Abstract
The mechanisms underlying exercise-induced increases in adipose tissue blood flow and lipolysis involve both β-adrenergic receptor (βAR)- and natriuretic peptide receptor (NPR)-dependent mechanisms. We hypothesied that daily wheel running (RUN) would increase the expression of NPR1, NPR2, βAR2, and βAR3 in retroperitoneal (RP) and epididymal (EPI) adipose tissues of obese Otsuka Long Evans Tokushima Fatty (OLETF) rats. Four-week old OLETF rats were assigned to sedentary (SED, n = 6), caloric restriction (CR, n = 8; fed 70% of SED), or RUN (n = 8) groups. Rats were sacrificed at 40 weeks of age. By design, body weight and adiposity were similar between RUN and CR animals, but each were lower than SED (P < 0.01). Compared to SED, RP depots of RUN rats exhibited 1.7-3.2-fold greater NPR1, NPR2, βAR2, and βAR3 mRNA levels (all P < 0.05). There were no differences between CR and SED in the expression of these genes in RP, and there were no differences in gene expression among groups in EPI. At the protein level, βAR2 and βAR3 were elevated in RUN and CR relative to SED in RP. To gain insights into mechanisms underlying the activity-induced increases in NPR and βAR mRNAs, RP explants from Wistar rats were treated with atrial natriuretic peptide (ANP), epinephrine, and/or S-Nitroso-N-acetyl-DL-penicillamine [SNAP; a nitric oxide (NO) donor] in organ culture experiments. SNAP synergistically enhanced epinephrine- and ANP-stimulated increases in NPR2 and βAR2 mRNA levels. Our data suggest that physical activity-induced increases in NO interact with epinephrine and ANP to trigger the induction of NPR and βAR mRNAs in the RP depot of the OLETF rat.
Keywords: obesity, lipolysis, blood flow, sympathetic nervous system
Regular physical activity favorably influences adipose tissue physiology (Thompson et al., 2012). Several physical activity-induced benefits, including increased fat oxidation, enhanced mitochondrial biogenesis, and reduced inflammation are thought to be at least partly due to reductions in adipocyte size resulting from an energy deficit. This idea stems from evidence that an energy deficit achieved through other means, such as caloric restriction or pharmacologic therapies, can also influence adipose tissue insulin sensitivity, metabolic flexibility, and inflammation (Reynisdottir et al., 1995; Stich et al., 2002; Lofgren et al., 2005; Jenkins et al., 2012). However, there are a number of exercise-specific, transient changes that occur as a result of acute dynamic activity that could have long term phenotypic implications for adipose tissue physiology independent of changes in adipose tissue mass.
Two exercise-specific signals involved in the modulation of adipose tissue phenotype are the natriuretic peptides such as atrial natriuretic peptide (ANP) and catecholamines (such as epinephrine) (Moro et al., 2004; Sutherland et al., 2009). Plasma concentrations of ANP and epinephrine are markedly increased during exercise (Tanaka et al., 1986; McMurray et al., 1987; Thamsborg et al., 1987; Thompson et al., 2012). These increased ANP and epinephrine concentrations signal increases in lipolysis (Arner, 1995; Moro et al., 2004) and stimulate the expression of metabolic genes (Moro et al., 2007; Sutherland et al., 2009; Wan et al., 2012). Although it is established that regular exercise increases the sensitivity of these pathways in overweight/obese humans (Stich et al., 1999; Moro et al., 2005), the mechanisms underlying this adaptation are largely unknown.
Importantly, ANP and epinephrine also are involved in exercise-induced increases in adipose tissue blood flow (Moritoki et al., 1992; Madhani et al., 2003). It has been postulated that epinephrine-mediated beneficial metabolic effects of exercise (e.g. increased lipolysis) are consequences of increases in blood flow (Galitzky et al., 1993; Thompson et al., 2012). In addition, it seems plausible that increased nitric oxide (NO) production resulting from the increased blood flow could exert beneficial effects not only on the vascular cells but potentially also on the underlying cells within the adipose tissue. Consistent with this idea, NO has been shown to have important regulatory effects on adipose tissue phenotype. For example, overexpression of endothelial NO synthase (eNOS) has been shown to prevent high-fat diet-induced obesity in mice in part through enhancing the metabolic activity of adipose tissue (Sansbury et al., 2012), and deletion of eNOS reduces the adipose tissue mitochondrial content (Nisoli et al., 2005). There also is evidence that NO, ANP, and epinephrine pathways converge on similar downstream intracellular signals that produce favorable changes in gene expression in a variety of cell types (Kiemer & Vollmar, 1998; Kone, 2001; Madhani et al., 2003; Pacher et al., 2007; Figueroa et al., 2009; Bordicchia et al., 2012).
Therefore, we tested the hypothesis that regular physical activity (wheel running) increases the expression of natriuretic peptide receptors (NPR1 and NPR2) and β-adrenergic receptors (βAR2 and βAR3) in retroperitoneal (RP) and epididymal (EPI) visceral adipose tissue depots of hyperphagic, obese Otsuka Long Evans Tokushima Fatty (OLETF) rats, an established rodent model of type 2 diabetes mellitus (Kawano et al., 1992). Given our initial result of enhanced expression of these mRNAs in the RP of physically active OLETF rats, we also tested the hypothesis that ANP, epinephrine, and NO can synergistically augment the expression of NPR and βAR mRNAs in RP adipose tissues using an ex vivo adipose tissue organ culture approach.
METHODS
Animals and Experimental Design
The animals used for the present study and the overall experimental design have been described in detail previously (Mikus et al., 2010). Briefly, male OLETF rats (Tokushima Research Institute, Otsuka Pharmaceutical; Tokushima, Japan) were obtained at 4 wk of age and were individually housed in cages maintained in temperature-controlled (21°C) animal quarters with 0600–1800 light and 1800–0600 dark cycles. At 4 wk of age, rats were randomized to one of three groups: 1) sedentary (SED; n = 6); 2) sedentary + caloric restriction (CR, fed ~70% of ad libitum-fed SED animals; n = 8); or 3) voluntary wheel running (RUN; n = 8). Animals in the RUN group were housed with running wheels connected to a Sigma Sport BC 800 bicycle computer (Cherry Creek Cyclery, Foster Falls, VA) for determination of daily running distance. All groups were provided with standard chow (Formulab 5008, Purina Mills, St Louis, MO) with approximately 26% protein, 18% fat, and 56% carbohydrate. The SED and RUN groups had ad libitum access to food, while the food provided to the CR group was adjusted weekly to ensure the mean body weights of the CR and RUN groups were closely matched. The wheels of the RUN group were locked and food was removed from the cages of all groups at 5 h before sacrifice. At 40 wk of age, rats were anesthetized with intraperitoneal administration of pentobarbital sodium (100 mg/kg). Tissues were harvested, and the animals were killed by exsanguination.
Adipose tissue organ culture
Adipose tissue organ culture experiments were carried out as described previously (Sutherland et al., 2009), with minor modifications. Briefly, RP fat pads were removed from male Wistar rats (~300 g, n = 6), weighed, and immediately placed in 50 ml conical tubes containing sterile ice cold PBS with 1% penicillin/streptomycin. Under sterile conditions, ~300 mg of tissue was and placed into each well of 24-well culture dishes containing 500 μl of M199 supplemented with 1% penicillin/streptomycin, 50 μIU/ml insulin and 2.5 nM dexamethasone. The explants were incubated in a 37°C, 5% CO2 atmosphere for a 24 h equilibration period. A 300 mg sample from each individual rat was treated with or without epinephrine, ANP, S-Nitroso-N-acetyl-DL-penicillamine (SNAP; a nitric oxide donor), and all possible treatment combinations for 6 hr, forming 8 treatment conditions: 1) vehicle control, 2) 10 μM epinephrine, 3) 100 nM ANP, 4) 100 μM SNAP, 5) epinephrine + ANP, 6) epinephrine + SNAP, 7) ANP + SNAP, 8) epinephrine + ANP + SNAP. After 6 h, the tissues were rapidly frozen and stored at -80°C until further analysis.
Quantitative Real-Time PCR
Total RNA was extracted from ~100 mg adipose tissue samples using RNeasy lipid tissue kits (Qiagen) with on-column DNase digestion. Purity and concentration were determined using a Nanodrop 1000 spectrometer (Thermo Scientific). 500 ng (from OLETF rats) and 100 ng (from adipose organ culture experiments) of total RNA was used to synthesize cDNA using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems) according to the manufacturer’s instructions. qRT-PCR was carried out using SYBR green assays on a Bio-Rad CFX-connect RT-PCR system. 18S was used as the reference gene and relative gene expression was calculated using the 2-ΔΔCT method. Gene-specific primer sequences are provided in Table 1.
Table 1.
Primer Sequences for qRT-PCR
| Gene Name | Forward Primer | Reverse Primer |
|---|---|---|
| βAR2 | TGGTGCGAGTTCTGGACTTC | TAAGGCCCGACACAATCCAC |
| βAR3 | GGAACCGCAACTCTCCAGAA | CGGCTGAGGTAGTAGCGAAG |
| NPR1 | TGCTCTATGCAGATCGGCTG | GCAGTAGCTTGGGGTAGTGG |
| NPR2 | ACGCATAGGCGTCCATACTG | ATCCTTGGTGGTCGAGGAGA |
| 18S | GCCGCTAGAGGTGAAATTCTTG | CATTCTTGGCAAATGCTTTCG |
Immunoblotting
Immunoblot analysis was performed on RP samples to confirm observations at the mRNA level. Briefly, RP samples were homogenized in lysis buffer (50 mM Tris-HCl, 0.1 mM EDTA, 0.1 mM EGTA, 0.1% mercaptoethanol, 1 mM PMSF, 2 μM leupeptin, 1 μM pepstatin, and 20 mM CHAPS, pH 7.4) using a tissue homogenizer (TissueLyser LT, Qiagen, Valencia, CA) and total protein content from RP samples was measured using the Bradford method. Samples were then diluted with Laemmli buffer and 5 μg of protein from each sample was loaded onto polyacrylamide gels for separation by electrophoresis. Proteins were transferred to PVDF membranes and probed with rabbit polyclonal antibodies NPR1 (1:125, Abcam), βAR2 (1:500), and βAR3 (1:100). Blots were re-probed for GAPDH (1:2500, Cell Signaling). Band intensities were quantified using ImageJ software, and values of target proteins were normalized to the intensity for GAPDH. Commercial antibodies for NPR2 were unsuccessful in Western blot analyses.
Statistics
Differences among SED, CR and RUN groups were analyzed using a one-way ANOVA and Fisher’s LSD post hoc tests. Data from the adipose tissue organ culture experiments were analyzed using a 3 factor ANOVA (epinephrine × ANP × SNAP), with each factor having two levels (treated or not). Post hoc analysis was performed using Dunnet’s test to determine differences between individual treatment conditions and the control condition (i.e., no treatments). Statistical significance was accepted at P ≤ 0.05, and a P value between 0.05 and 0.10 was regarded as a trend.
RESULTS
Body weight, body composition, and daily running distance
By design, body weight, percent body fat and fat pad masses did not differ between RUN and CR animals, but each were significantly lower compared with SED (P < 0.01, Fig 1A-D). As reported previously (Mikus et al., 2010), rats with access to running wheels increased daily running distance between weeks 4 (3.9 ± 0.2 km/day) and 10 (10.9 ± 4 km/day), after which running distance gradually declined to 3.6 ± 0.2 km/day at 40 wk.
Figure 1.
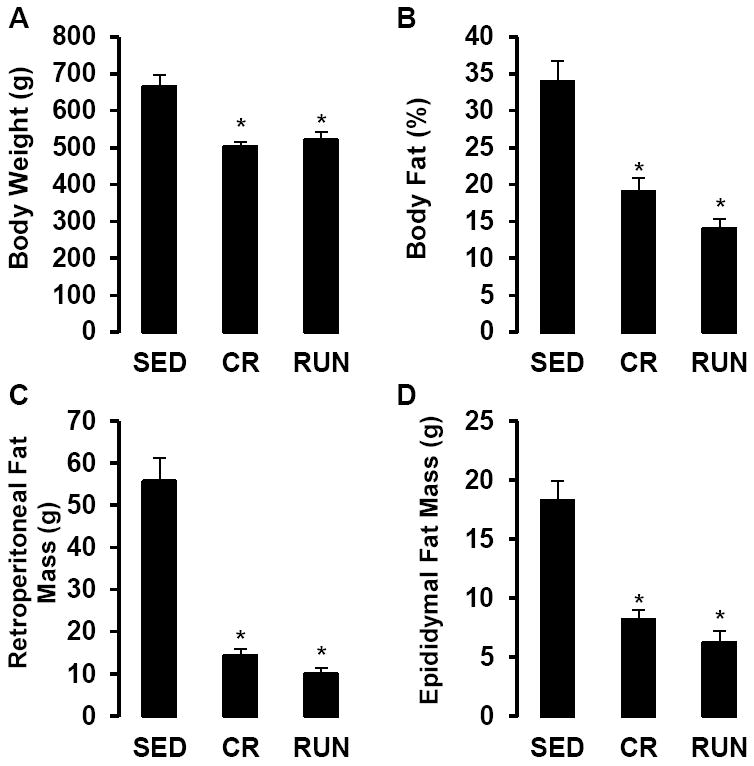
Body weight (A), % body fat (B), retroperitoneal fat mass (C) and epididymal fat mass (D), in sedentary (SED), caloric restriction (CR), and voluntary wheel running (RUN) OLETF rats. *P < 0.05 vs. SED.
Influence of daily activity on adipose tissue NPR and βAR mRNA and protein levels
Compared to SED, RP depots of RUN rats exhibited ~2-3 fold greater mRNA levels of NPR1, NPR2, βAR2, and βAR3 (all P < 0.05, Fig 2A-D). There were no statistically significant differences between CR and SED in the expression of these genes in RP. Adrenergic receptor mRNA levels tended to be increased in EPI of active OLETF but these differences did not attain statistical significance (Fig 2). For example, although EPI from OLETF RUN rats had ~2.5-fold greater βAR3 mRNA levels than SED this difference was not statistically significant (P = 0.08, Fig 2D). There were no statistically significant differences among groups in NPR1, NPR2, or βAR2 mRNA levels within EPI. It was confirmed that 18S expression was stably expressed across tissues and between groups in the present study, and that reaction efficiencies were similar for 18S and the genes of interest.
Figure 2.
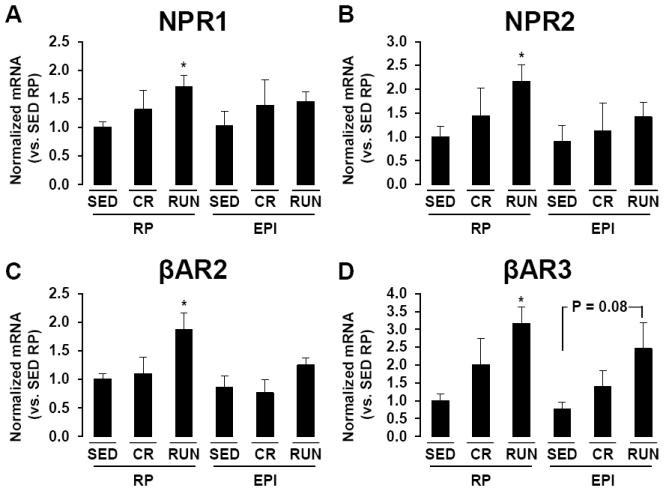
NPR1 (A), NPR2 (B), βAR2 (C), and βAR3 (D) mRNA levels in retroperitonal (RP) and epididymal (EPI) adipose tissue depots of sedentary (SED), caloric restriction (CR), and voluntary wheel running (RUN) OLETF rats. *P < 0.05 vs. SED.
At the protein level, RP depots of CR and RUN animals exhibited ~3.5 and ~4.5-fold greater βAR2 expression compared to SED, respectively (P < 0.05, Fig 3A). Similarly, βAR3 protein expression was ~1.7-fold greater in RP of both CR and RUN compared to SED (P < 0.05, Fig 3B). Although CR rats had ~2-fold greater expression of NPR1 than SED animals in RP, the difference between groups was not statistically significant (P = 0.24, Fig 3C).
Figure 3.
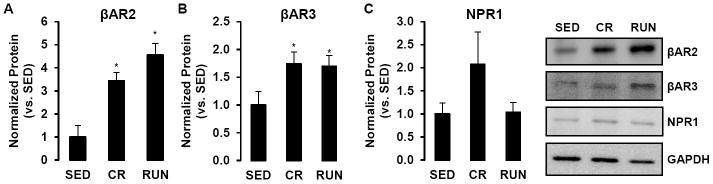
βAR2 (A), βAR3 (B), and NPR1 (C) protein levels in retroperitonal (RP) adipose tissue depots of sedentary (SED), caloric restriction (CR), and voluntary wheel running (RUN) OLETF rats. *P < 0.05 vs. SED.
Effects of epinephrine, ANP, and NO on NPR and βAR gene expression in retroperitoneal adipose tissue organ culture
To gain insights into possible mechanisms underlying the activity-induced increases in NPR and βAR mRNAs, RP explants from Wistar rats were treated for 6 hours with atrial natriuretic peptide (ANP), epinephrine, and/or a nitric oxide (NO) donor (SNAP) in adipose tissue organ culture experiments. There were no statistically significant effects of any treatment on NPR1 gene expression (Fig 4A). Epinephrine alone (~30%, P < 0.05) and in the presence of SNAP (~80%, P < 0.05) increased the expression of NPR2 (Fig 4B). Additionally, ANP tended to increase NPR2 by 37% alone (P = 0.06) and by ~2-fold in the presence of SNAP (P = 0.10; Fig 4B). For the βAR genes, epinephrine alone (~30%, P = 0.08) and in the presence of SNAP (P = 0.10) tended to increase the expression of βAR2 (Fig 4C). Analysis of main effects revealed a significant effect of ANP on βAR2 mRNA levels (P < 0.05, Fig 4C). Post hoc tests indicated that ANP in the presence of SNAP significantly increased βAR2 by ~2-fold (P < 0.05, Fig 4C). Further, the combination of epinephrine, ANP, and SNAP induced a similar ~2-fold increase in βAR2 gene expression (P = 0.10). Finally, there were no statistically significant effects of any treatment on the expression of βAR3 (Fig 4D).
Figure 4.
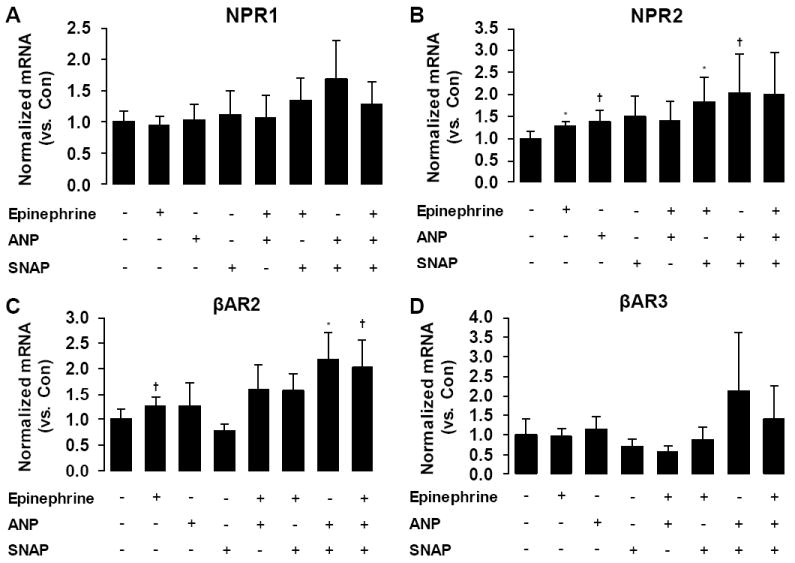
Effects of epinephrine, atrial natriuretic peptide (ANP), S-Nitroso-N-acetyl-DL-penicillamine (SNAP), and their combinations on NPR1 (A), NPR2 (B), βAR2 (C), and βAR3 (D) mRNA levels in retroperitoneal adipose tissue organ cultures. + denotes presence of treatment; - denotes absence of treatment. *P < 0.05 vs control. †P < 0.10 vs. control.
DISCUSSION
The major findings of this study are that (i) regular physical activity increases expression of NPR and βAR mRNAs in RP adipose tissue of OLETF rats, (ii) both CR and physical activity increase the level of βAR protein expression, and (iii) neither treatment significantly alters NPR1 protein expression. Thus, it appears that while CR and RUN produce differential effects on βAR and NPR genes at the mRNA level, these effects are not uniformly associated with changes at the protein level. In addition, our ex vivo experiments demonstrate that epinephrine and ANP can increase the mRNA expression of their receptors, especially in the presence of NO. Together, these data support the concept that exercise-induced increases in NO may synergize with circulating epinephrine and ANP to signal the induction of NPR and βAR mRNAs.
Our finding that physical activity increased NPR and βAR mRNA levels in RP (Fig 2) may partly explain the mechanism underlying previous observations that training enhances sensitivity of adipose tissue to the lipolytic actions of natriuretic peptides and catecholamines (Thompson et al., 2012). CR-induced reduction in adiposity did not produce the same effects, indicating that enhanced expression of these NPR and βAR mRNAs was the result of some chronic exercise specific signal. Nevertheless, CR was sufficient to enhance βAR2 and βAR3 protein content to a similar extent as RUN, suggesting that at the protein level, wheel-running induced enhancement in βAR expression may indeed be adiposity/body weight dependent. Additionally, the lack of differences among groups in NPR1 protein expression also suggests differential effects of RUN and CR on mRNA and protein levels. We speculate that the enhanced NPR1 mRNA in RUN with lack of differences among groups in NPR1 protein suggests a potential mechanism by which regular exercise serves to maintain NPR1 protein expression at a constant homeostatic level. Additional studies are required to further investigate the complex and multifactorial regulation of βAR and NPR gene expression in adipose tissue.
Interestingly, the enhanced expression of NPR and βAR mRNAs was observed in the RP but not the EPI, indicating that the effects of regular physical activity, at least on these genes, are not uniform among all visceral adipose tissue depots in the OLETF rat. This finding was contrary to our expectation that EPI and RP would exhibit similar adaptations given previous evidence that acute exercise and epinephrine increase mitochondria-related gene expression in both EPI and RP of Wistar rats (Sutherland et al., 2009). An important difference between our study and the previous study by Sutherland et al. is that they used a swim training program, whereas in the present study we used a wheel running exercise protocol. The differential influence of swimming and wheel running on plasma catecholamines may have contributed to our lack of robust increases in NPR and βAR genes in EPI. Furthermore, our group has previously shown that that voluntary wheel running increases mitochondrial content as well as cytochrome C and COXIV-subunit I protein expression in the omental fat depot of OLETF rats (Laye et al., 2009). Thus we would have expected all visceral depots (including RP and EPI in the present study) to have displayed similar adaptations in upstream mediators such as NPR and βAR genes. One possible explanation for our observed differential effects between RP and EPI could be related to the difference in fat mass between the depots. The physical activity-induced reduction in fat pad mass was similar between depots in relative terms, but in absolute terms the reduction was much greater in the RP (~46 g difference between SED and RUN; Fig 1C) than in the EPI (~12 g differences between SED and RUN; Fig 1D). It seems reasonable to speculate that between-depot differences in preferential fat mobilization during exercise may account for the differential effects of regular physical activity on NPR and βAR gene expression.
With the large body of evidence that exercise acutely increases ANP and epinephrine concentrations, we were intrigued by our observation that mRNA levels of these receptors in the RP would be increased as a result of regular physical activity. Based on the usual physiological response for these receptors to down-regulate upon increased exposure to their agonists (Hertel & Perkins, 1984; Kone, 2001) we would not expect the increased ANP and epinephrine concentrations alone to be fully responsible for the increased gene expression. Given the evidence that (i) exercise increases adipose tissue blood flow (Thompson et al., 2012) and therefore presumably shear stress in adipose tissue vascular beds, and (ii) the genomic regulation of NPR transcription involves NO-sensitive signaling pathways [e.g., there is a cGMP response element in the promoter region of the NPR1 gene (Hum et al., 2004)], we postulated that NO would augment ANP- and epinephrine-induced increases in NPR and βAR genes in organ culture experiments. Indeed, our adipose tissue organ culture data indicating that ANP increased NPR2 by 37% alone and by ~200% in the presence of SNAP (Fig 4B) support the hypothesis that these signals can synergistically increase NPR2 mRNA levels. Similarly, epinephrine in the presence of SNAP produced a synergistic increase in βAR2 mRNA (80% increase) compared to epinephrine alone (~30% increase, Fig 4C). Together these data suggest that NO in combination with NPR2 and βAR2 ligands can increase the mRNA levels of these receptors.
In an unexpected and interesting finding, ANP increased βAR2 mRNA levels and epinephrine increased expression of NPR2 mRNA in adipose tissue organ cultures, and these increases were enhanced in the presence of the NO donor, SNAP (Figs 3B and 3C). These data provide novel evidence of the potential cross-talk between the NPR and βAR signaling pathways in adipose tissue. Moreover, in the context of adaptations to regular physical activity, we speculate that localized exercise-induced increases in NO might participate in this coordinated up-regulation of NPR and βAR genes.
Some limitations of our study should be noted. The observations of the present study occurred 5 h after the last exercise bout, and it is therefore possible that the induction of the NPR and βAR genes could reflect an acute exercise effect. Furthermore, the influence of physical activity and caloric restriction on NPR and βAR genes in other major adipose tissue depots should be the focus of future studies. Additionally, although our adipose tissue organ culture experiment showed that the interactive effects of epinephrine, ANP, and NO are sufficient to induce gene expression of NPR2 and βAR2, we did not demonstrate that these signals are required for the physical activity effects. Future studies using in vivo experimental modifications of these pathways are warranted. Although our hypothesis that ANP and epinephrine would increase expression of their receptors was supported for NPR2 and βAR2, we found no effects of any treatments on NPR1 and βAR3 (Fig 4A and 4D). Thus, it seems that longer or repetitive stimulation and/or other signals are likely responsible for the selective physical activity inducedincreases in levels of NPR1 and βAR3 mRNA. Future research should investigate the genomic regulation of these important mediators of adipose tissue lipolysis, as our observation of differences between changes in mRNA and protein level expression of βAR and NPR genes in response to CR and RUN warrants further investigation. We did not measure NO levels in plasma or adipose tissue samples in the present study but previous work on this model in our laboratory reported that physical activity maintains normal plasma nitrite/nitrate levels (a marker of systemic NO bioavailability) in OLETF rats (Bunker et al., 2010).
In summary, the present study provides evidence of physical activity-induced increases in NPR and βAR mRNA levels in the RP, but not the EPI, of the OLETF rat. These effects on mRNA levels are independent of changes in adiposity given the lack of differences between CR and SED rats in NPR and βAR gene expression. Interestingly, it appears that both RUN and CR are sufficient to enhance protein expression of βAR2 and βAR3 in the RP of the OLETF rat, albeit through apparently different mechanisms. Finally, results from our ex vivo adipose tissue organ culture experiments support the hypothesis that increases in NO may synergistically interact with epinephrine and ANP signaling in control of expression of βAR2 and NPR2 mRNAs.
NEW FINDINGS.
-
What is the central question of this study?
The central question is: what are the effects of caloric restriction and chronic exercise on visceral adipose tissue of beta-adrenergic and natriuretic peptide receptor genes in obese rats?
-
What is the main finding and what is its importance?
physical activity-induced increases in NPR and βAR mRNA levels in retroperitoneal fat obese rats.
both chronic exercise and caloric restriction enhance protein expression of βAR2 and βAR3 in the RP of the OLETF rat, albeit through apparently different mechanisms.
Organ culture experiments support the hypothesis that increases in nitric oxide synergistically interact with epinephrine and ANP signaling in control of expression of βAR2 and NPR2 mRNAs.
Acknowledgments
We thank Nick Fleming, Claire Manse, Pam Thorne, and Grace Meers for excellent technical assistance. Funding was provided by NIH RO1HL036088 (M.H.L.), VA-CDA-IK2 BX001299-01 (R.S.R.), NIH T32-AR048523 (N.T.J.), and AHA 11POST5080002 (J.P.). This work was supported in part with resources and the use of facilities at the Harry S. Truman Memorial Veterans Hospital in Columbia, MO.
References
- Arner P. Impact of exercise on adipose tissue metabolism in humans. Int J Obes Relat Metab Disord. 1995;19(Suppl 4):S18–21. [PubMed] [Google Scholar]
- Bordicchia M, Liu D, Amri EZ, Ailhaud G, Dessi-Fulgheri P, Zhang C, Takahashi N, Sarzani R, Collins S. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest. 2012;122:1022–1036. doi: 10.1172/JCI59701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunker AK, Arce-Esquivel AA, Rector RS, Booth FW, Ibdah JA, Laughlin MH. Physical activity maintains aortic endothelium-dependent relaxation in the obese type 2 diabetic OLETF rat. Am J Physiol Heart Circ Physiol. 2010;298:H1889–1901. doi: 10.1152/ajpheart.01252.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa XF, Poblete I, Fernandez R, Pedemonte C, Cortes V, Huidobro-Toro JP. NO production and eNOS phosphorylation induced by epinephrine through the activation of beta-adrenoceptors. Am J Physiol Heart Circ Physiol. 2009;297:H134–143. doi: 10.1152/ajpheart.00023.2009. [DOI] [PubMed] [Google Scholar]
- Galitzky J, Lafontan M, Nordenstrom J, Arner P. Role of vascular alpha-2 adrenoceptors in regulating lipid mobilization from human adipose tissue. J Clin Invest. 1993;91:1997–2003. doi: 10.1172/JCI116421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertel C, Perkins JP. Receptor-specific mechanisms of desensitization of beta-adrenergic receptor function. Mol Cell Endocrinol. 1984;37:245–256. doi: 10.1016/0303-7207(84)90094-7. [DOI] [PubMed] [Google Scholar]
- Hum D, Besnard S, Sanchez R, Devost D, Gossard F, Hamet P, Tremblay J. Characterization of a cGMP-response element in the guanylyl cyclase/natriuretic peptide receptor A gene promoter. Hypertension. 2004;43:1270–1278. doi: 10.1161/01.HYP.0000126920.93207.53. [DOI] [PubMed] [Google Scholar]
- Jenkins NT, Padilla J, Arce-Esquivel AA, Bayless DS, Martin JS, Leidy HJ, Booth FW, Rector RS, Laughlin MH. Effects of endurance exercise training, metformin, and their combination on adipose tissue leptin and IL-10 secretion in OLETF rats. J Appl Physiol. 2012;113:1873–1883. doi: 10.1152/japplphysiol.00936.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano K, Hirashima T, Mori S, Saitoh Y, Kurosumi M, Natori T. Spontaneous longterm hyperglycemic rat with diabetic complications. Otsuka Long-Evans Tokushima Fatty (OLETF) strain. Diabetes. 1992;41:1422–1428. doi: 10.2337/diab.41.11.1422. [DOI] [PubMed] [Google Scholar]
- Kiemer AK, Vollmar AM. Autocrine regulation of inducible nitric-oxide synthase in macrophages by atrial natriuretic peptide. J Biol Chem. 1998;273:13444–13451. doi: 10.1074/jbc.273.22.13444. [DOI] [PubMed] [Google Scholar]
- Kone BC. Molecular biology of natriuretic peptides and nitric oxide synthases. Cardiovasc Res. 2001;51:429–441. doi: 10.1016/s0008-6363(01)00327-3. [DOI] [PubMed] [Google Scholar]
- Laye MJ, Rector RS, Warner SO, Naples SP, Perretta AL, Uptergrove GM, Laughlin MH, Thyfault JP, Booth FW, Ibdah JA. Changes in visceral adipose tissue mitochondrial content with type 2 diabetes and daily voluntary wheel running in OLETF rats. J Physiol. 2009;587:3729–3739. doi: 10.1113/jphysiol.2009.172601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofgren P, Hoffstedt J, Naslund E, Wiren M, Arner P. Prospective and controlled studies of the actions of insulin and catecholamine in fat cells of obese women following weight reduction. Diabetologia. 2005;48:2334–2342. doi: 10.1007/s00125-005-1961-6. [DOI] [PubMed] [Google Scholar]
- Madhani M, Scotland RS, MacAllister RJ, Hobbs AJ. Vascular natriuretic peptide receptor-linked particulate guanylate cyclases are modulated by nitric oxide-cyclic GMP signalling. Br J Pharmacol. 2003;139:1289–1296. doi: 10.1038/sj.bjp.0705365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurray RG, Forsythe WA, Mar MH, Hardy CJ. Exercise intensity-related responses of beta-endorphin and catecholamines. Med Sci Sports Exerc. 1987;19:570–574. [PubMed] [Google Scholar]
- Mikus CR, Rector RS, Arce-Esquivel AA, Libla JL, Booth FW, Ibdah JA, Laughlin MH, Thyfault JP. Daily physical activity enhances reactivity to insulin in skeletal muscle arterioles of hyperphagic Otsuka Long-Evans Tokushima Fatty rats. J Appl Physiol. 2010;109:1203–1210. doi: 10.1152/japplphysiol.00064.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritoki H, Yoshikawa T, Hisayama T, Takeuchi S. Possible mechanisms of age-associated reduction of vascular relaxation caused by atrial natriuretic peptide. Eur J Pharmacol. 1992;210:61–68. doi: 10.1016/0014-2999(92)90652-k. [DOI] [PubMed] [Google Scholar]
- Moro C, Crampes F, Sengenes C, De Glisezinski I, Galitzky J, Thalamas C, Lafontan M, Berlan M. Atrial natriuretic peptide contributes to physiological control of lipid mobilization in humans. FASEB J. 2004;18:908–910. doi: 10.1096/fj.03-1086fje. [DOI] [PubMed] [Google Scholar]
- Moro C, Klimcakova E, Lolmede K, Berlan M, Lafontan M, Stich V, Bouloumie A, Galitzky J, Arner P, Langin D. Atrial natriuretic peptide inhibits the production of adipokines and cytokines linked to inflammation and insulin resistance in human subcutaneous adipose tissue. Diabetologia. 2007;50:1038–1047. doi: 10.1007/s00125-007-0614-3. [DOI] [PubMed] [Google Scholar]
- Moro C, Pillard F, De Glisezinski I, Harant I, Riviere D, Stich V, Lafontan M, Crampes F, Berlan M. Training enhances ANP lipid-mobilizing action in adipose tissue of overweight men. Med Sci Sports Exerc. 2005;37:1126–1132. doi: 10.1249/01.mss.0000170124.51659.52. [DOI] [PubMed] [Google Scholar]
- Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, Falcone S, Valerio A, Cantoni O, Clementi E, Moncada S, Carruba MO. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310:314–317. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiological Reviews. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynisdottir S, Langin D, Carlstrom K, Holm C, Rossner S, Arner P. Effects of weight reduction on the regulation of lipolysis in adipocytes of women with upper-body obesity. Clin Sci (Lond) 1995;89:421–429. doi: 10.1042/cs0890421. [DOI] [PubMed] [Google Scholar]
- Sansbury BE, Cummins TD, Tang Y, Hellmann J, Holden CR, Harbeson MA, Chen Y, Patel RP, Spite M, Bhatnagar A, Hill BG. Overexpression of endothelial nitric oxide synthase prevents diet-induced obesity and regulates adipocyte phenotype. Circ Res. 2012;111:1176–1189. doi: 10.1161/CIRCRESAHA.112.266395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stich V, de Glisezinski I, Galitzky J, Hejnova J, Crampes F, Riviere D, Berlan M. Endurance training increases the beta-adrenergic lipolytic response in subcutaneous adipose tissue in obese subjects. Int J Obes Relat Metab Disord. 1999;23:374–381. doi: 10.1038/sj.ijo.0800829. [DOI] [PubMed] [Google Scholar]
- Stich V, Marion-Latard F, Hejnova J, Viguerie N, Lefort C, Suljkovicova H, Langin D, Lafontan M, Berlan M. Hypocaloric diet reduces exercise-induced alpha 2-adrenergic antilipolytic effect and alpha 2-adrenergic receptor mRNA levels in adipose tissue of obese women. J Clin Endocrinol Metab. 2002;87:1274–1281. doi: 10.1210/jcem.87.3.8349. [DOI] [PubMed] [Google Scholar]
- Sutherland LN, Bomhof MR, Capozzi LC, Basaraba SA, Wright DC. Exercise and adrenaline increase PGC-1{alpha} mRNA expression in rat adipose tissue. J Physiol. 2009;587:1607–1617. doi: 10.1113/jphysiol.2008.165464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H, Shindo M, Gutkowska J, Kinoshita A, Urata H, Ikeda M, Arakawa K. Effect of acute exercise on plasma immunoreactive-atrial natriuretic factor. Life Sci. 1986;39:1685–1693. doi: 10.1016/0024-3205(86)90166-9. [DOI] [PubMed] [Google Scholar]
- Thamsborg G, Storm T, Keller N, Sykulski R, Larsen J. Changes in plasma atrial natriuretic peptide during exercise in healthy volunteers. Acta Med Scand. 1987;221:441–444. doi: 10.1111/j.0954-6820.1987.tb01278.x. [DOI] [PubMed] [Google Scholar]
- Thompson D, Karpe F, Lafontan M, Frayn K. Physical activity and exercise in the regulation of human adipose tissue physiology. Physiol Rev. 2012;92:157–191. doi: 10.1152/physrev.00012.2011. [DOI] [PubMed] [Google Scholar]
- Wan Z, Frier BC, Williams DB, Wright DC. Epinephrine induces PDK4 mRNA expression in adipose tissue from obese, insulin resistant rats. Obesity (Silver Spring) 2012;20:453–456. doi: 10.1038/oby.2011.252. [DOI] [PubMed] [Google Scholar]


