Abstract
BACKGROUND
In this study, we asked whether anti-CD3-activated T cells (ATCs) from cord blood (CB) could be expanded and targeted to solid tumors or hematologic malignancies for infusions after unrelated CB stem cell transplant and whether cord blood ATCs (CBATCs) could reduce alloresponsiveness.
STUDY DESIGN AND METHODS
CB mononuclear cells (MNCs) were activated with anti-CD3 (20 ng/mL) and expanded for 14 days in interleukin-2 (100 IU/mL). CBATCs were armed with anti-CD3 × anti-CD20 (CD20Bi) or anti-CD3 × anti-Her2 (Her2Bi) bispecific antibodies (CBaATCs) and tested for specific cytotoxicity, cytokine secretion, and alloresponsiveness.
RESULTS
Our results show the mean expansion of CBATCs to be 37-fold after 14 days of culture from either frozen (n = 4) or fresh (n = 4) CB units. Cytotoxicity was optimal when CBATCs were armed with 50 ng of CD20Bi/106 cells. Cytotoxicity peaked between Day 8 and Day 10 for both bispecific antibodies. At an effector-to-target ratio of 25:1, the mean cytotoxicities of CBATCs armed with Her2Bi or CD20Bi were 40% (n = 4) and 30% (n = 4), respectively. CBaATCs exhibited peak specific interferon-γ enzyme-linked immunosorbent spots on Day 10. CBATCs and CBaATCs suppressed responsiveness to alloantigens by 20% to 50% when compared with normal allogeneic peripheral blood MNC response.
CONCLUSION
We showed that armed CBATCs mediate specific cytotoxicity, secrete low levels of cytokines and chemokines, and demonstrate attenuated response to alloantigens.
Umbilical cord blood transplantation (CBT) has emerged as a viable option for patients with hematologic and nonhematologic malignancies who do not have an HLA-matched sibling or matched unrelated donor.1–4 The advantages of CBT include rapid access to a donor and a greater tolerance for HLA-disparity due to the naivety of the newborn’s immune system. Outcomes with CBT are comparable to transplant with bone marrow or peripheral blood (PB) stem cells with an equal or lesser incidence of acute and chronic graft-versus-host disease (GVHD).2 Unfortunately, CBT has been limited by a greater incidence in transplant-related mortality from opportunistic infections, primarily due to delays in neutrophil engraftment and immune reconstitution.5,6 Thus, strategies to improve engraftment and immune reconstitution, while decreasing relapse rates, may significantly improve outcomes after CBT.
The number of total nucleated and CD34+ cells have been identified as the most critical variables that predict engraftment and outcomes after CBT.7 To date, CBT has been successfully employed in children, but its application has been limited in adults by insufficient number of stem cells in a single cord blood (CB) unit.8 Strategies that have been employed in an effort to circumvent the limitation of cell dose include the use of multiple CB units and coinfusion of an ex vivo expanded CB unit.3,7 While transplantation with ex vivo expanded CB cells has failed to enhance engraftment in clinical studies, both preclinical and clinical studies have shown that the cotransplantation of two or more CB units significantly improves the rate of engraftment over a single CB transplant when an insufficient cell dose of hematopoietic stem cells (HSCs) is administered.3,7,9,10 Furthermore, patients receiving double-CB transplants have similar rates of acute and chronic GVHD, transplant-related mortality, and disease-free and overall survival when similar cell doses are compared between single- and double-CB transplants.11,12 These findings support umbilical CB as a worthy stem cell source for allogeneic stem cell transplant (alloSCT) and warrant further investigation into methods to optimize engraftment and immune reconstitution.
Strategies that can boost immunity and outcomes after CBT include adoptive transfer of activated T cells or tumor primed T cells.13 Even though CB contains significantly higher absolute numbers of T, NK, and B lymphocytes than adult PB,14,15 CB T cells fundamentally differ from “naive” adult T cells due to a relative Th2 bias with fewer CB T cells expressing HLA-DR and CCR-5 activation markers.16 Moreover, a higher rate of CB T cells progress through cell cycle and enter apoptosis compared with adult blood, indicating high cell turnover.16 In vitro apoptosis of CB T lymphocytes can be prevented by cytokines signaling through the common γ-chain cytokine receptor family including IL-2, IL-4, IL-7, and IL-15.17–19 Circulating neonatal T cells express higher levels of the IL-7 receptor α-chain (CD127) than adult naive T cells.19 IL-7 is involved in thymocyte development at a stage preceding the T-cell receptor rearrangement.20 In contrast to IL-7, IL-15 induces the differentiation of CD8 T lymphocytes in vitro.21 Studies by Szabolcs and colleagues13,22 have reported significantly enhanced T-cell expansion with IL-7, along with IL-2 and CD3/CD28 costimulation. In addition, IL-7 promotes the preservation of a polyclonal T-cell receptor repertoire and a surface phenotype that favors lymph node homing and lacks alloreactivity while reducing the activation-induced cell death. Infusion of expanded CB T cells may alleviate posttransplant lymphopenia and qualitative T-cell defects until immune reconstitution is established.
In an earlier study,23 we showed that anti-CD3–activated murine splenocytes could enhance survival in lethally irradiated (9 Gy) 6- to 8-week-old female BDF1 (C57BL6 × DBA, H2b/H2d) mice after transplantation of syngeneic HSC-containing splenocytes. There was a significant enhancement in overall survival after transplant in mice given dose-limiting numbers of unmanipulated splenocytes. The infused anti-CD3–activated murine splenocyte population consisted of 77.6% CD3+ cells. More recently, Hexner and coworkers24 showed that T cells can enhance hematopoietic engraftment by stimulating stem-cell differentiation in human xenografts in immunodeficient mice. Consistent with these findings, immune reconstitution is significantly delayed in patients who receive T-cell-depleted alloSCT. These studies strongly suggest that T cells play an important role in facilitating engraftment and/or hematopoiesis.
A graft-versus-leukemia (GVL) effect after alloSCT plays a major role in eradicating residual disease after chemotherapy with both myeloablative and nonmyeloablative conditioning regimens.25 Although GVHD provides the benefit of overlapping GVL effect, it is restricted by its toxicity and high mortality rate. We hypothesize that CB anti-CD3–activated T cells (CBATCs) can be expanded ex vivo, armed with bispecific antibodies (BiAbs) and redirected to tumor targets while providing help for engraftment. In this study, we show that CBATCs and CBATCs armed with anti-CD3 × anti-CD20 BiAb exhibit significantly low alloreactivity and produce low levels of cytokines and chemokines yet mediate effective target-specific killing.
MATERIALS AND METHODS
CB units
Fresh or cryopreserved CB units were obtained from the JP McCarthy Cord Blood Bank at Karmanos Cancer Institute that were not qualified for banking according to the preprocessing content of nucleated cell of less than 10 × 108. The cryopreserved CB units were red blood cell depleted before cryopreservation and processed according to the established CB banking procedure for clinical use.26,27 The protocols for obtaining CB or PB from normal healthy donors for research use were approved by the Wayne State University Human Investigation Committee. For fresh CB units, mononuclear cells (MNCs) were separated by Ficoll-Hypaque density gradient centrifugation, washed 3× in RPMI 1640 (Lonza, Inc., Allendale, NJ), and reconstituted in RPMI 1640 supplemented with heat-inactivated 10% fetal bovine serum (FBS; Valley Biomedical, Winchester, VA). The cryopreserved CB units were thawed in a 37°C water bath, rapidly diluted in RPMI containing 20% serum, washed, and reconstituted in RPMI 1640 containing 10% FBS before use.
Production of anti-OKT3 × anti-CD20 and anti-OKT3 × anti-Her2 BiAbs
BiAbs were produced by chemical heteroconjugation of OKT3 (a murine IgG2a anti-CD3 monoclonal antibody, Ortho Biotech, Horsham, PA) and rituximab (a chimeric anti-CD20 IgG1, Genentech, Inc., San Francisco, CA) or trastuzumab (a humanized anti-Her2 IgG1, Genentech, Inc.) as described previously.28 Before use, ATCs were armed using an optimal concentration of BiAb (50 ng/106 ATC) for 30 minutes at room temperature with either anti-CD3 × anti-CD20 (CD20Bi) or anti-CD3 × anti-Her2 (Her2Bi) BiAbs. After arming, the armed ATCs (aATCs) were washed thrice to eliminate any unbound BiAb.
Expansion and generation of CBATCs
To establish the optimal dose of soluble anti-CD3 for activation, three CBMNCs were tested for growth and expansion in 100 IU/mL IL-2 for 14 days. CBMNCs were tested for activation with 5, 10, 20, 50, and 100 ng of OKT3/mL. Since our preliminary data show that CD3+ T cells can be activated with 20 ng/mL OKT3, we expanded and activated T cells from CBMNCs using 20 ng/mL OKT3 and 100 IU of IL-2 for 14 days at a concentration of 1 × 106 CBMNCs/mL in RPMI 1640 supplemented with 10% FBS and IL-2 every 2 to 3 days for all other experiments. CB T-cell expansion was determined for 4 frozen and 4 fresh CB units over the course of 14-day cultures.
Cell lines
The human lymphoblastoid B-cell lines (Daudi, B9C, and Raji from ATCC, Norcross, GA) and breast cancer cell line SK-BR-3 were maintained in RPMI 1640 (Lonza, Inc., Allendale, NJ) supplemented with 10% FBS (Lonza, Inc.), 2 mmol/L l-glutamine (Invitrogen, Carlsbad, CA), 50 units/mL penicillin, and 50 mg/mL streptomycin (Invitrogen).
51Cr release cytotoxicity assay
Cytotoxicity directed at CD20+ targets (B9C, Raji and Daudi) or Her2/neu+ target (SK-BR-3) were tested by arming ATC. ATC cultures were harvested from 5 to 6 CBMNC units after 6, 8, 10, and 14 days of culture. CBATCs armed with 50 ng of CD20Bi/106 ATCs or Her2Bi/106 ATCs were tested for cytotoxicity at various effector-to-target (E : T) ratios and at specified time points to define the optimal time in culture for the development of specific cytotoxicity.28,29
Enzyme-linked immunosorbent spots for interferon-γ–secreting T cells
Kinetics of interferon (IFN)-γ–producing CBATCs or CBATCs armed with CD20Bi were detected by IFN-γ specific enzyme-linked immunosorbent spot (ELISpot) assay (BD Biosciences, San Jose, CA) when stimulated with CD20+ target cells. ELISpots were assessed after 18 hours of exposure to the stimulant cells at 10:1 E : T in ELISpot plates as previously described.30 Briefly, 96-well plates (BD Biosciences) were coated with purified anti-human IFN-γ (BD Biosciences). After being washed and blocked with RPMI 1640 supplemented 10% FBS, appropriate numbers of specific or nonspecific targets and effectors were added to the plates and incubated overnight at 37°C. Plates were washed with deionized water before washing with phosphate-buffered saline/0.05% Tween 20 followed by incubation with IFN-γ–specific secondary antibody conjugated to horseradish peroxidase (BD Biosciences). The assay was developed with 3-amino-9-ethylcarbazole as a chromogenic substrate (BD Biosciences). Spots were captured and counted on a counter using its accompanying software (CTL ImmunoSpot and ImmunoSpot software, Version 4, Cellular Technology Ltd, Shaker Heights, OH).
Flow cytometry
Phenotyping was performed on CBMNCs on Day 0 or on Day 14 after expansion using standard flow cytometry to determine the proportions of CD3+, CD4+, CD8+, CD25+, CD56+, CD16+, CD45RA+, CD45RO+, CD19+, and CD20+ cells.
Mixed leukocyte cultures
Cultures were established in round-bottom 96-well plates. Responder peripheral blood MNCs (PBMNCs; 5.0 × 104) were cultured with 1.0 × 105 irradiated (2500 rads) stimulator PBMNCs in 200 mL RPMI 1640 supplemented with 10% autologous serum, 2 mmol/L l-glutamine (Invitrogen), 50 units/mL penicillin, and 50 mg/mL streptomycin (Invitrogen) in quadruplicate. On Day 5, cultures were pulsed with [3H]thymidine (2.0 mCi/well) for the final 18 hours and [3H]thymidine incorporation was plotted as the mean counts per minute of the 16 stimulator and responder combinations.
Measurement of cytokine secretion
Cytokines were measured in the supernatants of CBATCs unstimulated or stimulated with target cells using a 25-plex human cytokine by Luminex array (Invitrogen) and a Bio-Plex system (Bio-Rad Lab., Hercules, CA). The multiplex panel includes IL-1β, IL-1 receptor antagonist, IL-2, IL-2R, IL-4, IL-5, IL-6, IL-7, IL-8, IL-13, IL-17, tumor necrosis factor (TNF-α), IFN-α, IFN-γ, granulocyte-macrophage–colony-stimulating factor (GM-CSF), macrophage inhibitory protein (MIP)-1α, MIP-1β, IFN-inducible protein (IP)-10, MIG, eotaxin, RANTES, and monocyte chemotactic protein 1. The limit of detection for these assays is less than 10 pg/mL, based on a detectable signal of more than twofold above background (Bio-Rad). Cytokine concentration was automatically calculated from a standard curve by the computer software (BioPlex Manager, Bio-Rad).
Statistical analyses
All statistical analyses were performed using computer software (GraphPad Prism, Version 7.00 for Windows, GraphPad Software, San Diego, CA). Results from cytotoxicity assays, ELISpot assays, and mixed lymphocyte response were analyzed using two-way analysis of variance or t test.
RESULTS
Expansion and generation of CBATCs
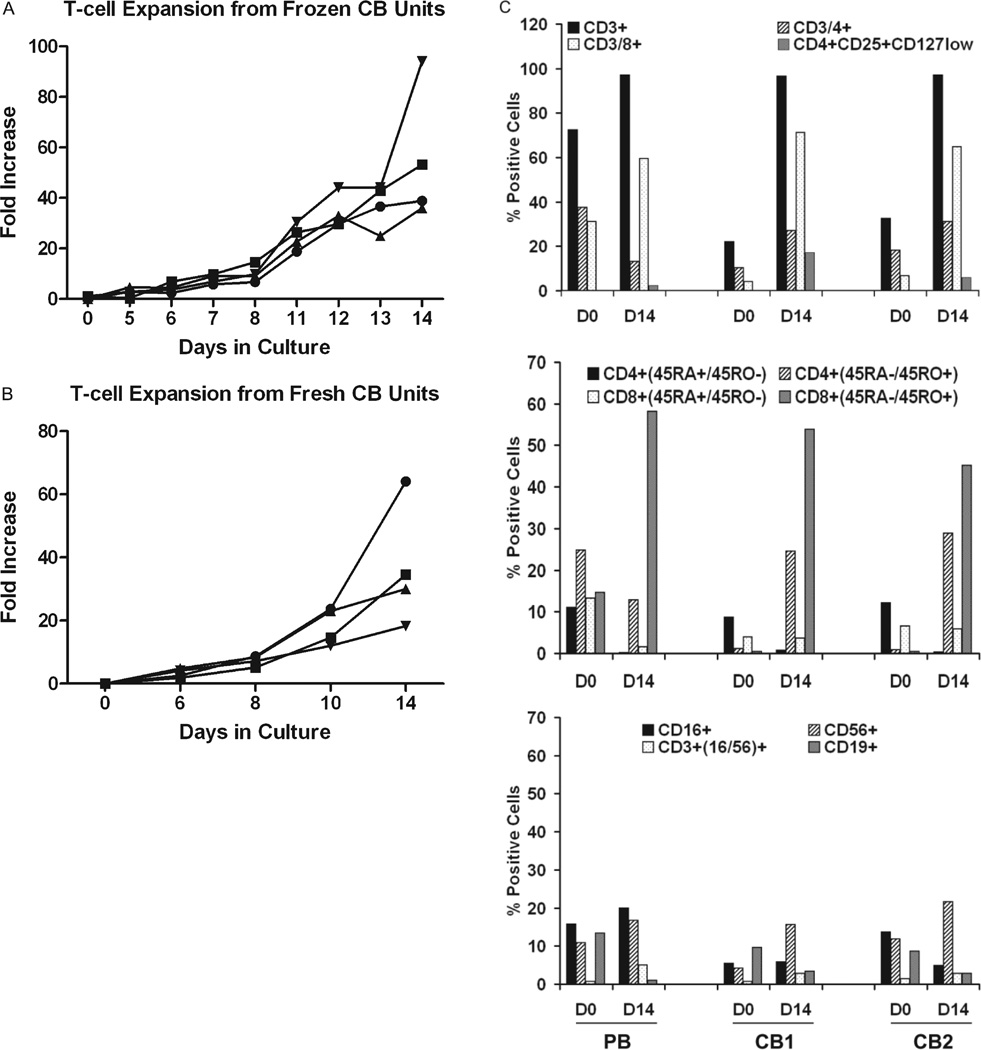
CBMNCs were activated with 20 ng/mL OKT3 and consistently expanded up to 40-fold in IL-2 for up to 14 days in culture from cryopreserved CB units (n = 4), as shown in Fig. 1A. These results show that cryopreserved T cells in the umbilical cord can be consistently expanded under the same conditions used to expand ATCs from PBMNCs.28–30 Therefore, cryopreserved CB can potentially be used to enhance engraftment and provide a GVL effect. Similar to frozen CB units, fresh CB units activated with 20 ng/mL OKT3 expanded 20- to 50-fold in IL-2 (Fig. 1B).
Fig. 1.
Fold expansion of anti-CD3-activated CB. (A) Four frozen CBMNC units were activated with 20 ng/106 OKT3 and expanded in 100IUIL-2/106 cells consistently expanded 30- to 40-fold in 14 days. (●) CB1; (■) CB2; (▲) CB3; (▼) CB4. (B) Twenty- to 60-fold expansion of 4 fresh CBMNC units in 14 days. (●) CB1; (■) CB2; (▲) CB3; (▼) CB4. (C, top) Two CB units and 1 PB unit were pheno-typed by flow cytometry before and after anti-CD3 activation by OKT3 and expansion in IL-2 for 13 days of culture. Results are representative of two separate experiments. PB and 2 CBMNC units or activated and expanded T cells on Day 14 were stained for total CD3+ cells, CD3/CD4+ CD3/CD8+, and CD4+/CD25hi/CD127lo T cells and analyzed by flow cytometry. CBMNCs showed decreased proportions of CD3+, CD4+, and CD8+T cells compared to PBMNCs. Day 14 CB ATCs contained higher proportions of CD4+ CD25hi/CD127lo cells compared to PB ATC. (C, middle) Staining for CD4+ or CD8+/CD45RO+ and CD4+ or CD8+/CD45RA+T cells showed extremely low proportions of memory phenotype CD4+ or CD8+/CD45RO+ in CBMNCs compared with PBMNCs but activated and expanded T cells showed similar proportions of CD4+ or CD8+/CD45RO+T cells. (C, bottom) NK-cell population in PBMNCs or CBMNCs and activated expanded T cells on Day 14. On Day 14, CBATCs showed a decreased proportion of CD16+ cells compared to PBATCs.
Phenotyping of CB before and after culture
To determine the phenotype of CBATCs, CBMNCs from 2 CB units and PBMNCs from a normal donor were activated with OKT3 and expanded in IL-2 for 13 to 14 days in culture and phenotyped for T-cell, B-cell, and NK-cell populations on Days 0 and 14 by flow cytometry. Figure 1C (top) shows the expansion of CD3+ cells and a marked increase in the proportion of CD8+ cells with a smaller proportion of CD4+ T cells. All CB-expanded CD4+ T cells showed positive staining for CD25+. We then tested whether expression of CD25 on T cells is due to the IL-2–mediated expansion or if there is a development of CD4+CD25+ T regulatory cells (Tregs). Surprisingly, a higher percentage of T regulatory cells (Tregs, CD4+CD25int/high-CD127low) were present in CBMNC-expanded ATCs ranging from 6% to 17% compared to approximately 2.5% to 5% in PBMNC-expanded ATCs. As expected, CB T cells were of naive phenotype (CD4+/CD45RA+ or CD8+/CD45RA+) on Day 0 compared to PBMNCs, which expressed higher (CD4+/CD45RO+ or CD8+/CD45RO+) memory phenotype compared to naive (CD4+/CD45RA+ or CD8+/CD45RA+) phenotype. After activation and expansion on Day 14, the majority of CB T cells were of memory phenotype (CD4+/CD45RO+ or CD8+/CD45RO+), which was comparable to PB ATCs (Fig. 1C, middle). The numbers of CD56+ NK cells also increased by twofold during the culture (Fig. 1C, bottom).
Cytotoxicity with CBATCs armed with Her2Bi is reproducible
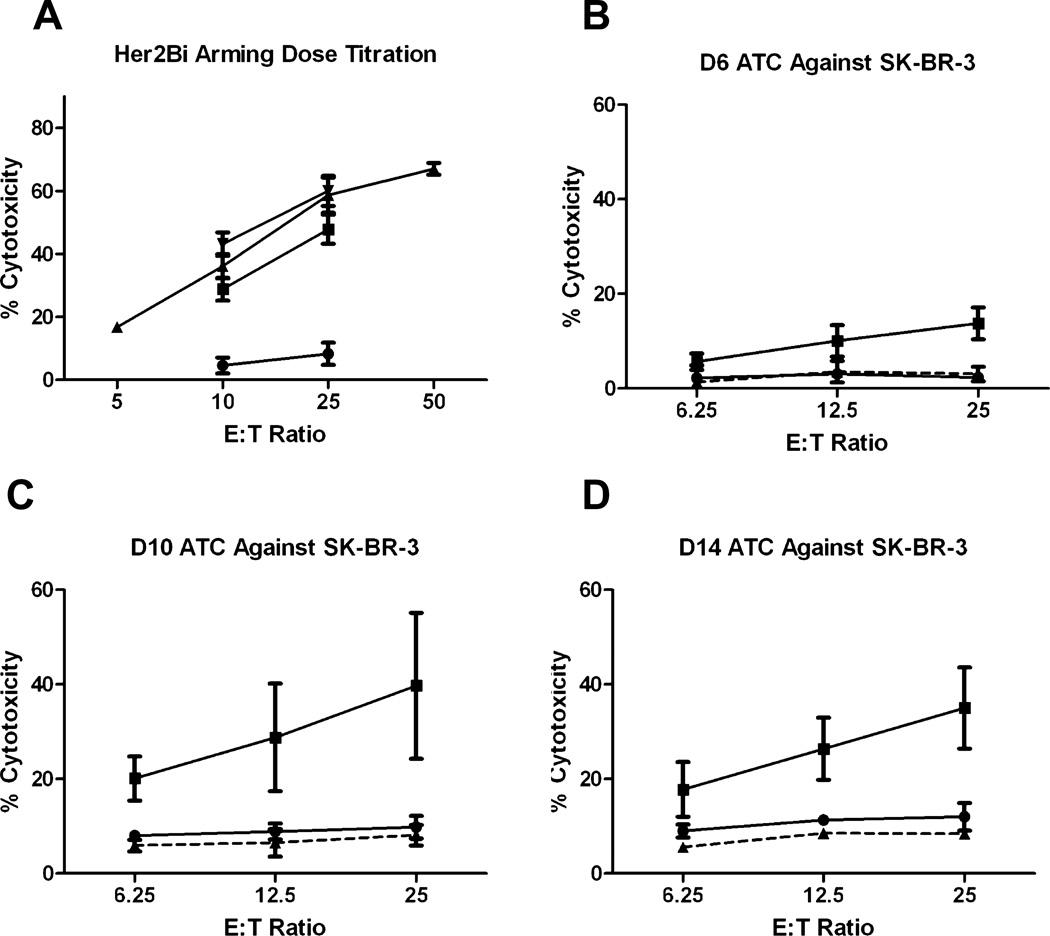
CB units were thawed, activated, and expanded for 14 days to produce CBATCs, armed with Her 2 Bidoses ranging from 5 to 500 ng/106 cells, and tested at the indicated E : T ratio for cytotoxicity directed at SK-BR-3 cells (n = 8). Since arming doses of 50 ng and 500 ng/106 cells showed similar cytotoxicity, we chose the 50-ng dose for all subsequent experiments. Her2Bi-armed ATCs derived from four randomly chosen frozen CBATC and four fresh CBATC samples were highly cytotoxic to SK-BR-3 ranging from 15% on Day 8, 40% on Day 10 (p < 0.009), and 35% on Day 14 (p < 0.0007) at an E : T of 25:1. These results show that cryopreserved CB units can be consistently expanded ex vivo and armed with BiAbs to mediate tumor-specific killing (Fig. 2).
Fig. 2.
Time course of the development of Her2Bi cytotoxicity directed at SK-BR-3. (A) ATCs were armed with 0, 5, 50, and 500 ng/106 cells with Her2Bi. 51Cr release assay was performed to determine cytotoxicity against SK-BR-3 target cells at different arming doses (●, 0 ng/mL; ■, 5 ng/mL; ▲ 50 ng/mL; ▼ 500 ng/mL) and E : T ratios. (B-D) CBATCs were harvested on the indicated days and armed with 50 ng/106 Her2Bi and tested for specific cytotoxicity against SK-BR-3 by 51Cr release assay. (●) ATCs; (■) Her2Bi ATCs; (▲) CD20Bi ATCs.
Development of CD20Bi-armed specific cytotoxicity
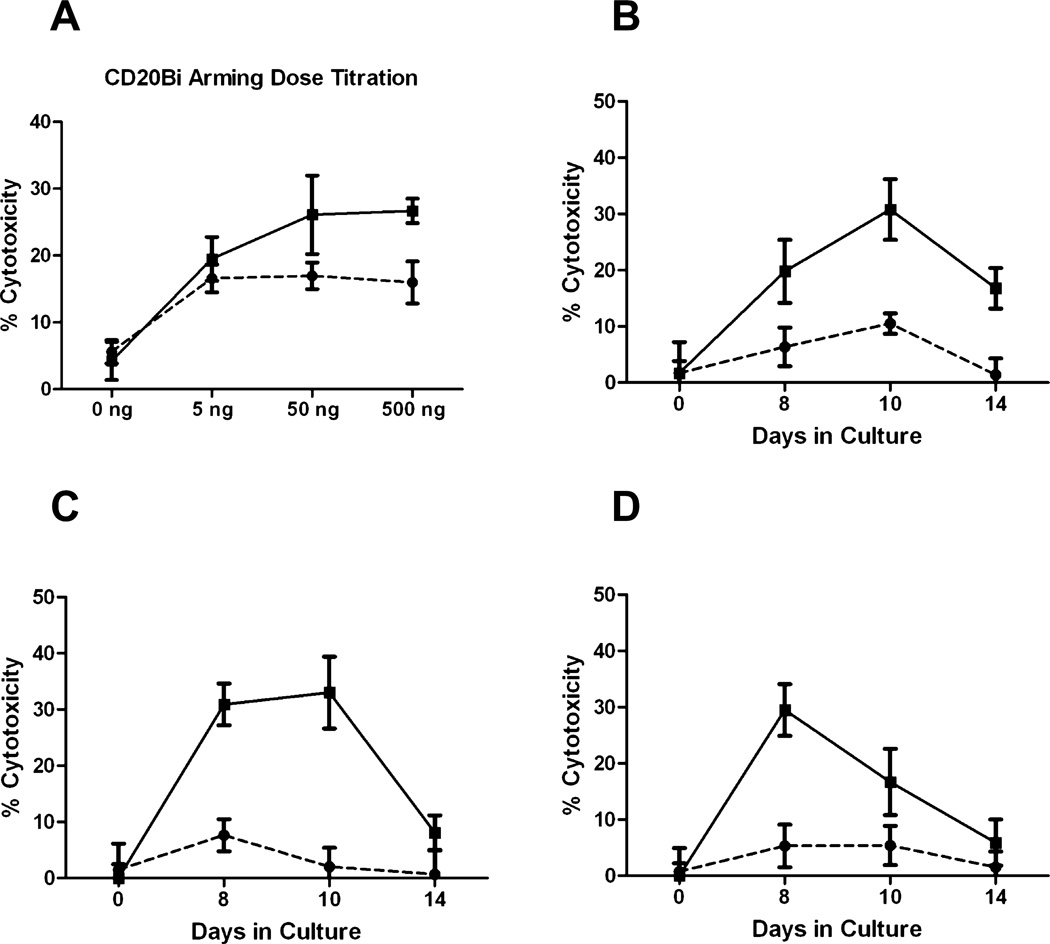
Similar to arming studies using Her2Bi, ATCs were armed with CD20Bi doses ranging from 0 to 500 ng/million ATCs and tested against Daudi cells at E : T ratios of 25:1 and 10:1. At an E : T of 25:1, ATCs armed with doses of 50 ng and 500 ng/million exhibited comparable cytotoxicity. Next, we defined the development of specific cytotoxicity in three different CD20+ cell lines (Raji, Daudi, and B9C) by testing CBATCs harvested on Days 8, 10, and 14, and armed with CD20Bi at 50 ng/106 ATC (Fig. 3). Cytotoxicity of CBATCs armed with CD20Bi was significantly elevated (30%; p < 0.0034) on Day 10 for Daudi cells, Days 8 to 10 for Raji cells (30%; p < 0.001), and approximately 30% for B9C cells on Day 8 (p < 0.003).
Fig. 3.
Time course of the development of CD20Bi cytotoxicity directed at Daudi, Raji, and B9C targets. (A) CD20Bi dose titration studies were performed with CB ATCs armed with 0, 5, 50, and 500 ng/106 cells. 51Cr-labeled targets (1 × 104 cells/round-bottomed microwell) were tested at the indicated E : T ratios (●, 10:1; ■, 25:1). (B-D) Specific cytotoxicity profiles of CD20Bi-armed ATCs harvested at 8, 10, and 14 days and armed with 50 ng CD20Bi/106 CB ATCs were evaluated against Daudi, Raji, and B9C targets. (●) ATCs; (■) aATCs. Results are expressed as mean (±SD) cytotoxicity for the indicated time point (n = 8).
Defining the time course and specificity of armed CBATC cytotoxicity using IFN-γ ELISpots
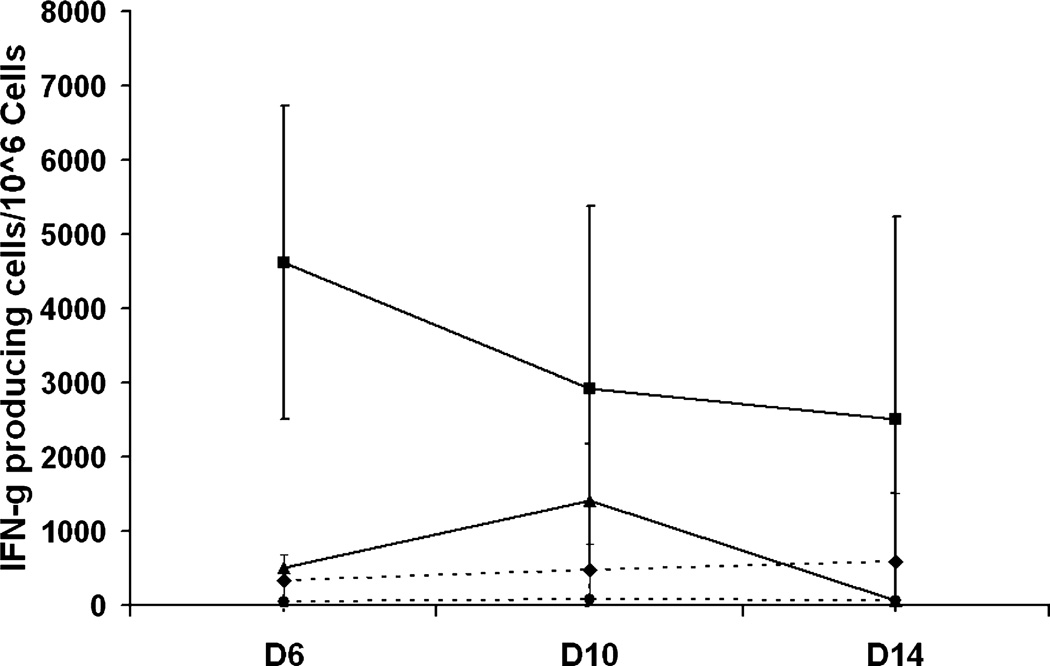
By using ELISpots as a surrogate marker of cytotoxic T cells and helper T cells that secrete IFN-γ, the number of T cells secreting IFN-γ can be enumerated. CBATCs armed with CD20Bi and unarmed ATCs were stimulated by coculture with the indicated target cells at 10:1 E : T ratio for 18 hours before ELISpot detection. CD20-armed CBATC results in a specific increase in IFN-γ ELISpots when stimulated with CD20+ targets (Daudi and B9C) on Day 10. The IFN-γ–producing cells declined by Day 14. These data show specificity of the CD20Bi-armed CBATCs and that responses peaked on Day 10 (Fig. 4).
Fig. 4.
Time course to determine for IFN-γ ELISpots in CD20Bi- (♦) or Her2Bi- (●) armed ATCs or unarmed ATCs stimulated with CD20+ Daudi cells (▲) and Her2/neu + SK-BR-3 cells (■). ELISpots were developed after exposing CBATCs or CD20Bi-armed CBATCs to Daudi and Her2Bi-armed CBATCs to SK-BR-3 targets at indicated time points (n = 4). Data are expressed as IFN-γ ELISpots per 106 cells.
CBMNCs and CBATCs are nonresponsive to alloantigens in mixed leukocyte culture assays
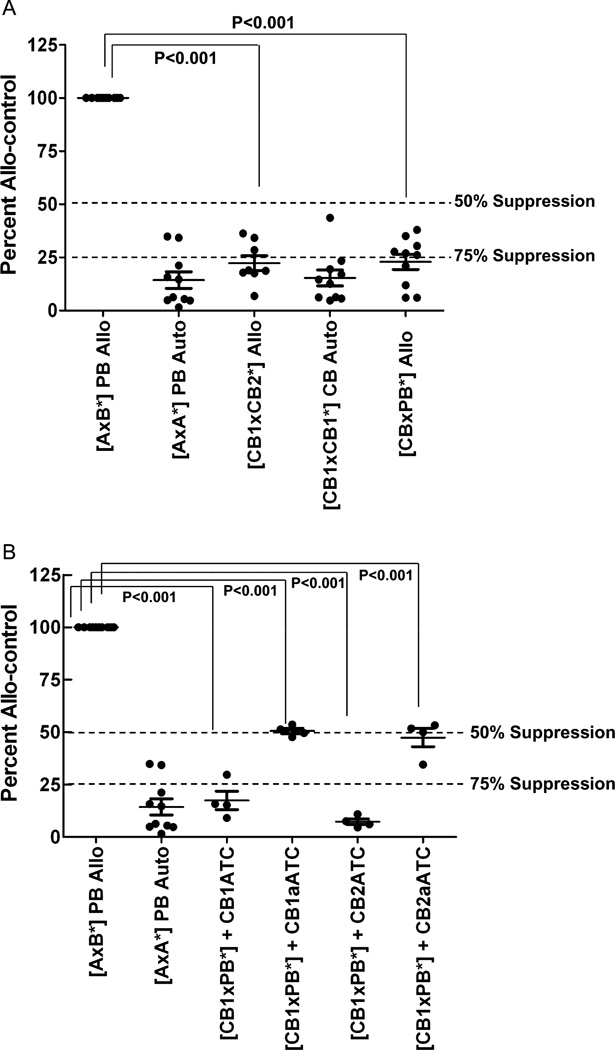
To determine the magnitudes of allogeneic responses, CBMNCs, CBATCs, and CD20Bi or Her2Bi armed CBATCs (CBaATCs) from six different normal donors were stimulated with irradiated (2500 rads) unrelated PBMNCs. As expected PBMNCs or CBMNCs did not respond to irradiated autologous PBMNCs or CBMNCs, respectively (Fig. 5, top). The allogeneic PBMNCs (mixed leukocyte culture [MLC] positive control) responded vigorously whereby PBMNCs of individual A are responding to irradiated PBMNCs of individual B. All of the data have been normalized to 100 = the allogeneic control (allocontrol) (A × B*) and presented as percent allocontrol. The mean percent response of CB to alloantigen was less than 50% (p < 0.001) of the allocontrol (Fig. 5A). Next, we tested whether CBATCs added to the allocontrol (A × B*) would suppress a third-party MLC. The mean alloresponsiveness of CBaATCs and CBATCs to alloantigen was approximately 50% (p < 0.001) and less than 75% (p < 0.001) of the allocontrol, respectively. Adding allo-CBATCs or -CBaATCs from the same CB or second CB in the MLC did not result in enhanced alloreactivity in the presence of ATCs (Fig. 5B). In 16 allostimulation combinations, CB, CBATCs, and CBaATCs were statistically less responsive than allo-PB control (p < 0.001). These data show that CBATCs and CBaATCs are hyporesponsive to alloantigens when compared with normal allo-PBMNCs.
Fig. 5.
(A) CBMNCs suppress MLC responses to alloantigens. The proliferative responses of 50,000 responders cocultured with 100,000 irradiated (2500 rad) stimulators were measured by 3H thymidine incorporation assay after 6 days of culture. The allocontrol was normalized to 100%, where PBMNCs of individual A are responding to irradiated PBMNCs of individual B (A × B*), and all data are presented as % allocontrol. Response of CB to alloantigen was less than 50% of the allo-control. (B) Armed or unarmed CB ATCs derived from 14-day cultures were added to MLCs. The proliferative index of responders (CB) to irradiated stimulator cells (*PB) showed attenuated responsiveness to alloantigens. Similarly, response of CBaATCs to alloantigens was 50% less than the allocontrol (A = PBMCs from individual A; B = PBMCs from individual B; CB1 = cord blood #1; CB2=cord blood #2; CB1 ATC =ATCs from cord blood #1, CB2 ATC = ATCs from cord blood #2.)
Cytokine profile of CBATCs and CBaATCs
Next, we tested whether reduced alloreactivity of CBATCs or CBaATCs is due to reduced ability of CBATCs and CBaATCs to produce cytokines and chemokines. Culture supernatants of CBATCs or CBaATCs with or without allo-stimulation were tested for cytokines and chemokines using multiplex cytokine array (n = 3). Both stimulated and unstimulated CBATCs and CBaATCs produced reduced levels of Th1 and Th2 cytokines, chemokines, and inflammatorycy tokines compared to PBATCs or PBaATCs. The only cytokine that showed higher levels compared to the unstimulated CBATCs or CBaATCs was IL-2 with comparable levels in both CBATCs and CBaATCs in the presence of Daudi cells (Table 1).
TABLE 1.
Cytokine profile of CBATCs and CBATCs armed with CD20Bi alone (unstimulated) or stimulated with Daudi cells (CBATC + Daudi or CBaATC +Daudi) in culture supernatant*
| Fold change |
||||
|---|---|---|---|---|
| Th1 cytokines | CBATCs + Daudi | CBaATCs + Daudi | CBATCs | CBaATCs |
| GM-CSF | −7.1 | −20.9 | −4.8 | −4.4 |
| IFN-γ | −6.9 | −37.9 | −5.6 | −5.2 |
| IL-2 | −2.4 | −1.0 | −15.2 | −13.3 |
| Th2 cytokine | ||||
| IL-13 | −4.5 | −18.7 | −6.0 | −3.0 |
| IL-5 | −6.0 | −2.3 | −5.1 | −7.4 |
| IL-4 | −4.3 | −4.5 | −4.0 | −5.5 |
| IL-10 | −11.4 | −98.1 | −8.3 | −15.0 |
| Chemokines | ||||
| CCL3/MIP-1α | −17.5 | −290.9 | −3.4 | −11.3 |
| CCL4/MIP-1β | −24.7 | −220.9 | −4.8 | −13.8 |
| CCL2/MCP-1 | −6.9 | −4.6 | −14.2 | −9.6 |
| CCL5/RANTES | −8.3 | −18.4 | −3.6 | −8.2 |
| CXCL9/MIG | −3.2 | −3.1 | −4.6 | −12.8 |
| CXCL10/IP-10 | −1.8 | −2.5 | −4.8 | −5.2 |
| Inflammatory cytokines | ||||
| IFN-α | −4.0 | −4.3 | −4.6 | −2.8 |
| IL-6 | −7.5 | −5.1 | −6.0 | −7.1 |
| TNF-α | −4.7 | −22.3 | −2.9 | −4.0 |
Negative number (−) indicates lower production in CBATCs or CBaATCs compared to PBATCs or PBaATCs.
IP-10 = IFN-inducible protein 10; MCP-1 = monocyte chemotactic protein 1.
DISCUSSION
In this study, we investigated: 1) the expansion of T cells from fresh and frozen CB units; 2) the functional ability of CBATCs or CBaATCs to mediate BiAb-redirected cytotoxicity after expansion and the development of BiAb-mediated cytotoxicity in CBaATCs directed at breast and lymphoma targets; 3) the phenotype of CBATCs after expansion; 4) the ability of CBATCs and CBaATCs to mount or suppress an alloresponse in MLCs; and 5) the pattern of cytokine and chemokine secretion after CBATCs and CBaATCs engage tumor targets. Dose titrations show that the optimal arming dose is approximately 50 ng for each BiAb/106 CBATCs. Specific cytotoxicity as measured by 51Cr release assays or by IFN-γ ELISpots responses peaked between 6 and 10 days for Her2Bi- and CD20Bi-armed ATCs, respectively. These studies show that functional and cytotoxic T cells can be generated from fresh or frozen CB and may be used to target tumor cells in vivo. Studies by others also support the feasibility to expand various cell populations from CB. Previous studies have reported that T, NK, and CD34+ cells could be expanded from CB.31,32 Kobari and colleagues33 showed that the ex vivo expansion of CB CD34+ cells did not alter the capacity to generate functional T lymphocytes or dendritic cells. Recently, IL-7 has been reported to enhance CB T-cell expansion significantly, in combination with IL-2 and CD3/CD28 costimulation. Furthermore, tumor-specific cytotoxic T cells could be generated by priming the expanded T cells against lymphoid and myeloid leukemia cells.34 CB T cells have also been expanded and engineered with chimeric receptors targeting CD19 to improve the antitumor properties. Serrano and colleagues35 demonstrated that CB T cells could be genetically modified to express a chimeric CD19 receptor that targets and kills CD19+ tumor targets in vitro and reduced the size of CD19+ tumors in vivo in NOD/SCID mice. Similarly, CB T cells can be redirected to kill leukemia and lymphoma cells by engineering with a single-chain chimeric antigen receptor for CD19 containing an intracellular domain of the CD3ζ chain with 4-1BB and intracellular domain of CD28.36 The study by Tammana and coworkers36 showed a synergistic role of 4-1BB and CD28 costimulation in engineered antileukemia CB effector cells. Our data show that CBATCs can be produced by anti-CD3 stimulation and expanded in IL-2 using the previously described methods28 and that CBATCs are functionally comparable with PBATCs.
Consistent with another study,37 our data show that T cells in CB are predominantly of naive phenotype (CD45RA+) with extremely low numbers of CD45RO+ memory phenotype when compared with adult PBT cells. CB T cells after activation (anti-CD3) and expansion (IL-2) showed a completely reversed pattern. CBATCs were predominantly CD45RO+ memory phenotype. Activated and expanded CBATCs (Day 14) were more than 90% positive for CD3+ T cells with the majority being CD8+ T cells, similar to PBATCs. Interestingly, the Treg population (CD4+CD25int/highCD127low) was higher in CBATCs (6 and 17%) as compared to PBATCs (2%) on Day 14.Treg cells play a central role in immunoregulation and have been shown to prevent transplant rejection and also prevent GVHD by suppressing allogeneic immune responses.38 It is likely that a higher percentage of Tregs in CB may be responsible for depressed alloresponses. Other studies support that CD25+ regulatory T cells inhibit the proliferation of CD4 and CD8 T lymphocytes and thereby may control allogeneic immune responses.39
Next, we tested the activity of human CBMNCs, CBATCs, or CBATCs armed with CD20Bi in 6-day MLCs (Fig. 5) along with fresh PBMNCs with fresh irradiated allogeneic PBMNCs. The data clearly show that despite a brisk allogeneic response between the PBMNCs of different donors, neither CBATCs nor CBaATCs exhibited any significant activity against allogeneic stimulators. This in vitro data demonstrate that CBATCs or CBaATCs are poor responders to allostimulation. The finding that CBaATCs do not proliferate in response to alloantigens in MLC reactions suggests that CBATCs or CBaATCs could facilitate engraftment and GVL without augmenting GVHD. Similarly, other studies show that human T cells or T cells activated with anti-CD3 and IL-2 can inhibit the generation of alloresponsiveness.24,40,41 More recently, limited Th1 maturation with low expression levels of 4-1BB/CD137, CD40L, and perforin during expansion have also been correlated with low alloreactivity.13,22 Our previous study in murine model shows that unfractionated murine ATC enhances engraftment when the number of stem cells are dose limiting, supporting the premise that CBATCs enhance engraftment.23 The MLC data strongly support the fact that CBaATCs derived from a second CB unit can help the primary CB unit engraft since there is little alloreactivity in whole CB, CBATCs, or armed CBATCs.
CBATCs or CBaATCs (either unstimulated or stimulated with Daudi cells) produce lower levels of both Th1 and Th2 cytokines and chemokines, which is in agreement with previous studies in purified T cells or expanded T cells from CB.37,42,43 Donor T cells that are activated by recipient alloantigen produce Th1-type cytokines and chemokines, which can recruit and activate other effector cells, thus amplify the alloreactivity resulting in tissue damage. Cytokines that are directly implicated in tissue damage during GVHD include TNF-α and IFN-γ.44–49 Levels of these cytokines are significantly increased in allogeneic recipients during GVHD reactions.46–49 Reduced incidence of GVHD and reduced severity after CB transplantation, despite greater MHC disparity, are likely to be due to lower expression of Th1 cytokines production in an alloreaction. Suen and coworkers50 also reported that the impaired ability of CB to produce IL-12 and IL-15 in response to stimulation may contribute to the decrease in IFN-γ, TNF-α production, and NK and LAK activities. The transcription of many cytokine genes is regulated by nuclear factor of activated T cells c2 (NFATc2), a member of the NFAT family.43,51 Kaminski and colleagues43 showed that reduced expression of cytokines in CB T cells was associated with the decreased expression of a transcription factor NFATc2. Low levels of Th1 and Th2 cytokine expression may also be attributed due to the reduced expression of Th1- and Th2-associated transcription factors STAT4 and T-bet in umbilical CB.52,53
In spite of low levels of cytokine production, functional responses of CBATCs were not compromised. Our data show that CBATCs derived from CB units exhibit specific cytotoxicity directed toward CD20+ and Her2/neu+ targets in vitro when armed with CD20Bi and Her2Bi, respectively. This finding is consistent with our preclinical studies using human PB. We have previously expanded autologous PB ATCs ex vivo and armed them with Her2Bi or CD20Bi for preclinical and Phase I and II trials28–30,54–56 and showed that Her2Bi- and/or CD20Bi-armed ATCs 1) specifically kill targeted cell lines via a perforin-mediated pathway, 2) can be safely administered to patients after chemotherapy and/or autologous stem cell transplant, and 3) induce specific CTL activity against tumor cell lines in patient PBMNCs. These data from our Phase I clinical trials involving aATC infusions directed at Her2/neu in metastatic breast cancer patients and directed at CD20 in non-Hodgkin’s lymphoma patients after autologous stem cell transplant suggest that armed CBATCs may be clinically useful.
In summary, our data show that CBATCs can be expanded ex vivo and armed with CD20Bi or Her2Bi to exhibit antigen-specific cytotoxicity toward CD20+ and Her2/neu+ tumor targets, respectively, and that CBATCs or CBaATCs do not proliferate in response to alloantigens. The attenuated response of CBATCs or CBaATCs to alloantigens and their ability to suppress MLCs may translate into reduced GVHD, while CBaATCs redirected to solid tumors or hematologic malignancies by BiAbs may enhance graft-versus-tumor effect after CB stem cell transplant; these warrant further investigations.
Acknowledgments
These studies were supported in part by funds from a Michigan Life Science Grant, Translational Grants #6092-09 (LGL) and #6066-06 (LGL) from the Leukemia and Lymphoma Society, NIH R01 CA 092344 (LGL), R01 CA 140314 (LGL), and Startup Funds from Karmanos Cancer Institute.
ABBREVIATIONS
- alloSCT
allogeneic stem cell transplant
- aATC(s)
armed anti-CD3–activated T cell(s)
- ATC(s)
anti-CD3–activated T cell(s)
- BiAb(s)
bispecific antibody(-ies)
- CBaATC(s)
anti-CD3 × anti-CD20 or anti-CD3 × anti-Her2 bispecific antibodies armed activated T cells
- CBT
cord blood transplantation
- CD20Bi
anti-CD3 × anti-CD20
- ELISpot(s)
enzyme-linked immunosorbent spot(s)
- E : T
effector-to-target (ratio)
- FBS
fetal bovine serum
- GVL
graft-versus-leukemia
- Her2Bi
anti-CD3 × anti-Her2
- MIP
macrophage inhibitory protein
- MLC(s)
mixed leukocyte culture(s)
- NFAT
nuclear factor of activated T cell
- PB
peripheral blood
- Tregs
T regulatory cells
Footnotes
CONFLICT OF INTEREST
None of the authors has any potential financial conflict of interest related to this article.
REFERENCES
- 1.Brunstein CG, Setubal DC, Wagner JE. Expanding the role of umbilical cord blood transplantation. Br J Haematol. 2007;137:20–35. doi: 10.1111/j.1365-2141.2007.06521.x. [DOI] [PubMed] [Google Scholar]
- 2.Brunstein CG, Baker KS, Wagner JE. Umbilical cord blood transplantation for myeloid malignancies. Curr Opin Hematol. 2007;14:162–169. doi: 10.1097/MOH.0b013e32802f7da4. [DOI] [PubMed] [Google Scholar]
- 3.Hofmeister CC, Zhang J, Knight KL, Le P, Stiff PJ. Ex vivo expansion of umbilical cord blood stem cells for transplantation: growing knowledge from the hematopoietic niche. Bone Marrow Transplant. 2007;39:11–23. doi: 10.1038/sj.bmt.1705538. [DOI] [PubMed] [Google Scholar]
- 4.Schoemans H, Theunissen K, Maertens J, Boogaerts M, Verfaillie C, Wagner J. Adult umbilical cord blood transplantation: a comprehensive review. Bone Marrow Transplant. 2006;38:83–93. doi: 10.1038/sj.bmt.1705403. [DOI] [PubMed] [Google Scholar]
- 5.Cornetta K, Laughlin M, Carter S, Wall D, Weinthal J, Delaney C, Wagner J, Sweetman R, McCarthy P, Chao N. Umbilical cord blood transplantation in adults: results of the prospective Cord Blood Transplantation (COBLT) Biol Blood Marrow Transplant. 2005;11:149–160. doi: 10.1016/j.bbmt.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 6.Komanduri KV, St John LS, de Lima M, McMannis J, Rosinski S, McNiece I, Bryan SG, Kaur I, Martin S, Wieder ED, Worth L, Cooper LJ, Petropoulos D, Molldrem JJ, Champlin RE, Shpall EJ. Delayed immune reconstitution after cord blood transplantation is characterized by impaired thymopoiesis and late memory T cell skewing. Blood. 2007;110:4543–4551. doi: 10.1182/blood-2007-05-092130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Majhail NS, Brunstein CG, Wagner JE. Double umbilical cord blood transplantation. Curr Opin Immunol. 2006;18:571–575. doi: 10.1016/j.coi.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 8.Rocha V, Gluckman E. Clinical use of umbilical cord blood hematopoietic stem cells. Biol Blood Marrow Transplant. 2006;12(Suppl 1):34–41. doi: 10.1016/j.bbmt.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Delaney C, Heimfeld S, Brashem-Stein C, Voorhies H, Manger RL, Bernstein ID. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat Med. 2010;16:232–236. doi: 10.1038/nm.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delaney C, Gutman JA, Appelbaum FR. Cord blood transplantation for haematological malignancies: conditioning regimens, double cord transplant and infectious complications. Br J Haematol. 2009;147:207–216. doi: 10.1111/j.1365-2141.2009.07782.x. [DOI] [PubMed] [Google Scholar]
- 11.Brunstein CG, Barker JN, Weisdorf DJ, Defor TE, Miller JS, Blazar BR, McGlave PB, Wagner JE. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 110 adults with hematologic disease. Blood. 2007;110:3064–3070. doi: 10.1182/blood-2007-04-067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Macmillan ML, Weisdorf DJ, Brunstein CG, Cao Q, Defor TE, Verneris MR, Blazar BR, Wagner JE. Acute graft-versus-host disease after unrelated donor umbilical cord blood transplantation: analysis of risk factors. Blood. 2009;113:2410–2415. doi: 10.1182/blood-2008-07-163238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis CC, Marti LC, Sempowski GD, Jeyaraj DA, Szabolcs P. Interleukin-7 permits Th1/Tc1 maturation and promotes ex vivo expansion of cord blood T cells: a critical step toward adoptive immunotherapy after cord blood transplantation. Cancer Res. 2010;70:5249–5258. doi: 10.1158/0008-5472.CAN-09-2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han P, Hodge G, Story C, Xu X. Phenotypic analysis of functional T-lymphocyte subtypes and natural-killer-cells in human cord-blood—relevance to umbilical-cord blood transplantation. Br J Haematol. 1995;89:733–740. doi: 10.1111/j.1365-2141.1995.tb08409.x. [DOI] [PubMed] [Google Scholar]
- 15.D’Arena G, Musto P, Cascavilla N, Di Giorgio G, Fusilli S, Zendoli F, Carotenuto M. Flow cytometric characterization of human umbilical cord blood lymphocytes: immunophenotypic features. Haematologica. 1998;83:197–203. [PubMed] [Google Scholar]
- 16.Szabolcs P, Park KD, Reese M, Marti L, Broadwater G, Kurtzberg J. Coexistent naive phenotype and higher cycling rate of cord blood T cells as compared to adult peripheral blood. Exp Hematol. 2003;31:708–714. doi: 10.1016/s0301-472x(03)00160-7. [DOI] [PubMed] [Google Scholar]
- 17.Hassan J, Reen DJ. Human recent thymic emigrants-identification, expansion, and survival characteristics. J Immunol. 2001;167:1970–1976. doi: 10.4049/jimmunol.167.4.1970. [DOI] [PubMed] [Google Scholar]
- 18.Soares MVD, Borthwick NJ, Maini MK, Janossy G, Salmon M, Akbar AN. IL-7-dependent extrathymic expansion of CD45RA(+) T cells enables preservation of a naive repertoire. J Immunol. 1998;161:5909–5917. [PubMed] [Google Scholar]
- 19.Fukui T, Katamura K, Abe N, Kiyomasu T, Iio J, Ueno H, Mayumi M, Furusho K. IL-7 induces proliferation, variable cytokine-producing ability and IL-2 responsiveness in naive CD4(+) T-cells from human cord blood. Immunol Lett. 1997;59:21–28. doi: 10.1016/s0165-2478(97)00093-x. [DOI] [PubMed] [Google Scholar]
- 20.von Freeden-Jeffry U, Solvason N, Howard M, Murray R. The earliest T lineage-committed cells depend on IL-7 for Bcl-2 expression and normal cell cycle progression. Immunity. 1997;7:147–154. doi: 10.1016/s1074-7613(00)80517-8. [DOI] [PubMed] [Google Scholar]
- 21.Cookson S, Reen D. IL-15 drives neonatal T cells to acquire CD56 and become activated effector cells. Blood. 2003;102:2195–2197. doi: 10.1182/blood-2003-01-0232. [DOI] [PubMed] [Google Scholar]
- 22.Szabolcs P, Cairo MS. Unrelated umbilical cord blood transplantation and immune reconstitution. Semin Hematol. 2010;47:22–36. doi: 10.1053/j.seminhematol.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin NR, Lum LG, Ratanatharathorn V, Sensenbrenner LL. Anti-CD3-activated splenocytes enhance survial in lethally irradiated mice after transplant of syngeneic hematopoietic stem cells. Exp Hematol. 1995;23:1331–1336. [PubMed] [Google Scholar]
- 24.Hexner EO, Danet-Desnoyers GA, Zhang Y, Frank DM, Riley JL, Levine BL, Porter DL, June CH, Emerson SG. Umbilical cord blood xenografts in immunodeficient mice reveal that T cells enhance hematopoietic engraftment beyond overcoming immune barriers by stimulating stem cell differentiation. Biol Blood Marrow Transplant. 2007;13:1135–1144. doi: 10.1016/j.bbmt.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 25.Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, Rimm AA, Ringdén O, Rozman C, Speck B, Truitt RL, Zwaan FE, Bortin MM. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555–562. [PubMed] [Google Scholar]
- 26.Cairo MS, Wagner EL, Fraser J, Cohen G, van de Ven C, Carter SL, Kernan NA, Kurtzberg J. Characterization of banked umbilical cord blood hematopoietic progenitor cells and lymphocyte subsets and correlation with ethnicity, birth weight, sex, and type of delivery: a Cord Blood Transplantation (COBLT) Study report. Transfusion. 2005;45:856–866. doi: 10.1111/j.1537-2995.2005.04429.x. [DOI] [PubMed] [Google Scholar]
- 27.Fraser JK, Cairo MS, Wagner EL, McCurdy PR, Baxter-Lowe LA, Carter SL. Cord Blood Transplantation Study (COBLT): cord blood bank standard operating procedures. 7th ed. Philadelphia: W.B. Saunders Co; 1998. pp. 521–561. [DOI] [PubMed] [Google Scholar]
- 28.Gall JM, Davol PA, Grabert RC, Deaver M, Lum LG. T cells armed with anti-CD3 x anti-CD20 bispecific antibody enhance killing of CD20+ malignant B cells and bypass complement-mediated rituximab resistance in vitro. Exp Hematol. 2005;33:452–459. doi: 10.1016/j.exphem.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Sen M, Wankowski DM, Garlie NK, Siebenlist RE, Van Epps D, LeFever AV, Lum LG. Use of anti-CD3 x anti-HER2/neu bispecific antibody for redirecting cytotoxicity of activated T cells toward HER2/neu tumors. J Hematother Stem Cell Res. 2001;10:247–260. doi: 10.1089/15258160151134944. [DOI] [PubMed] [Google Scholar]
- 30.Grabert RC, Cousens LP, Smith JA, Olson S, Gall J, Young WB, Davol PA, Lum LG. Human T cells armed with Her2/neu bispecific antibodies divide, are cytotoxic, and secrete cytokines with repeated stimulation. Clin Cancer Res. 2006;12:569–576. doi: 10.1158/1078-0432.CCR-05-2005. [DOI] [PubMed] [Google Scholar]
- 31.Spanholtz J, Tordoir M, Eissens D, Preijers F, van der Meer A, Joosten I, Schaap N, de Witte TM, Dolstra H. High log-scale expansion of functional human natural killer cells from umbilical cord blood CD34-positive cells for adoptive cancer immunotherapy. Plos ONE. 2010;5:e9221. doi: 10.1371/journal.pone.0009221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Introna M, Pievani A, Borleri G, Capelli C, Algarotti A, Mico C, Grassi A, Oldani E, Golay J, Rambaldi A. Feasibility and safety of adoptive immunotherapy with CIK cells after cord blood transplantation. Biol Blood Marrow Transplant. 2010;16:1603–1607. doi: 10.1016/j.bbmt.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 33.Kobari L, Giarratana MC, Gluckman JC, Douay L, Rosenzwajg M. Ex vivo expansion does not alter the capacity of umbilical cord blood CD34+ cells to generate functional T lymphocytes and dendritic cells. Stem Cells. 2006;24:2150–2157. doi: 10.1634/stemcells.2006-0102. [DOI] [PubMed] [Google Scholar]
- 34.Gluckman E, Rocha V. Cord blood transplantation: state of the art. Haematologica-Hematol J. 2009;94:451–454. doi: 10.3324/haematol.2009.005694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Serrano LM, Pfeiffer T, Olivares S, Numbenjapon T, Bennitt J, Kim D, Smith D, McNamara G, Al-Kadhimi Z, Rosenthal J, Forman SJ, Jensen MC, Cooper LJ. Differentiation of naive cord-blood T cells into CD19-specific cytolytic effectors for posttransplantation adoptive immunotherapy. Blood. 2006;107:2643–2652. doi: 10.1182/blood-2005-09-3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tammana S, Huang X, Wong M, Milone MC, Ma LN, Levine BL, June CH, Wagner JE, Blazar BR, Zhou X. 4-1BB and CD28 signaling plays a synergistic role in redirecting umbilical cord blood T cells against B-cell malignancies. Hum Gene Ther. 2010;21:75–86. doi: 10.1089/hum.2009.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chalmers IMH, Janossy G, Contreras M, Navarette C. Intracellular cytokine profile of cord and adult blood lymphocytes. Blood. 1998;92:11–18. [PubMed] [Google Scholar]
- 38.Godfrey WR, Spoden DJ, Ge YG, Baker SR, Liu BL, Levine BL, June CH, Blazar BR, Porter SB. Cord blood CD4(+)CD25(+)-derived T regulatory cell lines express FoxP3 protein and manifest potent suppressor function. Blood. 2005;105:750–758. doi: 10.1182/blood-2004-06-2467. [DOI] [PubMed] [Google Scholar]
- 39.Takahata Y, Nomura A, Takada H, Ohga S, Furuno K, Hikino S, Nakayama H, Sakaguchi S, Hara T. CD25(+)CD4(+)T cells in human cord blood: an immunoregulatory subset with naive phenotype and specific expression of forkhead box p3 (Foxp3) gene. Exp Hematol. 2004;32:622–629. doi: 10.1016/j.exphem.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 40.Ho VT, Soiffer RJ. The history and future of T-cell depletion as graft-versus-host disease prophylaxis for allogeneic hematopoietic stem cell transplantation. Blood. 2001;98:3192–3204. doi: 10.1182/blood.v98.12.3192. [DOI] [PubMed] [Google Scholar]
- 41.Martin PJ, Hansen JA, Torok-Storb B, Durnam D, Przepiorka D, O’Quigley J, Sanders J, Sullivan KM, Witherspoon RP, Deeg HJ. Graft failure in patients receiving T-cell-depleted HLA-identical allogeneic marrow transplants. Bone Marrow Transplant. 1988;3:445–456. [PubMed] [Google Scholar]
- 42.Tang Q, Grzywacz B, Wang HB, Kataria N, Cao Q, Wagner JE, Blazar BR, Miller JS, Verneris MR. Umbilical cord blood T cells express multiple natural cytotoxicity receptors after IL-15 stimulation, but only NKp30 is functional. J Immunol. 2008;181:4507–4515. doi: 10.4049/jimmunol.181.7.4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaminski BA, Kadereit S, Miller RE, Leahy P, Stein KR, Topa DA, Radivoyevitch T, Veigl ML, Laughlin MJ. Reduced expression of NFAT-associated genes in UCB versus adult CD4(+) T lymphocytes during primary stimulation. Blood. 2003;102:4608–4617. doi: 10.1182/blood-2003-05-1732. [DOI] [PubMed] [Google Scholar]
- 44.Krampera M, Tavecchia L, Benedetti F, Nadali G, Pizzolo G. Intracellular cytokine profile of cord blood T-, and NK-cells and monocytes. Haematologica. 2000;85:675–679. [PubMed] [Google Scholar]
- 45.Chipeta J, Komada Y, Zhang XL, Sakurai M, Azuma E. Intracellular cytokine profiles of cord and adult blood lymphocytes. Blood. 1999;93:1120–1121. [PubMed] [Google Scholar]
- 46.Niederwieser D, Herold M, Woloszczuk W, Aulitzky W, Meister B, Tilg H, Gastl G, Bowden R, Huber C. Endogenous IFN-gamma during human bone-marrow transplantation—analysis of serum levels of interferon and interferon-dependent secondary messages. Transplantation. 1990;50:620–625. doi: 10.1097/00007890-199010000-00019. [DOI] [PubMed] [Google Scholar]
- 47.Ferrara JLM, Deeg HJ. Graft-versus-host disease. N Engl J Med. 1991;324:667–674. doi: 10.1056/NEJM199103073241005. [DOI] [PubMed] [Google Scholar]
- 48.Dickinson AM, Sviland L, Hamilton PJ, Usher P, Taylor P, Jackson G, Dunn J, Proctor SJ. Cytokine involvement in predicting clinical graft-versus-host disease in allogeneic bone-marrow transplant recipients. Bone Marrow Transplant. 1994;13:65–70. [PubMed] [Google Scholar]
- 49.Holler E, Kolb HJ, Hintermeierknabe R, Mittermuller J, Thierfelder S, Kaul M, Wilmanns W. Role of tumor-necrosis-factor-alpha in acute graft-versus-host disease and complications following allogeneic bone-marrow transplantation. Transplant Proc. 1993;25:1234–1236. [PubMed] [Google Scholar]
- 50.Suen Y, Lee SM, Qian J, van de Ven C, Cairo MS. Dysregulation of lymphokine production in the neonate and its impact on neonatal cell mediated immunity. Vaccine. 1998;16:1369–1377. doi: 10.1016/s0264-410x(98)00094-2. [DOI] [PubMed] [Google Scholar]
- 51.Boise LH, Petryniak B, Mao XH, June CH, Wang CY, Lindsten T, Bravo R, Kovary K, Leiden JM, Thompson CB. The NFAT-1 DNA-binding complex in activated T-cells contains Fra-1 and JunB. Mol Cell Biol. 1993;13:1911–1919. doi: 10.1128/mcb.13.3.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang Y, Ochando JC, Bromberg JS, Ding YZ. Of a distant T-bet enhancer responsive to IL-12/Stat4 and IFN gamma/Stat1 signals. Blood. 2007;110:2494–2500. doi: 10.1182/blood-2006-11-058271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Szabo SJ, Sullivan BM, Stemmann C, Satoskar AR, Sleckman BP, Glimcher LH. Distinct effects of T-bet in T(H)1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science. 2002;295:338–342. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- 54.Lum LG, Rathore R, Dizon D, Wang D, Al-Kadhimi Z, Uberti JP, Ratanatharathorn V. Induction of immune responses and improved survival after infusions of T cells armed with anti-CD3 X anti-Her2/neu bispecific antibody in stage IV breast cancer patients (phase I) ASH Annu Meet Abstr. 2007;110:2747. [Google Scholar]
- 55.Lum LG, Rathore R, Dizon D, Wang D, Al-Kadhimi Z, Uberti JP, Skuba C, Steele P, Sandborg R, Ratanatharathorn V. Phase I trial of multiple infusions of autologous activated T cells with anti-CD3 x anti-CD20 bispecific antibody (CD20Bi) after autologous peripheral blood stem cell transplant for non-Hodgkin’s lymphoma to improve graft-vs-lymphoma effects. 2007;110 ed. [Google Scholar]
- 56.Lum LG, Al-Kadhimi Z, Skuba C, Ratanatharathorn V, Uberti JP Roger Williams Hospital BMT/Immunotherapy Team. Enhanced anti-breast cancer cytotoxicity after autologous peripheral blood stem cell transplant (PBSCT) by boosting with multiple infusions of activated T cells (ATC) armed with anti-CD3 x anti-Her2 bispecific antibody (Her2Bi) prior to PBSCT. ASH Annu Meet Abstr. 2007;110:2748. [Google Scholar]