [39]. At the end of oogenesis, FCs detach from the eggshell when the mature egg exits the ovariole through the oviduct and into the uterus. Detached FCs accumulate at the entrance of the oviduct, where they can be engulfed by epithelial cells and/or macrophages [38, 39]. Most of the PCD pathways in FCs remain poorly understood.
Molecular mechanisms of germline cell death in mid-oogenesis
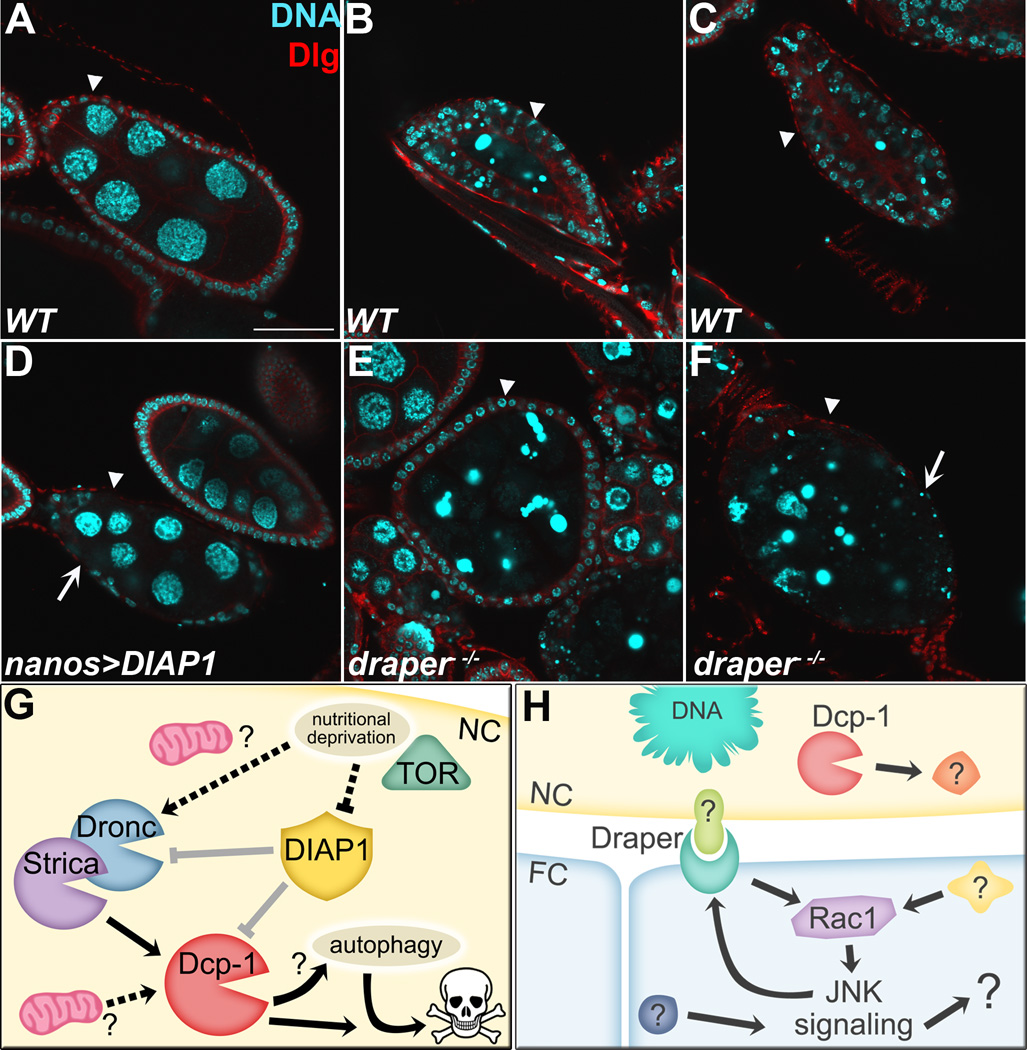
Cell death can be induced in mid-oogenesis by developmental abnormalities, drug treatment, or poor environmental conditions. However, almost everything we know about cell death in midoogenesis has been uncovered by inducing death via protein starvation. Lack of protein in the fly’s diet activates pro-death signaling, causing degeneration of egg chambers in the germarium and at stages 7–9, and slows production of new oocytes by germline stem cells, causing a reversible decrease in fertility [30]. In the germarium, cell death is observed primarily of germline cyst cells, perhaps because the germline stem cells do not slow cell division as much as the follicle cell stem cells, and the ratio of germ cells to follicle cells needs to be precisely matched [30]. In midoogenesis, it has been proposed that the stage-specificity of PCD is due to a specific “checkpoint” where conditions are monitored before the onset of energetically expensive vitellogenesis (yolk production) starting in stage 8 [30, 35]. Mid-stage death is easily identified by morphological changes. Unlike a healthy mid-stage egg chamber (Figure 2A), a dying egg chamber’s NC chromatin condenses and fragments (Figure 2B) [35, 40]. As this happens, the surrounding FCs switch to a phagocytic role to enlarge and engulf the dying NC material (Figure 2B–C), eventually consuming the entire germline [35–37].
Figure 2. Overview of starvation induced cell death during mid-oogenesis.
Mid-stage egg chambers from starved flies stained with DAPI (cyan) to label DNA and anti-Discs large (Dlg, red) to mark the cell membranes. A–C) WT egg chambers. A) Healthy stage 8 egg chamber has large NC nuclei surrounded by a thin layer of FCs. Arrowhead indicates FC layer in all panels. B) Dying egg chamber has condensed and fragmented NC DNA, and the surrounding layer of FCs has enlarged and begun to engulf germline material. C) Late dying egg chamber has few NC nuclear fragments remaining and the FCs have completed engulfment. D) An undead egg chamber (arrowhead) where the NC nuclei have failed to condense and fragment, and many of the surrounding FCs have disappeared (arrow), resulting from overexpression of DIAP1 in the NCs. E) draper−/− mid-dying egg chamber contains a thin layer of FCs (arrowhead) that have failed to enlarge (compare to WT in B). F) Late dying draper−/− egg chamber (arrowhead) has lingering germline material and pyknotic FCs (arrow). Scale bar = 50 µm. G) Model of mid-stage death, showing suppression of caspases by DIAP1 (in gray) and potential regulatory mechanisms for mitochondria and nutritional deprivation (dotted lines). H) Model of mid-stage engulfment, showing activation of Draper-Rac1-JNK pathway by an unknown signal.
Cell death in mid-oogenesis resembles apoptosis morphologically and is caspase-dependent, although the upstream activators differ from those used in most apoptotic cell deaths in the fly (Figure 2G) [20, 29]. The first gene shown to be required for cell death in mid-oogenesis was the effector caspase gene Dcp-1 [41]. Dcp-1 null flies produce “undead” egg chambers with uncondensed NC nuclei and a loss of the surrounding FCs. Undead egg chambers are also observed with over-expression of DIAP1 (Figure 2D) or p35 [36, 42, 43]. Surprisingly, whereas Dcp-1 mutants are completely defective in mid-stage cell death, mutations in initiator caspases have a much milder phenotype, suggesting a novel mechanism of effector caspase activation [43]. Furthermore, the RHG pro-death proteins are not required for mid-stage cell death in the ovary [44]; thus, other pathways must conduct the pro-apoptotic signal to mid-stage egg chambers.
The upstream signals for cell death in mid-oogenesis have only been partially identified. Nutrition availability information is transmitted by insulin and ecdysone signaling pathways [29, 45]. However, egg chambers deficient in insulin signaling cannot develop to mid-oogenesis, and they also cannot die correctly before then [46]. Mutations in Target of rapamycin (Tor), which encodes a highly conserved Ser/Thr kinase that integrates signaling via insulin, AMP-activated protein kinase (AMPK), AKT, and other growth and stress pathways [47], more accurately mimic the normal degeneration of mid-stage egg chambers [31, 46, 48]. The phenotypic differences between Insulin-like receptor and Tor mutants suggest that Tor is the key regulator of mid-stage cell death. Treatment with rapamycin or a derivative compound (which blocks Tor) produces varying results: adults fed rapamycin show an alternative form of cell death where FCs invade and engulf otherwise healthy germline cysts [49], whereas injection with the derivative RAD produces ovaries lacking vitellogenic egg chambers [31]. The differences in phenotypes between Tor nulls and rapamycin treatments may be due to different levels of Tor inhibition, tissue specificity, or contributions of TorC2 which is not blocked by rapamycin. Tor also regulates mammalian germ cell death (see Box 1).
Box 1: The fly: a model for human fertility?
There is a surprising amount of similarity between oocyte development, cell death, and infertility in the fly and mammalian ovary, making Drosophila a novel model for human oocyte loss. As in flies, mammals show developmental cell death of germline cyst cells and follicular atresia of maturing oocytes. The developmental germ cell loss affects the initial pool of available oocytes, whereas follicular atresia further reduces the number of oocytes during aging. Somatic granulosa cells surround the germ cells and contribute to their death.
In the mammalian embryonic ovary, primordial germ cells first develop into germ cell cysts via incomplete cytokinesis [81]. Shortly before birth, germline cells inside the cyst begin to die by apoptosis (“cyst breakdown”) until only a few remain, with each germ cell surrounded by somatic granulosa cells forming a structure known as a follicle. Multiple pathways have been found to be involved in this germ cell death, including apoptosis and autophagy, as well as BMP and Notch signaling [81]. Knock down of Notch2 in somatic granulosa cells leads to impaired apoptosis during cyst breakdown, resulting in follicles that contain multiple oocytes [82]. As mutant mice approach sexual maturity, their ovaries become hemorrhagic, and as adults their litter size is dramatically smaller than WT. Thus, during mammalian oogenesis, somatic cells can non-autonomously contribute to developmental cell death of the germline. In flies, FCs surrounding the NC nuclei in late oogenesis may also contribute non-autonomously to NC death, which could explain the weak phenotype caused by inhibiting caspases in the NCs with excess DIAP1 (Figure 3E).
In adult humans, the vast majority of follicles will degenerate during the antral stage (“follicular atresia”) before they fully mature [81, 83, 84]. Follicular atresia, like fly mid-stage death, features apoptotic and autophagic mechanisms, as well as engulfment of dead tissue. When a follicle degenerates, the granulosa cells are the first to show apoptotic features, but some survive to engulf other dead cells, eventually including the oocyte [85, 86]. As in flies, decreased Tor signaling causes excessive germ cell death. Culturing strips of human ovary in rapamycin (which blocks Tor signaling) results in increased degenerating or “empty” follicles, suggesting that a subsection of granulosa cells phagocytosed a viable oocyte [87]. This is very similar to the effect of rapamycin injection in female flies, where FCs engulf germline cysts, rendering the flies infertile [49].
These studies suggest that oocyte loss in adults does not necessarily happen because an oocyte is inferior or unviable, but that some die because of non-autonomous interactions that could perhaps be blocked pharmacologically. Studying how the inappropriate destruction of healthy oocytes can be prevented is of particular relevance to extending human fertility as women age [88].
Upstream apoptotic signals in mammals converge on mitochondria through the Bcl-2 family of proteins. There is increased evidence for the role of mitochondria in regulating PCD in Drosophila, but their exact contributions are still controversial [14, 50, 51]. As in mammals [52] and worms [53], Bcl-2 proteins and mitochondrial remodeling contribute to cell death in Drosophila, although their mechanisms may differ. Fragmentation of the mitochondrial network (which drives membrane permeabilization) is mediated by the mitochondrial fission protein Drp-1 before caspase activation [12, 13]. In fly, worm, and mammalian models, cells lacking Drp-1 have impaired mitochondrial fragmentation, caspase activation, and cell death [50]. In Drosophila mid-stage death in the ovary, mitochondria remodel into clusters, which are engulfed and then degraded by the FCs [54]. Formation of normal clusters is dependent on the fly Bcl-2 genes debcl and buffy, mitochondrial remodeling genes, caspases, and autophagy genes [54]. Mutations in the Bcl-2 genes and mitochondrial remodeling genes also inhibit mid-stage cell death, suggesting that mitochondrial signaling plays a role in promoting PCD in the ovary.
Autophagy occurs alongside apoptotic events during mid-stage death. Starvation increases the number of acidic vesicles (marked by LysoTracker) and autophagosomes/autolysosomes (marked by Autophagy (Atg) fusion proteins) in egg chambers prior to and during degeneration [18, 19, 31]. Autophagy is reduced in Dcp-1 mutants, and even without starvation, over-expression of Dcp-1 causes degenerating egg chambers with increased LysoTracker and GFP-LC3 puncta (marking autophagosomes), indicating that autophagy can be triggered by caspase activity [18]. Dying midstage egg chambers lacking the autophagy genes Atg1 or Atg7 in NCs show chromatin condensation but less DNA fragmentation, indicating that some but not all cell death events are affected by autophagy [18, 19]. It is possible that autophagy enhances the speed of nurse cell destruction but this has not been reported. Furthermore, how the caspase Dcp-1 interfaces with the autophagic machinery and/or mitochondria remains to be determined (Figure 2G).
The final step in PCD is the selective removal of dying cells, a process known as engulfment [2, 55]. The engulfment of cell corpses is generally executed by professional phagocytes, such as macrophages. Interestingly, in the absence of professional phagocytes, adjacent cells such as epithelial cells can transition to the role of non-professional phagocytes and remove apoptotic corpses [56, 57]. The Drosophila ovary has few circulating macrophages [26], so engulfment is completed by the epithelial FCs acting as non-professional phagocytes. During starvation-induced PCD in mid-oogenesis, FCs synchronously enlarge and engulf the dying NCs, a remarkable transformation (Figure 2B–C) [35–37, 54].
The genetic pathways required for engulfment by FCs have recently begun to be elucidated. The engulfment receptor Draper, the ortholog of Ced-1 in C. elegans, is required for engulfment by FCs and likely acts through Rac1, which promotes cytoskeletal rearrangements [37]. In draper mutant egg chambers, FCs fail to enlarge or engulf any germline material (Figure 2E), leading to the persistence of dead egg chambers. A similar phenotype is seen when draper is knocked down specifically in the FCs, demonstrating a requirement for draper in the phagocytic FCs. Egg chambers that are defective in engulfment contain lingering germline material, and FCs die prematurely (Figure 2F). It is possible that FCs are programmed to die following engulfment of the germline and that this program is activated prematurely when engulfment is not completed.
The JNK pathway is activated downstream of Draper, and it is required in the FCs during engulfment. Remarkably, expression of constitutively activated hemipterous, a JNK kinase, is sufficient to induce engulfment by the FCs in the absence of draper, indicating that JNK can activate Draper-independent pathways to promote engulfment. Moreover, overexpression of activated hemipterous in a WT background causes the FCs to engulf and kill the NCs in the absence of starvation, suggesting that the FCs have the ability to induce NC death. In addition, several engulfment mutants show defects in NC nuclear breakdown, implying that the phagocytic FCs contribute to the process of cell death of the underlying germline. It is plausible that the starved state of the follicle cells primes them for engulfing the germline; they may need to “eat or be eaten.” Under starvation conditions, the follicle cells show increased autophagy [31], and the autophagic machinery may participate in engulfment. Furthermore, selective inhibition of Tor promotes engulfment of the oocyte by the follicle cells [49]. FC involvement in Drosophila germ cell death is reminiscent of the role of granulosa cells in oocyte death in mammals (see Box 1). Together, these data suggest a model where Draper recognizes an unknown signal from the NCs, which leads to the activation of Rac1 and JNK signaling, which promotes engulfment and the progression of germline PCD. In a feed-forward loop, JNK signaling also leads to an increase in Draper as engulfment proceeds (Figure 2H) [37]. Further investigation is necessary to determine whether any additional known or novel genes are involved in engulfment of the NCs by the FCs during mid-oogenesis programmed cell death.
Genetics of nurse cell death in late oogenesis
During late stages of oogenesis, the NCs that provide nutrients, proteins, mRNAs, and organelles for the developing oocyte transport (“dump”) their contents into the oocyte and undergo PCD (Figure 3A–D) [26, 27]. By the time that egg chambers reach maturity at stage 14 (characterized by the formation of dorsal appendages), the NCs are completely eliminated, while the oocyte is protected (Figure 3A–D)[26, 27]. The developmental cell death of NCs has been studied since at least the 1930s [29], yet the exact mechanisms of developmental NC death still remain unclear.
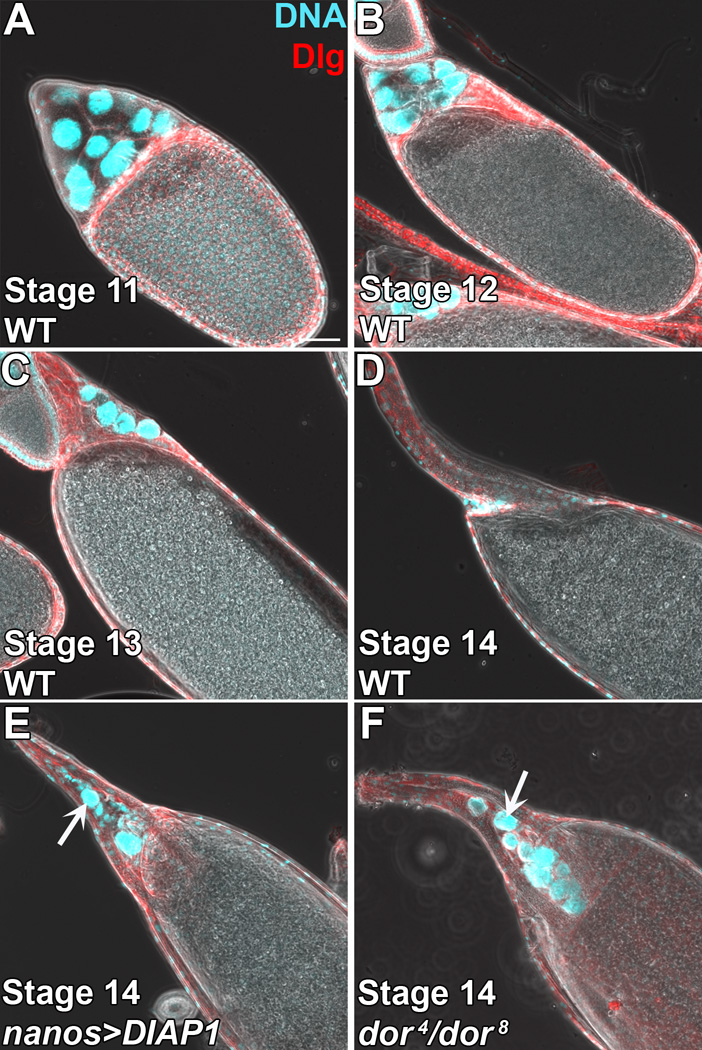
Figure 3. Overview of developmental programmed cell death during late oogenesis.
Egg chambers from well-fed flies stained with DAPI (cyan) to mark the DNA and anti-Discs large (Dlg, red) to mark the cell membranes. A–D) WT egg chambers. A) Stage 11 egg chamber has several NCs that still contain cytoplasm. B) Stage 12 egg chamber has completed dumping and retains NC nuclei. C) Stage 13 egg chamber has begun to form dorsal appendages and has a few NC nuclei remaining. D) Stage 14 egg chamber has fully formed dorsal appendages and no longer contains any NCs. E) Overexpression of DIAP1 in the NCs leads to a weak persisting nuclei phenotype where stage 14 egg chambers still contain a few NC nuclei (arrow). F) deep orange transheterozygous (dor8/dor4) stage 14 egg chamber has a strong persisting nuclei phenotype with many NC nuclei (arrow).
During NC dumping, a cytoplasmic network of actin bundles provides support for the NC nuclei while the cytoplasm streams into the oocyte through the ring canals. It has been suggested that the flattening of the FCs may help drive NC dumping during late oogenesis [27, 58]. While several mutants that affect NC dumping have been described, the upstream signals that initiate dumping are largely unknown. Furthermore, whether NC dumping plays a role in NC death or simply occurs concurrently is also unclear. One of the first indications that the NCs have begun to undergo PCD during late oogenesis is the permeabilization of the nuclear envelope occurring towards the end of stage 10B [20, 28, 35, 59, 60]. However, NC nuclear breakdown and DNA fragmentation have been shown to occur independently of actin bundle formation, indicating that dumping is not necessary for NC death [20, 28, 29, 59, 61, 62].
One possibility that has been explored extensively is that the NCs undergo apoptosis. The NC nuclei that remain behind after cytoplasmic dumping and in stages 12–13 become TUNEL positive, a hallmark of apoptosis [29, 61, 63]. However, several findings suggest that apoptosis executed autonomously within the NCs provides only a minor contribution to developmental NC death. The three major regulators of apoptosis (RHG genes) in most Drosophila tissues are not required for NC death during late oogenesis [61]. Additionally, overexpression of the caspase inhibitors p35 or DIAP1 (Figure 3E) or mutations in caspases lead to only partial defects in NC death [36, 41–43]. Together, these data suggest that apoptosis only plays a minor role in NC death during late oogenesis, or works in conjunction with other forms of cell death.
Another possibility is that autophagic cell death occurs in the NCs during late oogenesis. In late stage egg chambers from Drosophila virilis and melanogaster, autophagosomes are present [64, 65]. In Drosophila melanogaster, LysoTracker-positive puncta appear around the NC nuclei, indicating the presence of acidified organelles [66–68]. Furthermore, germline specific knockdown of several autophagy genes produces stage 14 egg chambers with some persisting NC nuclei that do not undergo DNA fragmentation [26, 27, 65, 67]. DNA fragmentation of the NCs has been shown to be dependent on the autophagic degradation of dBruce [65]. However, more recently it has been shown that germline clones of autophagy mutants generated using pole cell transplantation do not cause a persisting nuclei phenotype, and that the OvoD/heat shock method leads to the generation of FC clones that may contribute to persisting nuclei [69]. Thus, autophagy or apoptosis alone provide only minor contributions to NC death. Recent findings suggest that autophagy and apoptosis do not function redundantly [70], indicating that they must act in conjunction with other pathways to carry out NC death during late oogenesis.
While LysoTracker is often used as a marker for autophagic cell death, it can also be indicative of acidification that occurs during necrosis or during corpse processing following phagocytosis [71]. The progression of LysoTracker staining to include entire NC remnants during late oogenesis elicits the intriguing possibilities that NC death occurs via programmed necrosis [67] or that the FCs may engulf the NCs during late oogenesis [40, 66] and contribute non-autonomously to NC death. The idea that programmed necrosis occurs is supported by the presence of reactive oxygen species and uptake of propidium iodide (a marker for compromised membrane integrity) in the NCs during late oogenesis [68]. Additionally, calcium is redistributed from the NC nuclei to the NC cytoplasm following nuclear membrane permeabilization [72]. Calcium release is known to occur during necrosis [73]. However, further studies are necessary to understand whether programmed necrosis is contributing to developmental NC death. Interestingly, germ cell death in the testis shows many similarities to NC death (see Box 2).
Box 2: Programmed cell death in the male germline.
An unusual type of PCD in the Drosophila male germline has many similarities to death of the NCs during late oogenesis. During spermatogenesis, 20–30% of newly generated spermatogonial cysts die [89]. These cysts show a mix of apoptotic (condensed chromatin, TUNEL staining), autophagic (acidification), and necrotic (deformed mitochondria, increased reactive oxygen species) characteristics, but require few components of the canonical apoptotic or autophagic pathways. The initiator caspase Dronc acts independently of effector caspases, which are not required. Surprisingly, over-expression of caspase inhibitors p35 or Diap1 actually increase the frequency of dying cysts. Cysts died normally in Atg7 and Atg8 loss-of-function mutants, but still showed acidified vesicles and required lysosomal genes including cathepsin D and deep orange, which have both been shown to participate in developmental NC death (Figure 3F) [67]. A genetic modifier screen for this PCD phenotype found that the loss of HtrA2/Omi, a mitochondrial metalloprotease, reduced germ cell death and rendered males infertile. HtrA2/Omi has previously been shown to interact with IAPs through an IAP-binding motif (IBM) [90]. However, flies expressing HtrA2/Omi without its IBM had WT levels of germ cell death and fertility, indicating that HtrA2/Omi acts through its protease activity and not via suppressing DIAP1 [89]. It is interesting that both the male and female germline use non-apoptotic pathways to remove extraneous cells. Perhaps the requirement for mitochondria and lysosomes reflect a more ancient form of cell death not dependent on caspases.
During late oogenesis, a specific population of FCs known as the stretch FCs (Figure 1D) surround the NCs and may phagocytose them [29, 40, 66]. The presence of LysoTracker staining in and around the NCs during late oogenesis might be indicative of acidified vesicles present during corpse processing. Mutations in the lysosomal trafficking genes spinster and deep orange (dor, Figure 3F) lead to a strong persistence of NC nuclei in stage 14 egg chambers, further suggesting that lysosomal processing of the dying NCs is important for their removal [67]. While spinster is required in the germline for proper NC death/clearance [67], the tissue specificity of deep orange has not been reported. Whether engulfment of the NCs by the FCs is a contributing factor to NC death, or simply a consequence is unknown.
Concluding remarks
Even though the Drosophila ovary is a simple tissue composed of only three cell types, there are at least five different forms of cell death that occur without genetic or pharmacological manipulations. Two of these PCD events, the death of supernumerary polar FCs and mid-stage death of the germline, are apoptotic. However, the other examples of ovarian PCD can occur in the absence of caspase activity and provide a valuable opportunity to decipher non-apoptotic cell death pathways. The contribution of non-apoptotic cell death to disease in humans has become increasingly appreciated, inspiring several recent reviews and conferences [74–76].
Non-apoptotic cell death is common in the Drosophila germline, having now been revealed in primordial germ cells [77–79], the testis (see Box 2), and during the developmental PCD of the NCs. Why might germ cells rely on non-apoptotic cell death mechanisms when the vast majority of cell deaths in the fly occur by apoptosis? It is possible that germ cells rely on ancient cell death pathways; interestingly, lysosomal and mitochondrial pathways have been shown to be involved in both the testis and the ovary. Non-apoptotic pathways may be used in the ovary because high levels of caspase activity could endanger surviving oocytes, which remain connected through intercellular bridges.
The close proximity of NCs and FCs and their isolation from macrophages provide an excellent opportunity to dissect engulfment mechanisms in non-professional phagocytes. Furthermore, the non-autonomous contribution of somatic cells to germ cell death has been uncovered in both mammals and flies and may reveal another form of non-apoptotic cell death. Such a “phagoptotic” form of cell death may contribute to diseases such as Alzheimer’s disease, and manipulation of phagocytic cells may lead to cell survival [80]. Study of cell death in the fly ovary opens windows into understanding the diversity of cell death mechanisms occurring in nature.
Highlights.
At least five distinct types of cell death occur in the fly ovary.
Cell death of mid-stage egg chambers uses a novel caspase-dependent pathway that involves autophagy.
Developmental programmed cell death of ovarian nurse cells occurs independently of canonical cell death pathways.
These forms of cell death are similar to germline cell death in other organisms.
Acknowledgements
We thank members of the lab for helpful comments on the manuscript. Our research is supported by NIH grants R01 GM060574 and R01 GM094452 (KM).
Abbreviations
- Dlg
Discs large
- FC
follicle cell
- Hid
Head involution defective
- IAP
Inhibitor of Apoptosis Protein
- IBM
IAP-binding motif
- NC
nurse cell
- PCD
programmed cell death
- RHG
reaper hid grim
- WT
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Victoria Kathryn Jenkins, Email: jenkinsv@bu.edu.
Allison K Timmons, Email: timmonal@bu.edu.
Kimberly McCall, Email: kmccall@bu.edu.
References
- 1.Kroemer G, et al. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009;16:3–11. doi: 10.1038/cdd.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elliott MR, Ravichandran KS. Clearance of apoptotic cells: implications in health and disease. J Cell Biol. 2010;189:1059–1070. doi: 10.1083/jcb.201004096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steller H. Regulation of apoptosis in Drosophila . Cell Death Differ. 2008;15:1132–1138. doi: 10.1038/cdd.2008.50. [DOI] [PubMed] [Google Scholar]
- 4.Taylor RC, et al. Apoptosis: controlled demolition at the cellular level. Nature Reviews. Molecular Cell Biology. 2008;9:231–241. doi: 10.1038/nrm2312. [DOI] [PubMed] [Google Scholar]
- 5.Lee TV, et al. Drosophila IAP1-mediated ubiquitylation controls activation of the initiator caspase DRONC independent of protein degradation. PLoS Genetics. 2011;7:e1002261. doi: 10.1371/journal.pgen.1002261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bao Q, Shi Y. Apoptosome: a platform for the activation of initiator caspases. Cell Death Differ. 2007;14:56–65. doi: 10.1038/sj.cdd.4402028. [DOI] [PubMed] [Google Scholar]
- 7.Xu D, et al. Genetic control of programmed cell death (apoptosis) in Drosophila . Fly. 2009;3:78–90. doi: 10.4161/fly.3.1.7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryoo HD, Baehrecke EH. Distinct death mechanisms in Drosophila development. Curr Opin Cell Biol. 2010;22:889–895. doi: 10.1016/j.ceb.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colin J, et al. Mitochondria, Bcl-2 family proteins and apoptosomes: of worms, flies and men. Front Biosci. 2009;14:4127–4137. doi: 10.2741/3517. [DOI] [PubMed] [Google Scholar]
- 10.Mukae N, et al. Identification and developmental expression of inhibitor of caspaseactivated DNase (ICAD) in Drosophila melanogaster . J Biol Chem. 2000;275:21402–21408. doi: 10.1074/jbc.M909611199. [DOI] [PubMed] [Google Scholar]
- 11.Adrain C, et al. Caspase-dependent inactivation of proteasome function during programmed cell death in Drosophila and man. J Biol Chem. 2004;279:36923–36930. doi: 10.1074/jbc.M402638200. [DOI] [PubMed] [Google Scholar]
- 12.Abdelwahid E, et al. Mitochondrial disruption in Drosophila apoptosis. Dev Cell. 2007;12:793–806. doi: 10.1016/j.devcel.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Goyal G, et al. Role of mitochondrial remodeling in programmed cell death in Drosophila melanogaster. Dev Cell. 2007;12:807–816. doi: 10.1016/j.devcel.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinou JC, Youle RJ. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev Cell. 2011;21:92–101. doi: 10.1016/j.devcel.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mizushima N. Autophagy: process and function. Genes Dev. 2007;21:2861–2873. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- 16.Das G, et al. Regulation and function of autophagy during cell survival and cell death. Cold Spring Harb Perspect Biol. 2012;4:a008813. doi: 10.1101/cshperspect.a008813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147:728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 18.Hou YC, et al. Effector caspase Dcp-1 and IAP protein Bruce regulate starvation-induced autophagy during Drosophila melanogaster oogenesis. J Cell Biol. 2008;182:1127–1139. doi: 10.1083/jcb.200712091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nezis IP, et al. Cell death during Drosophila melanogaster early oogenesis is mediated through autophagy. Autophagy. 2009;5:298–302. doi: 10.4161/auto.5.3.7454. [DOI] [PubMed] [Google Scholar]
- 20.Pritchett TL, et al. Cracking open cell death in the Drosophila ovary. Apoptosis. 2009;14:969–979. doi: 10.1007/s10495-009-0369-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCall K. Genetic control of necrosis - another type of programmed cell death. Curr Opin Cell Biol. 2010;22:882–888. doi: 10.1016/j.ceb.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuan J, Kroemer G. Alternative cell death mechanisms in development and beyond. Genes Dev. 2010;24:2592–2602. doi: 10.1101/gad.1984410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blum ES, et al. Noncanonical cell death programs in the nematode Caenorhabditis elegans . Cell Death Differ. 2008;15:1124–1131. doi: 10.1038/cdd.2008.56. [DOI] [PubMed] [Google Scholar]
- 24.Hitomi J, et al. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell. 2008;135:1311–1323. doi: 10.1016/j.cell.2008.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanda H, et al. Conserved metabolic energy production pathways govern Eiger/TNFinduced nonapoptotic cell death. Proc Natl Acad Sci USA. 2011;108:18977–18982. doi: 10.1073/pnas.1103242108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.King RC. Ovarian Development in Drosophila melanogaster. New York: Academic Press; 1970. [Google Scholar]
- 27.Spradling AC. Developmental genetics of oogenesis. In: Bate M, Martinez Arias A, editors. The Development of Drosophila melanogaster. Cold Spring Harbor Laboratory Press; 1993. pp. 1–70. [Google Scholar]
- 28.Buszczak M, Cooley L. Eggs to die for: cell death during Drosophila oogenesis. Cell Death Differ. 2000;7:1071–1074. doi: 10.1038/sj.cdd.4400755. [DOI] [PubMed] [Google Scholar]
- 29.McCall K. Eggs over easy: cell death in the Drosophila ovary. Dev Biol. 2004;274:3–14. doi: 10.1016/j.ydbio.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 30.Drummond-Barbosa D, Spradling AC. Stem cells and their progeny respond to nutritional changes during Drosophila oogenesis. Dev Biol. 2001;231:265–278. doi: 10.1006/dbio.2000.0135. [DOI] [PubMed] [Google Scholar]
- 31.Barth JM, et al. Autophagy in Drosophila ovaries is induced by starvation and is required for oogenesis. Cell Death Differ. 2011;18:915–924. doi: 10.1038/cdd.2010.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Besse F, Pret AM. Apoptosis-mediated cell death within the ovarian polar cell lineage of Drosophila melanogaster . Development. 2003;130:1017–1027. doi: 10.1242/dev.00313. [DOI] [PubMed] [Google Scholar]
- 33.Khammari A, et al. Physiological apoptosis of polar cells during Drosophila oogenesis is mediated by Hid-dependent regulation of Diap1. Cell Death Differ. 2011;18:793–805. doi: 10.1038/cdd.2010.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borensztejn A, et al. JAK/STAT autocontrol of ligand-producing cell number through apoptosis. Development. 2013;140:195–204. doi: 10.1242/dev.079046. [DOI] [PubMed] [Google Scholar]
- 35.Giorgi F, Deri P. Cell death in ovarian chambers of Drosophila melanogaster . J Emb Exp Morph. 1976;35:521–533. [PubMed] [Google Scholar]
- 36.Mazzalupo S, Cooley L. Illuminating the role of caspases during Drosophila oogenesis. Cell Death Differ. 2006;13:1950–1959. doi: 10.1038/sj.cdd.4401892. [DOI] [PubMed] [Google Scholar]
- 37.Etchegaray JI, et al. Draper acts through the JNK pathway to control synchronous engulfment of dying germline cells by follicular epithelial cells. Development. 2012;139:4029–4039. doi: 10.1242/dev.082776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nezis IP, et al. Dynamics of apoptosis in the ovarian follicle cells during the late stages of Drosophila oogenesis. Cell Tissue Res. 2002;307:401–409. doi: 10.1007/s00441-001-0498-3. [DOI] [PubMed] [Google Scholar]
- 39.Nezis IP, et al. Autophagy is required for the degeneration of the ovarian follicular epithelium in higher Diptera. Autophagy. 2006;2:297–298. doi: 10.4161/auto.2858. [DOI] [PubMed] [Google Scholar]
- 40.Nezis IP, et al. Stage-specific apoptotic patterns during Drosophila oogenesis. Eur J Cell Biol. 2000;79:610–620. doi: 10.1078/0171-9335-00088. [DOI] [PubMed] [Google Scholar]
- 41.Laundrie B, et al. Germline cell death in inhibited by P element insertions in the dcp-1/pita nested gene pair in Drosophila . Genetics. 2003;165:1881–1888. doi: 10.1093/genetics/165.4.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peterson JS, et al. Stage-specific regulation of caspase activity in Drosophila oogenesis. Dev Biol. 2003;260:113–123. doi: 10.1016/s0012-1606(03)00240-9. [DOI] [PubMed] [Google Scholar]
- 43.Baum JS, et al. The Drosophila caspases Strica and Dronc function redundantly in programmed cell death during oogenesis. Cell Death Differ. 2007;14:1508–1517. doi: 10.1038/sj.cdd.4402155. [DOI] [PubMed] [Google Scholar]
- 44.Peterson JS, et al. Noncanonical cell death pathways act during Drosophila oogenesis. Genesis. 2007;45:396–404. doi: 10.1002/dvg.20306. [DOI] [PubMed] [Google Scholar]
- 45.LaFever L, Drummond-Barbosa D. Direct control of germline stem cell division and cyst growth by neural insulin in Drosophila . Science. 2005;309:1071–1073. doi: 10.1126/science.1111410. [DOI] [PubMed] [Google Scholar]
- 46.Pritchett TL, McCall K. Role of the insulin/Tor signaling network in starvation-induced programmed cell death in Drosophila oogenesis. Cell Death Differ. 2012;19:1069–1079. doi: 10.1038/cdd.2011.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jewell JL, et al. Amino acid signalling upstream of mTOR. NatureReviews. Molecular Cell Biology. 2013;14:133–139. doi: 10.1038/nrm3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Y, et al. Drosophila target of rapamycin kinase functions as a multimer. Genetics. 2006;172:355–362. doi: 10.1534/genetics.105.051979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thomson TC, Johnson J. Inducible somatic oocyte destruction in response to rapamycin requires wild-type regulation of follicle cell epithelial polarity. Cell Death Differ. 2010;17:1717–1727. doi: 10.1038/cdd.2010.49. [DOI] [PubMed] [Google Scholar]
- 50.Krieser RJ, White K. Inside an enigma: do mitochondria contribute to cell death in Drosophila ? Apoptosis. 2009;14:961–968. doi: 10.1007/s10495-009-0362-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thomenius M, et al. Mitochondrial fusion is regulated by Reaper to modulate Drosophila programmed cell death. Cell Death Differ. 2011;18:1640–1650. doi: 10.1038/cdd.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Galluzzi L, et al. Mitochondrial control of cellular life, stress, and death. Circ Res. 2012;111:1198–1207. doi: 10.1161/CIRCRESAHA.112.268946. [DOI] [PubMed] [Google Scholar]
- 53.Rolland SG, Conradt B. New role of the BCL2 family of proteins in the regulation of mitochondrial dynamics. Curr Opin Cell Biol. 2010;22:852–858. doi: 10.1016/j.ceb.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tanner EA, et al. Bcl-2 proteins and autophagy regulate mitochondrial dynamics during programmed cell death in the Drosophila ovary. Development. 2011;138:327–338. doi: 10.1242/dev.057943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lauber K, et al. Clearance of apoptotic cells: getting rid of the corpses. Mol Cell. 2004;14:277–287. doi: 10.1016/s1097-2765(04)00237-0. [DOI] [PubMed] [Google Scholar]
- 56.Mangahas PM, Zhou Z. Clearance of apoptotic cells in Caenorhabditis elegans . Semin Cell Dev Biol. 2005;16:295–306. doi: 10.1016/j.semcdb.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 57.Fullard JF, et al. Clearance of apoptotic corpses. Apoptosis. 2009;14:1029–1037. doi: 10.1007/s10495-009-0335-9. [DOI] [PubMed] [Google Scholar]
- 58.Gutzeit HO. The microfilament pattern in the somatic follicle cells of mid-vitellogenic ovarian follicles of Drosophila . Eur J Cell Biol. 1990;53:349–356. [PubMed] [Google Scholar]
- 59.Cooley L, et al. chickadee encodes a profilin required for intercellular cytoplasm transport during Drosophila oogenesis. Cell. 1992;69:173–184. doi: 10.1016/0092-8674(92)90128-y. [DOI] [PubMed] [Google Scholar]
- 60.Guild GM, et al. Actin filament cables in Drosophila nurse cells are composed of modules that slide passively past one naother during dumping. J Cell Biol. 1997;138:783–797. doi: 10.1083/jcb.138.4.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Foley K, Cooley L. Apoptosis in late stage Drosophila nurse cells does not require genes within the H99 deficiency. Development. 1998;125:1075–1082. doi: 10.1242/dev.125.6.1075. [DOI] [PubMed] [Google Scholar]
- 62.Hudson AM, Cooley L. Understanding the function of actin-binding proteins through genetic analysis of Drosophila oogenesis. Annu Rev Genet. 2002;36:455–488. doi: 10.1146/annurev.genet.36.052802.114101. [DOI] [PubMed] [Google Scholar]
- 63.McCall K, Steller H. Requirement for DCP-1 caspase during Drosophila oogenesis. Science. 1998;279:230–234. doi: 10.1126/science.279.5348.230. [DOI] [PubMed] [Google Scholar]
- 64.Velentzas AD, et al. Apoptosis and autophagy function cooperatively for the efficacious execution of programmed nurse cell death during Drosophila virilis oogenesis. Autophagy. 2007;3:130–132. doi: 10.4161/auto.3582. [DOI] [PubMed] [Google Scholar]
- 65.Nezis IP, et al. Autophagic degradation of dBruce controls DNA fragmentation in nurse cells during late Drosophila melanogaster oogenesis. J Cell Biol. 2010;190:523–531. doi: 10.1083/jcb.201002035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cummings MR, King RC. Ultrastructural changes in nurse and follicle cells during late stages of oogenesis in Drosophila melanogaster . Z Zellforsch Mikrosk Anat. 1970;110:1–8. doi: 10.1007/BF00343981. [DOI] [PubMed] [Google Scholar]
- 67.Bass BP, et al. Cell-autonomous requirement for DNaseII in nonapoptotic cell death. Cell Death Differ. 2009;16:1362–1371. doi: 10.1038/cdd.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Timmons AK, Meehan TL, Gartmond TD, McCall K. Use of Necrosis Markers in the Drosophila Ovary. In. In: McCall K, Klein C, editors. Necrosis Methods and Protocols. Vol. 1004. Humana Press; 2013. pp. 215–228. [Google Scholar]
- 69.Barth JM, et al. The lack of autophagy triggers precocious activation of Notch signaling during Drosophila oogenesis. BMC Dev Biol. 2012;12:35. doi: 10.1186/1471-213X-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Peterson JS, McCall K. Combined inhibition of autophagy and caspases fails to prevent nurse cell death in the Drosophila melanogaster ovary, PLOS ONE. 2013 doi: 10.1371/journal.pone.0076046. under revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Klionsky DJ, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4:151–175. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Matova N, et al. Drosophila Quail, a villin-related protein, bundles actin filaments in apoptotic nurse cells. Development. 1999;126:5645–5657. doi: 10.1242/dev.126.24.5645. [DOI] [PubMed] [Google Scholar]
- 73.Golstein P, Kroemer G. Cell death by necrosis: towards a molecular definition. Trends Biochem Sci. 2007;32:37–43. doi: 10.1016/j.tibs.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 74.Blum ES, et al. PolyQ disease: misfiring of a developmental cell death program? Trends Cell Biol. 2013;23:168–174. doi: 10.1016/j.tcb.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tsujimoto Y. Multiple ways to die: non-apoptotic forms of cell death. Acta Oncol. 2012;51:293–300. doi: 10.3109/0284186X.2011.648340. [DOI] [PubMed] [Google Scholar]
- 76.Green DR, Victor B. The pantheon of the fallen: why are there so many forms of cell death? Trends Cell Biol. 2012;22:555–556. doi: 10.1016/j.tcb.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Coffman CR. Cell migration and programmed cell death of Drosophila germ cells. Ann N Y Acad Sci. 2003;995:117–126. doi: 10.1111/j.1749-6632.2003.tb03215.x. [DOI] [PubMed] [Google Scholar]
- 78.Sano H, et al. Control of lateral migration and germ cell elimination by the Drosophila melanogaster lipid phosphate phosphatases Wunen and Wunen 2. J Cell Biol. 2005;171:675–683. doi: 10.1083/jcb.200506038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yamada Y, et al. Programmed cell death of primordial germ cells in Drosophila is regulated by p53 and the Outsiders monocarboxylate transporter. Development. 2008;135:207–216. doi: 10.1242/dev.010389. [DOI] [PubMed] [Google Scholar]
- 80.Brown GC, Neher JJ. Eaten alive! Cell death by primary phagocytosis: 'phagoptosis'. Trends Biochem Sci. 2012;37:325–332. doi: 10.1016/j.tibs.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 81.Pepling ME. Follicular assembly: mechanisms of action. Reproduction. 2012;143:139–149. doi: 10.1530/REP-11-0299. [DOI] [PubMed] [Google Scholar]
- 82.Xu J, Gridley T. Notch2 is required in somatic cells for breakdown of ovarian germ-cell nests and formation of primordial follicles. BMC Biol. 2013;11:13. doi: 10.1186/1741-7007-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Craig J, et al. Gonadotropin and intra-ovarian signals regulating follicle development and atresia: the delicate balance between life and death. Front Biosci. 2007;12:3628–3639. doi: 10.2741/2339. [DOI] [PubMed] [Google Scholar]
- 84.Matsuda F, et al. Follicular growth and atresia in mammalian ovaries: regulation by survival and death of granulosa cells. Journal Reprod Dev. 2012;58:44–50. doi: 10.1262/jrd.2011-012. [DOI] [PubMed] [Google Scholar]
- 85.Inoue S, et al. Elimination of atretic follicles from the mouse ovary: a TEM and immunohistochemical study in mice. J Anat. 2000;196(Pt 1):103–110. doi: 10.1046/j.1469-7580.2000.19610103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Choi JY, et al. The role of autophagy in follicular development and atresia in rat granulosa cells. Fertil Steril. 2010;93:2532–2537. doi: 10.1016/j.fertnstert.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 87.McLaughlin M, et al. mTOR kinase inhibition results in oocyte loss characterized by empty follicles in human ovarian cortical strips cultured in vitro. Fertil Steril. 2011;96:1154–1159. e1151. doi: 10.1016/j.fertnstert.2011.08.040. [DOI] [PubMed] [Google Scholar]
- 88.Thomson TC, et al. Intrinsic and extrinsic mechanisms of oocyte loss. Mol Hum Reprod. 2010;16:916–927. doi: 10.1093/molehr/gaq066. [DOI] [PubMed] [Google Scholar]
- 89.Yacobi-Sharon K, et al. Alternative Germ Cell Death Pathway in Drosophila Involves HtrA2/Omi, Lysosomes, and a Caspase-9 Counterpart. Dev Cell. 2013;25:29–42. doi: 10.1016/j.devcel.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 90.Srinivasula SM, et al. Inhibitor of apoptosis proteins are substrates for the mitochondrial serine protease Omi/HtrA2. J Biol Chem. 2003;278:31469–31472. doi: 10.1074/jbc.C300240200. [DOI] [PubMed] [Google Scholar]