Abstract
If left untreated, symptomatic, severe aortic stenosis (AS) is associated with a dismal prognosis. Open-heart surgical valve replacement is the treatment of choice and is associated with excellent short and long-term outcome. However, many older patients with multiple co-morbidities and anticipated increased surgical risk are excluded from surgical intervention. For these patients, transcatheter aortic valve implantation (TAVI) is emerging as a viable treatment alternative. Transcatheter valvular heart procedures are characterized by lack of exposure and visualization of the operative field, therefore relying on image guidance, both for patient selection and preparation and the implantation procedure itself. This article describes the role of multi-detector row computed tomography (MDCT) for detailed assessment of the aortic valve, aortic root, and iliac arteries in the context of TAVI.
Key Words: Aortic stenosis, transcatheter aortic valve implantation, imaging, computed tomography
Aortic stenosis and transcatheter aortic valve replacement
Degenerative valvular heart disease, including aortic stenosis (AS), is a major cause of morbidity and mortality in the aging population of industrialized countries (1,2). If left untreated, symptomatic, severe aortic stenosis is associated with poor prognosis (3,4). Therefore, surgical aortic valve replacement is indicated in these patients. However, many older patients with AS have multiple co-morbidities with associated increased surgical risk and are therefore not considered surgical candidates (5). For these patients, transcatheter aortic valve implantation (TAVI) is emerging as a viable treatment alternative, with improved outcome compared to medical management.
In the decade following the first implantation in 2002, more than 30.000 TAVI procedures have been performed worldwide. Short and medium term results in patient populations with high surgical risk are encouraging (6-10). In patients not considered surgical candidates, the PARTNER trial cohort-B demonstrated a 20% absolute reduction of 1-year all-cause mortality in the TAVI cohort as compared with medical management (30.7% vs. 50.7%, respectively; P<0.001) (6). In the PARTNER trial cohort-A, which randomized high-risk patients with severe aortic stenosis to transcatheter or surgical procedures, both treatments were associated with similar rates of survival at 1 year, although there were important differences in periprocedural risks, specifically a higher risk of neurological events with TAVI (7).
The two most commonly used stent/valve systems are the balloon-expandable Edwards Sapien valve (Edwards Lifesciences, Irvine, California), and the self-expandable CoreValve ReValving system (Medtronic, Minneapolis, Minnesota). Both are approved for clinical use in the EU, but not yet in the US. Depending on the system, a retrograde transarterial technique (femoral or subclavian artery), or an ante-grade transapical implantation technique via the tip of the left ventricle are utilized (11-13). Prior to positioning of the stent-valve within the aortic root, balloon aortic valvuloplasty is typically performed to prepare the landing zone (14). The Edwards Sapien valve (Edwards Lifesciences) is then deployed and expanded by a balloon during rapid ventricular pacing to minimize cardiac output and prevent migration of the valve during deployment. The self-expanding CoreValve (Medtronic) is typically deployed without pacing.
The stent-valve is anchored within the annulus and, depending on stent design, additional stability is provided by the displaced calcified leaflets and/or the sinotubular junction. Optimal positioning of the transcatheter aortic prosthesis relative to the annulus is critical. If valve deployment is too high, increased risk of paravalvular regurgitation, aortic injury, or embolization into the aorta have been described. If positioning is too low, mitral valve dysfunction, heart block, paravalvular regurgitation, or embolization into the left ventricular cavity have been observed (15,16). The different valve systems have specific requirements regarding aortic annulus size, height and width of the aortic sinuses, as well as dimensions of the sinotubular junction. In addition, knowledge about the relationship between aortic valve leaflet height and distance between the insertion of the left/right coronary cusp and the coronary ostia is important to avoid coronary complications.
The relatively large delivery catheters currently required for insertion of the crimped valve are associated with the risk of vascular complications. It is therefore important to evaluate the size of the ilio-femoral or subclavian arteries, vessel calcification, and tortuosity. Recent developments of lower profile delivery systems (≤18-F sheaths, outer diameter of approximately 7 mm) are expected to reduce vascular complications and expand patient eligibility for the procedure.
Image Acquisition
For dedicated cardiovascular imaging, CT systems with at least 64-detector technology are recommended. The spatial resolution of these systems is approximately 0.5 to 0.6 mm, which is sufficient for the anatomic evaluation of the aortic root and the iliofemoral arteries. However, the temporal resolution of current systems (about 75 ms for dual-source systems and >135 ms for single source scanners) is limited in comparison to echocardiography. CT imaging is associated with the administration of iodinated contrast and exposure to ionizing radiation exposure. Risk and benefit should be considered for individual patients (17,18).
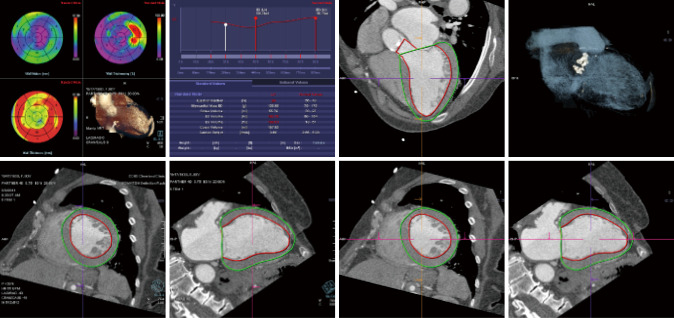
The specific scan protocols used for TAVI assessment vary, but typically include imaging of the entire aorta including the aortic root, thoraco-abdominal aorta, and iliofemoral arteries. Depending on the desired detail of the valve/root images, this information is acquired with standard aortic protocols or separate standard aortic and high-resolution (4-D) root acquisitions. Because of the cyclic motion of the aortic valve, root, and ascending aorta, ECG-synchronized imaging of the root is critical to avoid image degradation secondary to motion artifacts. Two-options to achieve synchronization to the ECG are available, retrospective gating and prospective triggering. ECG-synchronization with retrospective gating acquires CT data throughout the entire cardiac cycle, and subsequently reconstructs images gated to a specific phase of the cardiac cycle. Reconstructions in specific phases are described in percent relative to the position in the R-R interval, e.g., a systolic 30%-phase for valve area measurements and annular assessment corresponding to echocardiographic guidelines. Retrospective gating also allows 4-D image reconstruction of multiple adjacent reconstructions along the cardiac cycle displayed as cine-loops for evaluation of valvular and ventricular function (Figure 1, movies 1,2) (19,20).
Figure 1.
Valvular and LV function: retrospective gated image acquisition allows 4-D image reconstruction of multiple adjacent reconstructions along the cardiac cycle displayed as cine-loops for evaluation of valvular and LV ventricular function. This figure shows images of LV analysis (left panel) and images focused on the mitral valve
Movie 1.
Retrospective gated image acquisition allows 4-D image reconstruction of multiple adjacent reconstructions along the cardiac cycle displayed as cine-loops for evaluation of valvular function. This movie shows the star-shaped opening of a tricuspid valve
Movie 2.
In contrast to Movie 1, this movie shows an example of a bicuspid valve with fusion of the non- and right coronary cusp and eccentric, slit-like opening
Alternatively, ECG-synchronization can be achieved with prospective triggering, which is associated with significantly lower radiation exposure (21). With this acquisition mode, images are acquired only during a limited, pre-specified phase of the cardiac cycle (e.g. systole), with the tube turned off in-between. Therefore, reconstruction in other phases or 4-D cine-loops cannot be reconstructed. However, novel protocols with second-generation dual-source scanners allow prospective acquisition with a wider window and subsequent display of cine-loops (22).
While radiation exposure is important to consider with any CT acquisition, it is less a concern in the elderly patient populations currently considered for TAVI (18). Nonetheless, advanced scanner technology and imaging protocols, allowing lower dose imaging should be used (23). Such advances include dual-source and volume scanners (256-320 slice), imaging with prospective triggering, high-pitch helical acquisition, and novel image reconstruction with iterative image reconstruction techniques (24,25).
In the frail patient populations currently considered for TAVI, it is critically important to weigh risk and benefit of contrast administration. Contrast is necessary for precise luminal diameter measurements of the annulus, aorta, and iliofemoral arteries. The amount of contrast depends on the specific protocol. A standard contrast-enhanced scan of the aorta uses commonly a volume of 80-120 mL. However, lower contrast volume protocols are being tested for reducing contrast-associated side effects. A scan of the pelvic arteries after intra-arterial contrast injection into the infrarenal abdominal aorta (left in place after coronary catheterization) can be performed with low-dose (15 mL) of contrast (26). If contrast administration is not feasible, a non-contrast scan still allows the assessment of overall vessel size, calcification, and tortuosity.
Pre-medication with beta-blocker or nitrates, which is standard for coronary CTA angiography, should be avoided in patients with severe AS at the time of scanning, in order to avoid haemodynamic complications. With modern scanner systems, reliable imaging of the aortic root, aorta, and iliofemoral arteries is possible even in patients with higher heart rates.
Imaging and tavi
Because of the lack of exposure and visualization of the operative field, transcatheter valvular procedures rely on image guidance for patient selection, pre-procedural planning, and intra-operative decision-making. In the context of TAVI, anatomic measurements of the aortic annulus, aortic root, aortic valve, coronary ostia, and vascular access site are of critical importance. Conventional angiography and echocardiography are essential prior and during the procedure. In addition, novel 3-dimensional imaging approaches, including computed tomography, magnetic resonance imaging, 3-D echocardiography, and C-arm CT are increasingly used (27-31).
The role and relative importance of these imaging modalities is evolving. Multidetector computed tomography (CT) has assumed an increasingly important, complementary role before and after TAVI (32). It provides detailed anatomic assessment of the aortic root structures, the course of the descending aorta and the iliofemoral access, adding to the information obtained with echocardiography and angiography. Therefore routine screening with multidetector computed tomography (MDCT) is routinely performed in most large-volume centers for TAVI.
Image reconstruction
CT image acquisition results in (isotropic) 3-dimensional image data. The entire 3-D data can be reviewed in shaded, volume-rendered images giving depth perception (32). However, for measurement purposes the data is reconstructed along precisely defined 2D imaging planes, including those typically used in echocardiography or angiography. The strength of such 3-D-derived data analysis is the reconstruction along and perpendicular to the center axis of e.g., the aortic root or LV or the centerline of a vessel. These ‘double-oblique’ reconstructions allow precise diameter measurements, orthogonal to the axis of the structure. In particular in non-circular structures, e.g. the oval-shaped annulus, such 3-D derived diameter measurements allow to identify minimal and maximal diameter, circumference, and area. Importantly, these diameter measurements are not limited by the acquisition plane, as 2-D derived images from angiography and echocardiography are. Although, the differences between 2-D and 3-D derived measurements appear to be small, such differences might impact device selection and outcome in a subgroup of patients. However, to date most current clinical recommendations are based on 2-D imaging with echocardiography and angiography and the incremental value of 3-D derived data is incompletely understood (27). Several groups increasingly rely on additional 3-D derived CT-measurements for device selection and sizing. A particular interesting area in imaging research is the finite element analysis of clinical imaging data, for device development and simulation of device implantation (33,34).
Annulus
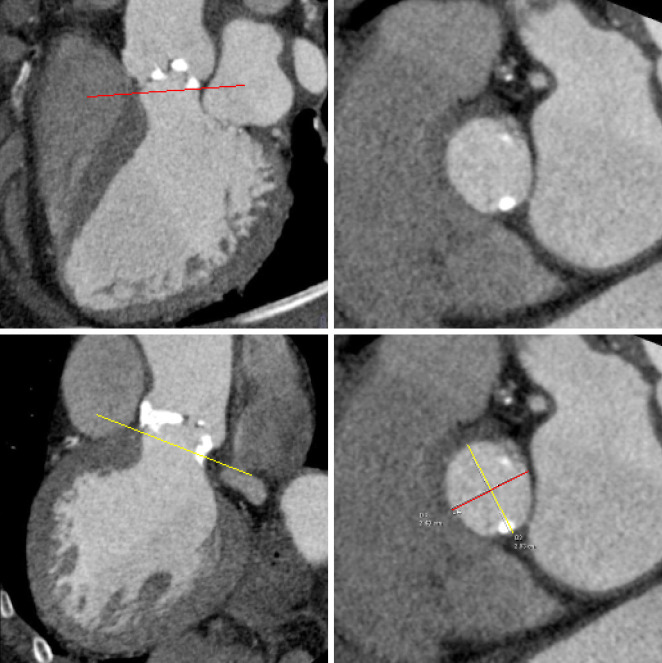
Dedicated analysis and measurement of the annulus is important for correct selection of prosthesis size and type and to avoid damage of the annulus (15,16,35). Anatomically, the annulus is a crown-shaped 3-dimensional structure rather than a circular plane. The attachment of the aortic cusps is semi-lunar, extending throughout the aortic root from the left ventricle distally to the sinotubular junction. For clinical purposes, the annulus is defined as the plane at the lowest insertion point of the aortic valve leaflets, just above the left ventricular outflow tract (LVOT) (‘basal ring’) (Figure 2), (36,37).
Figure 2.
Annulus Measurements: this figure demonstrates measurements at the annulus. For imaging, the annulus is defined at the level immediately below the lowest insertion point of the aortic leaflets. Measurements are taken from the phase with maximum valve opening. In this plane minimal, maximal, and mean diameter are described, with the mean diameter having the best correlation with echocardiography
Annulus measurements with 2 dimensional (2-D) transthoracic or transesophageal echocardiography, and aortic angiography provide a single diameter measurement, assuming a circular annular orifice (38). In contrast, 3-dimensional CT reconstruction of the annulus orthogonal to the center-axis of the LVOT allows for the assessment of minimal and maximal diameter, circumference, and area measurements. The mean of the maximum and minimum diameter measurements is closest to 2-D measurement with echocardiography (27,36,37,39). In analogy to echocardiography, measurements are taken from systolic phase reconstructions at about 30% of the R-R interval, using the phase with maximum valve opening (40). Recent papers describe the geometric change of the annulus during the cardiac cycle (41).
Despite the theoretical advantage of 3-D measurements as performed by CT (42), most experience and clinical recommendations regarding patient eligibility and sizing of the prosthesis is currently based upon measurements with TEE and angiography (27). Considering and comparing measurements from both TEE and MDCT may reduce the chance for error and provide a more comprehensive definition of the annular size and shape in patients considered for TAVI.
Coronary ostia
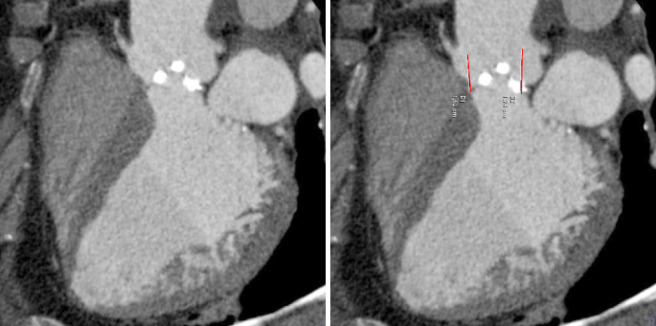
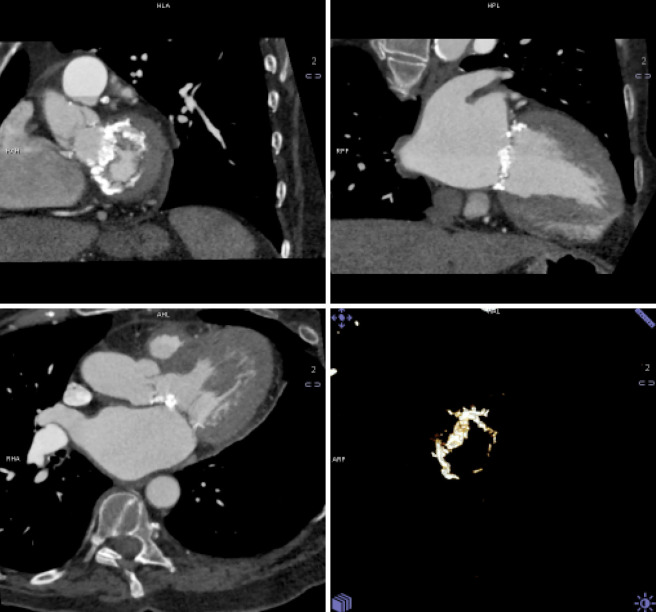
The pre-procedural evaluation for TAVI includes a complete assessment of coronary anatomy with conventional coronary angiography. Complete coronary assessment with CT is limited in the current population evaluated for TAVI because of the nearly universal presence of advanced calcified disease, which preclude precise assessment of luminal stenosis. However, CT allows to assess the relationship between leaflet height and distance between annulus and coronary ostia, which identifies patients at risk for coronary occlusion during the TAVI procedure. (Figure 3, Movie 3)
Figure 3.
Coronary Ostia: CT allows to assess the relationship between leaflet height and distance between annulus and coronary ostia, which identifies patients at risk for coronary occlusion during the TAVI procedure. This figure demonstrated measurements of the distance between the annulus and ostia of the left main (LM) and right coronary artery (RCA)
Movie 3.
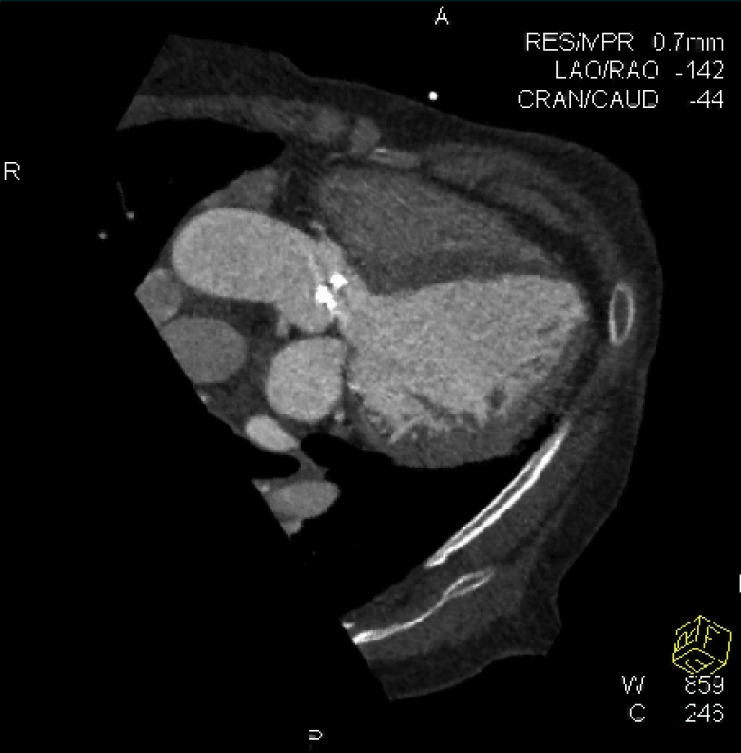
Coronary ostia and relationship to valve leaflets: CT allows to assess the relationship between leaflet height and distance between annulus and coronary ostia, which identifies patients at risk for coronary occlusion during the TAVI procedure. The movie files demonstrate cine-loops reconstructed at multiple phases of the cardiac cycle. This movie shows the relationship between the coronary ostia and the valve leaflets
While no definite criteria exist to exclude patients, a distance <10 mm distance may identify increased risk (43). In these patients, peri-deployment placement of a guidewire in the left main should be considered to ensure access in case of complications.
Aortic valve
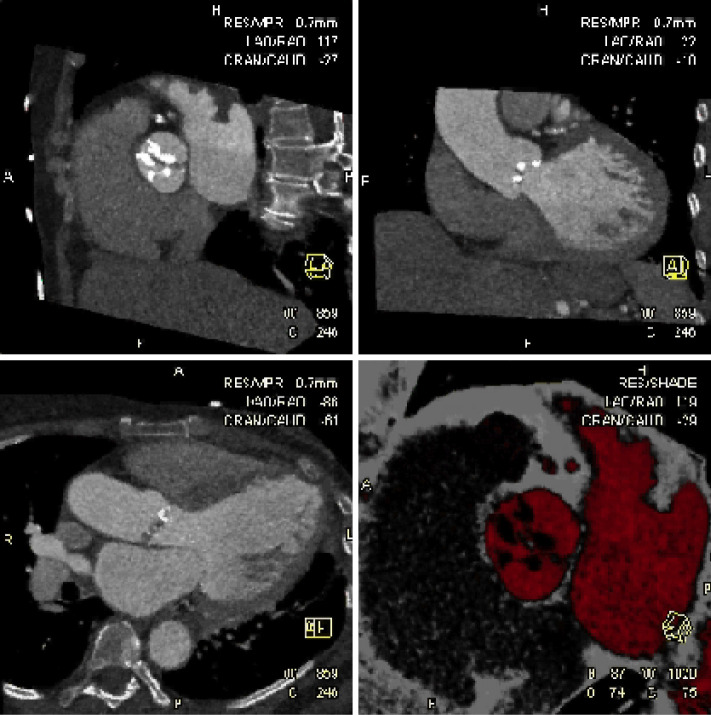
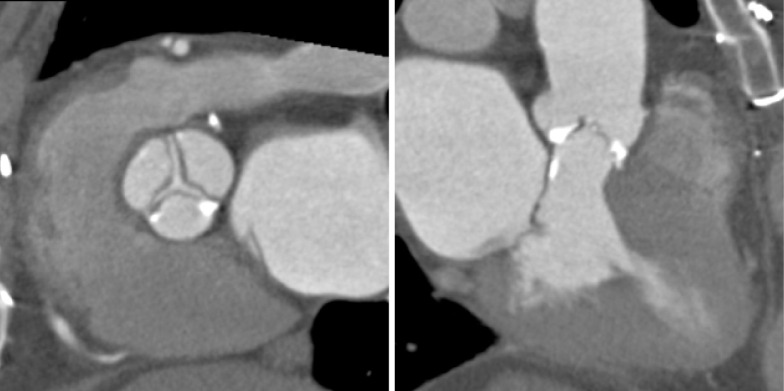
The aortic valve can be assessed in detail with CT. On single systolic reconstructions, and better on 4-D cineloops, the presence of a tricuspid valve can be confirmed. A tricuspid valve is characterized by symmetric leaflet height and star-shaped systolic opening versus a bicuspid valve with eccentric slit-like opening (Figures 4,5; Movies 1,2).
Figure 4.
Aortic Valve Area: detailed analysis of data in reconstructions along the cardiac cycle allows to identify the systolic phase with maximum valve opening (typically 20-30% RR-interval). In this reconstruction, a plane perpendicular to the valve plane is placed at the leaflet tips to measures the aortic valve opening area (AVA) by planimetry. This figure shows the star-shaped opening of a tricuspid valve
Figure 5.
Bicuspid Aortic Valve: in contrast to Figure 4, this figure shows an example of a bicuspid valve with fusion of the non- and right coronary cusp and eccentric, slit-like opening. A systolic (left panel) and diastolic (right panel) reconstruction is shown
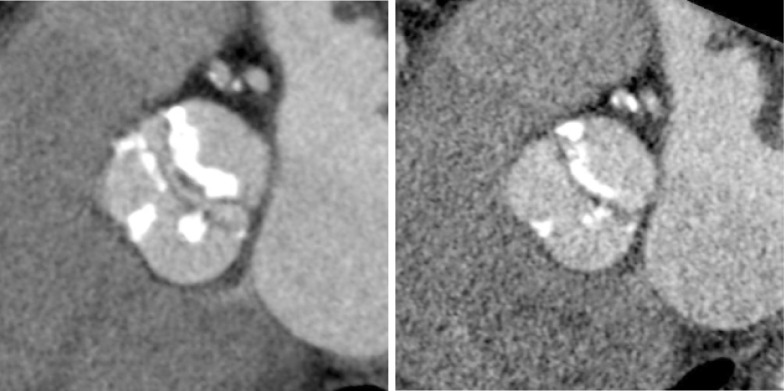
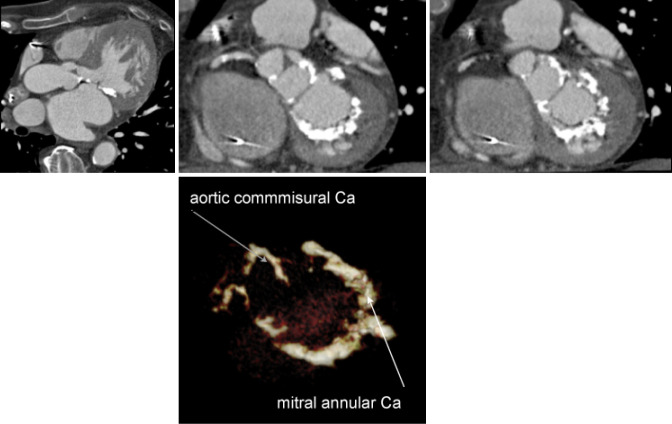
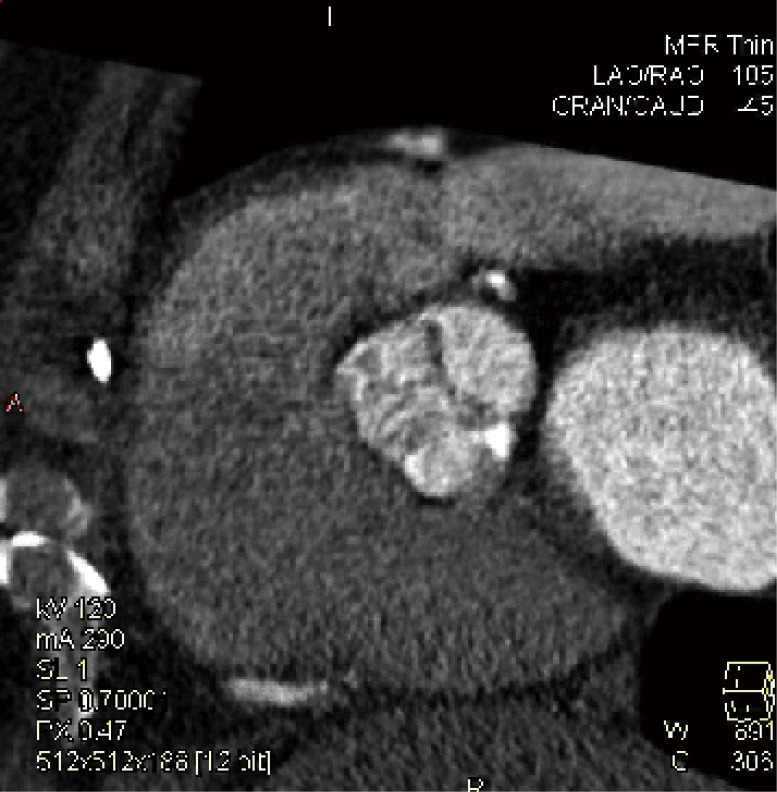
While CT is limited by blooming artifact from dense calcification, CT allows precise localization of calcification, e.g. extending from the commissure to the base of the anterior mitral leaflet and the mitral annulus (Figures 6,7).
Figure 6.
Aortic Valve Commisure Calcification: while CT is limited by blooming artifact from dense calcification, CT allows quantification and precise localization of calcification. The relationship between the calcification and postprocedural aortic regurgitation are unclear
Figure 7.
Mitral Annular Calcification: CT allows precise localization of mitral annular calcification. The impact of mitral annular calcification remains unclear
A non-contrast acquisition allows quantitative assessment of the aortic valve calcium score (44). Several investigators have started to investigate the potential relationship between aortic valve calcification and postprocedural aortic regurgitation and also potential impact or mitral annular calcification. However more data are needed before its impact on the TAVI procedure is fully understood (45,46).
For planimetry of the AVA, detailed analysis of data in reconstructions along the cardiac cycle allows to identify the systolic phase with maximum valve opening (typically 20-30% RR-interval). In this reconstruction, a plane perpendicular to the valve plane is placed at the leaflet tips to measures the aortic valve opening area (AVA) by planimetry. (42,47) Functional valvular assessment with CT is possible and demonstrates the relationship between valve calcification and leaflet motion.
Aortic dimensions and plaque
Measurement of the aortic sinus diameter, sinotubular junction, ascending and descending aorta are performed using centerline reconstructions. The presence of significant aneurysmal dilatation is considered a contraindication for TAVI. The extent of atherosclerotic plaque (48) is likely associated with complications including stroke (49).
Root angulation
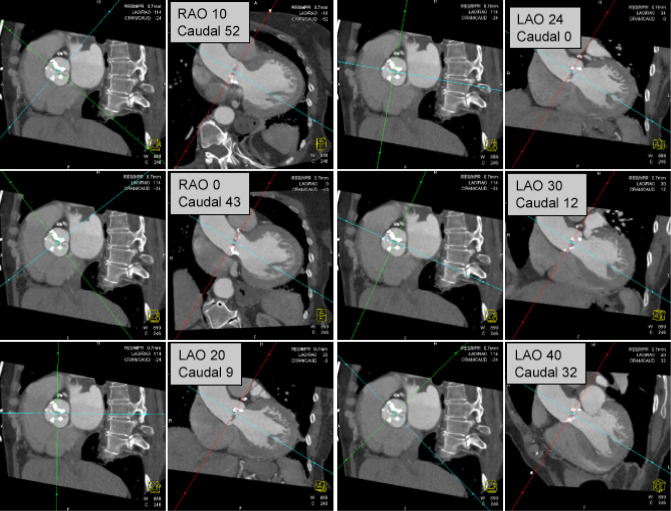
Precise coaxial alignment of the stent-valve along the centerline of the aortic valve and aortic root is important during positioning. Inappropriate alignment of the device is associated with increased risk of procedural complications such as stent embolization (15,16,35). Root orientation is typically assessed using multiple repeat catheter aortograms in 1 or 2 orthogonal planes before starting the procedure or during the pre-procedural diagnostic angiogram. Typically a caudal angulation is chosen when in a right anterior oblique (RAO) projection and cranial angulation when in the left anterior oblique (LAO) projection. However, there are significant variations in patient anatomy. MDCT allows to assess the aortic root in relation to the body axis (50,51). Double oblique transverse multiplanar reconstructions are performed at the level of the root and then are rotated through a series of any angles (Figure 8).
Figure 8.
Root Angulation: for the description of the aortic valve plane, double oblique transverse multiplanar reconstructions at the level of the aortic root are performed and rotated through a series of any angles. The right hand images show images perpendicular to the valve plane corresponding to angiographic images
Pre-procedural angle prediction with MDCT may decrease the number of aortograms required during the procedure, therefore shortening both procedure time and contrast usage, and potentially increases the likelihood of coaxial implantation by optimizing the orientation during device placement.
Iliofemroral arteries
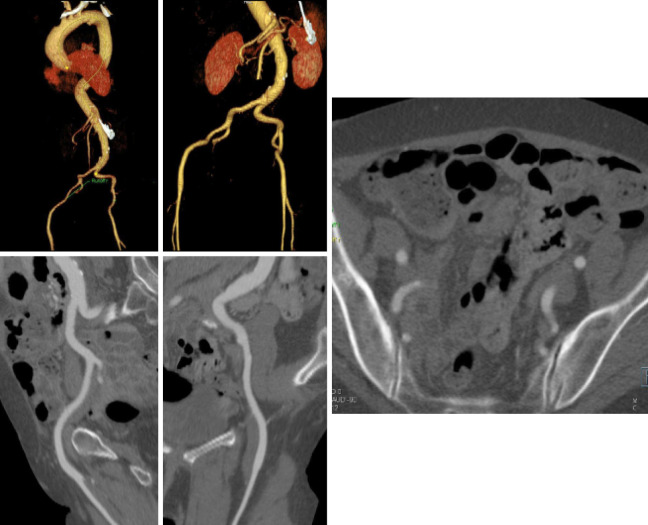
Because of the relatively large diameter of the delivery sheaths (≥18F), appropriate vascular access is critical. Small luminal diameter, dense and circumferential and/or horseshoe calcification, and severe tortuosity are common in this patient population and increase the risk of access site complications (Figure 9) and central embolization.
Figure 9.
Iliac Arteries: at least 1/3 of patients with critical aortic stenosis have unfavorable iliofemoral arteries. Centerline image processing of the iliac arteries (left panels) and cross-sectional images at the different levels allow assessment of dimensions, calcification, and angulation (right)
Kurra et al. (52) reported that at least 1/3 of patients with critical aortic stenosis had unfavorable iliofemoral arteries, with the majority of those patients having minimal luminal diameters of 8 mm. In such patient alternative access approaches may include surgical sidegraft on the iliac arteries, transaxillary, or transapical access.
Postprocedural imaging
Postprocedural imaging relies primarily on echocardiography, which allows assessment of the valve and valvular dysfunction. CT allows to assess position of the stent-valve and its relationship to the annulus and coronary artery (47).
Conclusion
Because of the lack of exposure and visualization of the operative field, transcatheter valvular procedures rely on image guidance for patient selection, pre-procedural planning, and intra-operative decision-making. Anatomic measurements of the aortic annulus, aortic root, aortic valve, coronary ostia, and vascular access site are of critical importance. The role and relative importance of different imaging modalities is evolving. Multidetector computed tomography (CT) has assumed an increasingly important, complementary role before and after TAVI, and provides detailed anatomic assessment of the aortic root structures and iliofemoral access, adding to the information obtained with echocardiography and angiography. Therefore routine screening with multidetector computed tomography (MDCT) is utilized by several leading groups.
However, further evaluation of the clinical impact of MDCT and other 3-D modalities is necessary, anticipating future growth of transcatheter procedures. Similar to recent guidelines developed for the procedures itself (53), this evaluation of the role of imaging should be guided by international consensus.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet 2006;368:1005-11. [DOI] [PubMed] [Google Scholar]
- 2.Bonow RO, Carabello BA, Chatterjee K, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing Committee to Revise the 1998 guidelines for the management of patients with valvular heart disease) developed in collaboration with the Society of Cardiovascular Anesthesiologists endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol 2006;48:e1-148. [DOI] [PubMed] [Google Scholar]
- 3.Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J 2003;24:1231-43. [DOI] [PubMed] [Google Scholar]
- 4.Kapadia SR, Goel SS, Svensson L, et al. Characterization and outcome of patients with severe symptomatic aortic stenosis referred for percutaneous aortic valve replacement. J Thorac Cardiovasc Surg 2009;137:1430-5. [DOI] [PubMed] [Google Scholar]
- 5.Tornos P, Iung B, Permanyer-Miralda G, Baron G, Delahaye F, Gohlke-Bärwolf Ch, et al. Infective endocarditis in Europe: lessons from the Euro heart survey. Heart 2005;91:571-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597-607. [DOI] [PubMed] [Google Scholar]
- 7.Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011;364:2187-98. [DOI] [PubMed] [Google Scholar]
- 8.Grube E, Schuler G, Buellesfeld L, et al. Percutaneous aortic valve replacement for severe aortic stenosis in high-risk patients using the second- and current third-generation self-expanding CoreValve prosthesis: device success and 30-day clinical outcome. J Am Coll Cardiol 2007;50:69-76. [DOI] [PubMed] [Google Scholar]
- 9.Tamburino C, Capodanno D, Ramondo A, et al. Incidence and predictors of early and late mortality after transcatheter aortic valve implantation in 663 patients with severe aortic stenosis. Circulation 2011;123:299-308. [DOI] [PubMed] [Google Scholar]
- 10.Gurvitch R, Wood DA, Tay EL, et al. Transcatheter aortic valve implantation: durability of clinical and hemodynamic outcomes beyond 3 years in a large patient cohort. Circulation 2010;122:1319-27. [DOI] [PubMed] [Google Scholar]
- 11.Petronio AS, De Carlo M, Bedogni F, et al. Safety and efficacy of the subclavian approach for transcatheter aortic valve implantation with the CoreValve revalving system. Circ Cardiovasc Interv 2010;3:359-66. [DOI] [PubMed] [Google Scholar]
- 12.Svensson LG, Dewey T, Kapadia S, et al. United States feasibility study of transcatheter insertion of a stented aortic valve by the left ventricular apex. Ann Thorac Surg 2008;86:46-54;discussion 54-5. [DOI] [PubMed] [Google Scholar]
- 13.Walther T, Simon P, Dewey T, et al. Transapical minimally invasive aortic valve implantation: multicenter experience. Circulation 2007;116:I240-5. [DOI] [PubMed] [Google Scholar]
- 14.Grube E, Naber C, Abizaid A, et al. Feasibility of transcatheter aortic valve implantation without balloon pre-dilation a pilot study. JACC Cardiovasc Interv 2011;4:751-7. [DOI] [PubMed] [Google Scholar]
- 15.Al Ali AM, Altwegg L, Horlick EM, et al. Prevention and management of transcatheter balloon-expandable aortic valve malposition. Catheter Cardiovasc Interv 2008;72:573-8. [DOI] [PubMed] [Google Scholar]
- 16.Tuzcu EM. Transcatheter aortic valve replacement malposition and embolization: innovation brings solutions also new challenges. Catheter Cardiovasc Interv 2008;72:579-80. [DOI] [PubMed] [Google Scholar]
- 17.Bagur R, Webb JG, Nietlispach F, et al. Acute kidney injury following transcatheter aortic valve implantation: predictive factors, prognostic value, and comparison with surgical aortic valve replacement. Eur Heart J 2010;31:865-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Einstein AJ, Henzlova MJ, Rajagopalan S. Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. JAMA 2007;298:317-23. [DOI] [PubMed] [Google Scholar]
- 19.Chenot F, Montant P, Goffinet C, et al. Evaluation of anatomic valve opening and leaflet morphology in aortic valve bioprosthesis by using multidetector CT: comparison with transthoracic echocardiography. Radiology 2010;255:377-85. [DOI] [PubMed] [Google Scholar]
- 20.Feuchtner GM, Alkadhi H, Karlo C, et al. Cardiac CT angiography for the diagnosis of mitral valve prolapse: comparison with echocardiography1. Radiology 2010;254:374-83. [DOI] [PubMed] [Google Scholar]
- 21.Husmann L, Valenta I, Gaemperli O, et al. Feasibility of low-dose coronary CT angiography: first experience with prospective ECG-gating. Eur Heart J 2008;29:191-7. [DOI] [PubMed] [Google Scholar]
- 22.Feuchtner G, Goetti R, Plass A, et al. Dual-step prospective ECG-triggered 128-slice dual-source CT for evaluation of coronary arteries and cardiac function without heart rate control: a technical note. Eur Radiol 2010;20:2092-9. [DOI] [PubMed] [Google Scholar]
- 23.Halliburton SS, Schoenhagen P. Cardiovascular imaging with computed tomography: responsible steps to balancing diagnostic yield and radiation exposure. JACC Cardiovasc Imaging 2010;3:536-40. [DOI] [PubMed] [Google Scholar]
- 24.Rybicki FJ, Otero HJ, Steigner ML, et al. Initial evaluation of coronary images from 320-detector row computed tomography. Int J Cardiovasc Imaging 2008;24:535-46. [DOI] [PubMed] [Google Scholar]
- 25.Leipsic J, Labounty TM, Heilbron B, et al. Estimated radiation dose reduction using adaptive statistical iterative reconstruction in coronary CT angiography: the ERASIR study. AJR Am J Roentgenol 2010;195:655-60. [DOI] [PubMed] [Google Scholar]
- 26.Joshi SB, Mendoza DD, Steinberg DH, et al. Ultra-low-dose intra-arterial contrast injection for iliofemoral computed tomographic angiography. JACC Cardiovasc Imaging 2009;2:1404-11. [DOI] [PubMed] [Google Scholar]
- 27.Messika-Zeitoun D, Serfaty JM, Brochet E, et al. Multimodal assessment of the aortic annulus diameter: implications for transcatheter aortic valve implantation. J Am Coll Cardiol 2010;55:186-94. [DOI] [PubMed] [Google Scholar]
- 28.Ng AC, Delgado V, van der Kley F, et al. Comparison of aortic root dimensions and geometries before and after transcatheter aortic valve implantation by 2- and 3-dimensional transesophageal echocardiography and multislice computed tomography. Circ Cardiovasc Imaging 2010;3:94-102. [DOI] [PubMed] [Google Scholar]
- 29.Wood DA, Tops LF, Mayo JR, et al. Role of multislice computed tomography in transcatheter aortic valve replacement. Am J Cardiol 2009;103:1295-301. [DOI] [PubMed] [Google Scholar]
- 30.Siegel RJ, Luo H, Biner S. Transcatheter valve repair/implantation. Int J Cardiovasc Imaging 2011;27:1165-77. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz JG, Neubauer AM, Fagan TE, et al. Potential role of three-dimensional rotational angiography and C-arm CT for valvular repair and implantation. Int J Cardiovasc Imaging 2011;27:1205-22. [DOI] [PubMed] [Google Scholar]
- 32.Schoenhagen P, Numburi U, Halliburton SS, et al. Three-dimensional imaging in the context of minimally invasive and transcatheter cardiovascular interventions using multi-detector computed tomography: from pre-operative planning to intra-operative guidance. Eur Heart J 2010;31:2727-40. [DOI] [PubMed] [Google Scholar]
- 33.Wang Q, Book G, Contreras Ortiz SH, Primiano, et al. Dimensional Analysis of Aortic Root Geometry During Diastole Using 3D Models Reconstructed from Clinical 64-Slice Computed Tomography Images. Cardiovascular Engineering and Technology 2011. DOI: 10.1007/s13239-011-0052-8Online First™ [DOI] [Google Scholar]
- 34.Schoenhagen P, Hill A, Kelley T, et al. In vivo imaging and computational analysis of the aortic root. Application in clinical research and design of transcatheter aortic valve systems. J Cardiovasc Transl Res 2011;4:459-69. [DOI] [PubMed] [Google Scholar]
- 35.Bleiziffer S, Ruge H, Hörer J, et al. Predictors for new-onset complete heart block after transcatheter aortic valve implantation. JACC Cardiovasc Interv 2010;3:524-30. [DOI] [PubMed] [Google Scholar]
- 36.Tops LF, Wood DA, Delgado V, et al. Noninvasive evaluation of the aortic root with multislice computed tomography implications for transcatheter aortic valve replacement. JACC Cardiovasc Imaging 2008;1:321-30. [DOI] [PubMed] [Google Scholar]
- 37.Akhtar M, Tuzcu EM, Kapadia SR, et al. Aortic root morphology in patients undergoing percutaneous aortic valve replacement: evidence of aortic root remodeling. J Thorac Cardiovasc Surg 2009;137:950-6. [DOI] [PubMed] [Google Scholar]
- 38.Moss RR, Ivens E, Pasupati S, et al. Role of echocardiography in percutaneous aortic valve implantation. JACC Cardiovasc Imaging 2008;1:15-24. [DOI] [PubMed] [Google Scholar]
- 39.Ng AC, Delgado V, van der Kley F, et al. Comparison of aortic root dimensions and geometries before and after transcatheter aortic valve implantation by 2- and 3-dimensional transesophageal echocardiography and multislice computed tomography. Circ Cardiovasc Imaging 2010;3:94-102. [DOI] [PubMed] [Google Scholar]
- 40.Bahlmann E, Nienaber CA, Cramariuc D, et al. Aortic root geometry in aortic stenosis patients (a SEAS substudy). Eur J Echocardiogr 2011;12:585-90. [DOI] [PubMed] [Google Scholar]
- 41.de Heer LM, Budde RP, Mali WP, et al. Aortic root dimension changes during systole and diastole: evaluation with ECG-gated multidetector row computed tomography. Int J Cardiovasc Imaging 2011;27:1195-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Brien B, Schoenhagen P, Kapadia SR, et al. Integration of Three-Dimensional Imaging Data in the Assessment of Aortic Stenosis: Impact on Classification of Disease Severity. Circ Cardiovasc Imaging 2011 Jul 7.[Epub ahead of print] [DOI] [PubMed]
- 43.Masson JB, Kovac J, Schuler G, et al. Transcatheter aortic valve implantation: review of the nature, management, and avoidance of procedural complications. JACC Cardiovasc Interv 2009;2:811-20. [DOI] [PubMed] [Google Scholar]
- 44.Cueff C, Serfaty JM, Cimadevilla C, et al. Measurement of aortic valve calcification using multislice computed tomography: correlation with haemodynamic severity of aortic stenosis and clinical implication for patients with low ejection fraction. Heart 2011;97:721-6. [DOI] [PubMed] [Google Scholar]
- 45.Latsios G, Gerckens U, Buellesfeld L, et al. “Device landing zone” calcification, assessed by MSCT, as a predictive factor for pacemaker implantation after TAVI. Catheter Cardiovasc Interv 2010;76:431-9. [DOI] [PubMed] [Google Scholar]
- 46.John D, Buellesfeld L, Yuecel S, et al. Correlation of Device landing zone calcification and acute procedural success in patients undergoing transcatheter aortic valve implantations with the self-expanding CoreValve prosthesis. JACC Cardiovasc Interv 2010;3:233-43. [DOI] [PubMed] [Google Scholar]
- 47.Delgado V, Ng AC, van de Veire NR, et al. Transcatheter aortic valve implantation: role of multi-detector row computed tomography to evaluate prosthesis positioning and deployment in relation to valve function. Eur Heart J 2010;31:1114-23. [DOI] [PubMed] [Google Scholar]
- 48.Kurra V, Lieber ML, Sola S, et al. Extent of thoracic aortic atheroma burden and long-term mortality after cardiothoracic surgery: a computed tomography study. JACC Cardiovasc Imaging 2010;3:1020-9. [DOI] [PubMed] [Google Scholar]
- 49.Ghanem A, Müller A, Nähle CP, et al. Risk and fate of cerebral embolism after transfemoral aortic valve implantation: a prospective pilot study with diffusion-weighted magnetic resonance imaging. J Am Coll Cardiol 2010;55:1427-32. [DOI] [PubMed] [Google Scholar]
- 50.Kurra V, Kapadia SR, Tuzcu EM, et al. Pre-procedural imaging of aortic root orientation and dimensions: comparison between X-ray angiographic planar imaging and 3-dimensional multidetector row computed tomography. JACC Cardiovasc Interv 2010;3:105-13. [DOI] [PubMed] [Google Scholar]
- 51.Gurvitch R, Wood DA, Leipsic J, et al. Multislice computed tomography for prediction of optimal angiographic deployment projections during transcatheter aortic valve implantation. JACC Cardiovasc Interv 2010;3:1157-65. [DOI] [PubMed] [Google Scholar]
- 52.Kurra V, Schoenhagen P, Roselli EE, et al. Prevalence of significant peripheral artery disease in patients evaluated for percutaneous aortic valve insertion: Preprocedural assessment with multidetector computed tomography. J Thorac Cardiovasc Surg 2009;137:1258-64. [DOI] [PubMed] [Google Scholar]
- 53.Leon MB, Piazza N, Nikolsky E, et al. Standardized endpoint definitions for transcatheter aortic valve implantation clinical trials: a consensus report from the Valve Academic Research Consortium. Eur Heart J 2011;32:205-17. [DOI] [PMC free article] [PubMed] [Google Scholar]