Abstract
Purpose
Intravascular ultrasound (IVUS) and optical coherence tomography (OCT) define neointima as the tissue encompassed between the stent and the lumen boundaries. This approach differs from the gold-standard histopathology, where neointima is traditionally calculated as the tissue between the internal elastic lamina (IEL) and the lumen. We aimed to investigate whether the neointimal assessment using IVUS and OCT-like definitions would correlate with the traditional histopathological quantification of neointima.
Methods
Histopathological analysis was obtained from a porcine model of 28-day coronary in-stent neointimal proliferation (n=13 bare stents). Traditional histopathology neointimal area (NIHPATH area) was calculated as the lumen area minus the IEL area, while the percent neointimal obstruction was defined as NIHPATH area divided by the IEL area. The IVUS/OCT-like neointima area (NIHIVUS/OCT area) was defined as the lumen area minus the stent area, while the percent neointimal obstruction was defined as NIHIVUS/OCT area divided by the stent area.
Results
The neointimal area as well as the percent obstruction were significantly correlated between histopathology and IVUS/OCT-like definitions (R2=0.89 and 0.95 respectively; P<0.01 for both). The average absolute difference between the IVUS/OCT-like and the pathology-like measurements was close to zero, however with a relatively wide dispersion (difference for neointimal area: 0.41 mm2 [95% CI 1.72 to –0.90 mm2]; difference for percent neointimal obstruction: 2.5% [95% CI 11.5% to –6.5%]).
Conclusions
The present findings support the use of stent area in replacement to IEL area, as in IVUS & OCT imaging protocols, for the calculation of neointimal parameters in experimental model of restenosis.
Key Words: Pathology, restenosis, intravascular ultrasound, optical coherence tomography
Introduction
In-stent restenosis has long been recognized as one of the most important factors limiting the long-term efficacy of coronary stent implantation. Different from post-balloon restenosis, where both negative vessel remodeling and neointimal proliferation contribute to decrease the lumen size, the late luminal reduction after stenting is basically related to neointimal tissue growth. Therefore, several interventional strategies have been tested over the last years in an attempt to inhibit neointima and ultimately decrease the risk of in-stent restenosis.
The continuing introduction of novel anti-restenosis technologies has triggered the need to develop diagnostic methods to quantify neointimal proliferation. Intravascular ultrasound (IVUS) is largely used for the in vivo quantification of in-stent neointima, both in experimental and in clinical studies. Also, optical coherence tomography (OCT) has been increasingly utilized to quantitatively evaluate in-stent neointimal tissue accumulation (1).
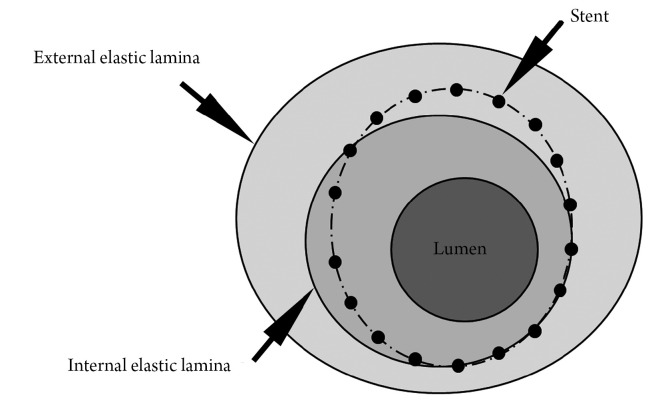
Both IVUS and OCT define neointima as the tissue encompassed between the stent and the lumen boundaries (Figure 1). However, although globally accepted in the literature, this definition differs from the criteria commonly used in histopathological analysis (2,3), the current gold-standard for the quantification of in-stent neointima. In histopathology, neointima is normally defined as the tissue encompassed between the limits of the internal elastic lamina (IEL) and the lumen (Figure 1). In this study, therefore, we aimed to investigate in pathological specimens whether the neointimal assessment using IVUS/OCT-like definitions would correlate with the traditional histopathological quantification of neointima.
Figure 1.
Schematic representation of an artery cross-section. The lumen, stent, internal elastic lamina (IEL), and external elastic lamina boundaries are traced. Note that the stent and the IEL borders are not coincident in this illustration. Neointima hyperplasia area, as traditionally defined by histopathology, is calculated as the lumen area minus the IEL area (NIHPATH area), while the traditional histopathological percent neointimal obstruction is defined as NIHPATH area divided by the IEL area. The IVUS/OCT-like neointima area (NIHIVUS/OCT area) is defined as the lumen area minus the stent area, while the IVUS/OCT-like percent neointimal obstruction is defined as the NIHIVUS/OCT area divided by the stent area
Methods
A total of 9 juvenile domestic pigs (25 to 35 kg) were used in the present study and underwent implantation of one or more coronary stents under fluoroscopic guidance. Animals were pre-anesthetized with bromazepan and thiopental based on animal weight. After orotracheal intubation, the anesthetic plane was maintained with inhaled isoflurane. An arterial sheath (6 French) was placed in the right common femoral artery by cut-down with general sterile technique. Intracoronary manipulation was performed after the administration of intravenous heparin (7,500 IU) and intracoronary nitroglycerin (200 µg). Six pigs received 1 stent, 2 pigs received 2 stents, and one pig received 3 stents, summing up a total of 13 cobalt-chromium bare metal stents (Cronus™, Scitech, Goiania, Brazil) implanted. Stents were placed in the left anterior descending, circumflex, or right coronary artery (one stent per vessel) according to a randomization chart. All stents were slightly oversized to induce a moderate vessel injury, aiming to obtain a 1.1:1 to 1.2:1 stent-to-artery ratio, compared with the reference vessel diameter. All animals were pre-treated with aspirin and clopidogrel, respectively maintained for 7 days or until euthanasia at 28 days. After completion of the 28-day control angiography, the animals under deep anesthesia were euthanized with a lethal dose of potassium chloride. The experiments were in compliance with the Animal Welfare Act and the Principles of Laboratory Animal Care, and the study protocol was approved in the Institution’s ethics committee.
Histopathological processing and analysis
Immediately after euthanasia, the hearts were excised and the coronaries were pressure-perfused (~80 mmHg) via ascending aorta with 0.9% saline, for clearing of blood, followed by 10% buffered formalin for a minimum of 1 hour. The stented arterial segments were then excised from the heart by dissection and fixed by immersion in 10% formalin overnight. The artery-stent specimens were dehydrated with ethanol solutions of increasing concentrations and embedded in methyl methacrylate resin. A total of three cross-sections (proximal, mid, and distal within the stented segment) were obtained from each vessel on a rotary microtome (cut thickness 3 to 6 µm) and stained with hematoxylin and eosin and Verhoeff stains. All sections were considered of diagnostic quality.
For each cross-section, the degree of arterial injury at the site of stent struts was graded according to the methods proposed by Schwartz et al. (4,5) and Gunn et al. (6). Microscopic digital morphometry was used to measure the external elastic lamina (EEL), IEL, and lumen areas. Neointima hyperplasia area, as traditionally defined by histopathology (NIHPATH area), and traditional histopathological percent neointimal obstruction (NIHPATH percent) were defined as (Figure 1):
| NIHPATH area=lumen area–IEL area |
| NIHPATH percentdxs=100 × (NIHPATH area/IEL area) |
The IVUS/OCT-like neointima area (NIHIVUS/OCT area) and the IVUS/OCT-like percent neointimal obstruction (NIHIVUS/OCT percent) were calculated as (Figure 1):
| NIHIVUS/OCT area=lumen area–stent area |
| NIHIVUS/OCT percent=100 × (NIHIVUS/OCT area/stent area) |
It is therefore clear that the divergence among the definitions above is restricted to the fact that IVUS/OCT-like calculations use the stent area instead of the IEL area (as used in histopathology studies).
Statistical analysis
Continuous variables were presented as means and standard deviations. The relationships between variables were assessed by linear modeling, and the strength of the relationship expressed by the corresponding model R2 correlation coefficient. Because the main objective was to compare different neointimal metrics in the same cross-sectional slice, each cut was considered as the unit for analysis and no adjustment for a clustering or intra-individual effect was applied. A priori, a P value <0.05 was considered to be significant. The method proposed by Bland and Altman (7,8) was utilized to evaluate the differences between the traditional histopathological and IVUS/OCT-like measurements. In Bland and Altman’s method, the average of the two measurements is plotted against the difference between them. In the Bland-Altman graphs, the continued lines represent the mean difference in measurements and the traced lines represent the 95% confidence interval (95% CI) for the difference in measurements. All analysis and graphs were generated by the SPSS 13.0 for Windows statistical package (SPSS Inc, Chicago, USA).
Results
The quantitative histomorphometric parameters are shown in Table 1. On average, the stent area was slightly larger than the IEL area, and thus the resulting NIHIVUS/OCT area was larger than the NIHPATH area. Also, the NIHIVUS/OCT percent was larger than the NIHPATH percent. When analyzing each individual cross-section, the measured stent area was larger than the IEL area in 69% but smaller in the remaining 31% of the slides.
Table 1. Histomorphometric parameters.
| Parameter | Average ± SD |
|---|---|
| Lumen area, mm2 | 4.01±1.46 |
| IEL area, mm2 | 6.70±1.31 |
| EEL area, mm2 | 8.42±1.64 |
| Stent area, mm2 | 7.11±1.62 |
| NIHPATH area, mm2 | 2.69±1.43 |
| NIHPATH percent, % | 39.9±18.8 |
| NIHIVUS/OCT area, mm2 | 3.10±1.82 |
| NIHIVUS/OCT percent, % | 42.4±19.5 |
| Difference between Stent and IEL areas, mm2 | 0.41±0.67 |
| Ratio between Stent and IEL areas | 1.06±0.10 |
| Difference between NIHIVUS/OCT and NIHPATH areas, mm2 | 0.41±0.67 |
| Difference between NIHIVUS/OCT percent and NIHPATH percent, % | 2.5±4.6 |
EEL=external elastic lamina; IEL=internal elastic membrane; NIHIVUS/OCT area=IVUS/OCT-like neointima area (lumen area minus the stent area); NIHIVUS/OCT percent=IVUS/OCT-like percent neointimal obstruction (NIHIVUS/OCT area divided by the stent area); NIHPATH area=neointima area traditionally defined by histopathology (lumen area minus IEL area); NIHPATH percent=traditional histopathological percent neointimal obstruction (NIHPATH area divided by IEL area)
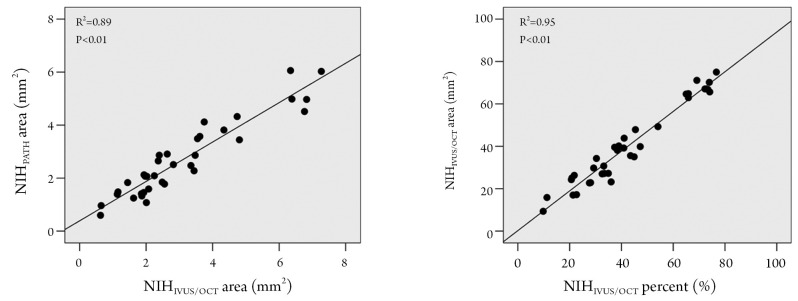
The neointimal area measured according to IVUS/OCT definition was significantly correlated with the neointima by the traditional pathological definition (R2=0.89; P<0.01) (Figure 2). Even more markedly, the percent neointimal obstruction by IVUS/OCT definition also significantly correlated with the percent obstruction by the traditional pathological criterion (R2 =0.95; P<0.01) (Figure 2).
Figure 2.
Correlations between measurements by IVUS/OCT and traditional pathological definitions. In the left panel, correlation graph between neointimal areas, measured according to IVUS/OCT definition and traditional pathological definition. In the right panel, correlation graph of percent neointimal obstruction by IVUS/OCT definition versus the percent obstruction by the traditional pathological criterion (for definitions see Figure 1)
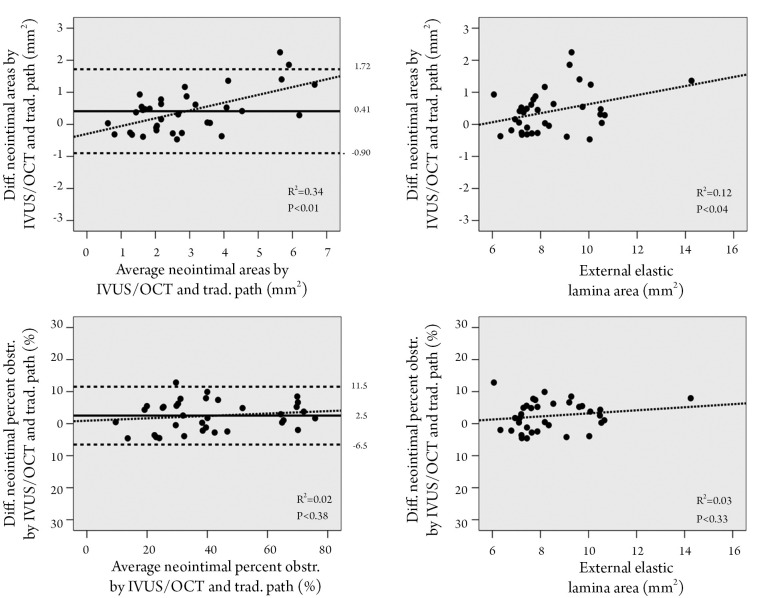
The average absolute difference between the IVUS/OCT-like and the pathology-like measurements was close to zero, however, a relatively wide dispersion of the differences was observed, particularly for the neointimal area measurements (difference for neointimal area: 0.41±0.67 mm2 [95% CI 1.72 to –0.90 mm2]; difference for percent neointimal obstruction: 2.5±4.6% [95% CI 11.5% to –6.5%]) (Table 1 and Figure 3). The difference in measurements of neointima area tended to increase with increasing vessel sizes (Figure 3). Conversely, the difference in percent neointimal obstruction between the IVUS/OCT-like and the pathology-like definitions remained constant across the range of vessels sizes (Figure 3).
Figure 3.
Analysis of the differences between measurements by IVUS/OCT and traditional pathological definitions. A. Bland and Altman graphs to evaluate the differences between the traditional histopathological and IVUS/OCT-like measurements for neointimal area (upper panel) and for percent neointimal obstruction (lower panel). The average of the two measurements is plotted against the difference between them. Continued lines represent the mean difference in measurements and the horizontal traced lines represent the 95% confidence interval for the difference in measurements. The additional traced line represents the correlation between the variables; B. Difference between the IVUS/OCT-like and the pathology-like measurements for neointimal area (upper panel) and for percent neointimal obstruction (lower panel), as a function of vessel size (inferred by the external elastic lamina area. The additional traced line represents the correlation between the variables (for definitions see Figure 1)
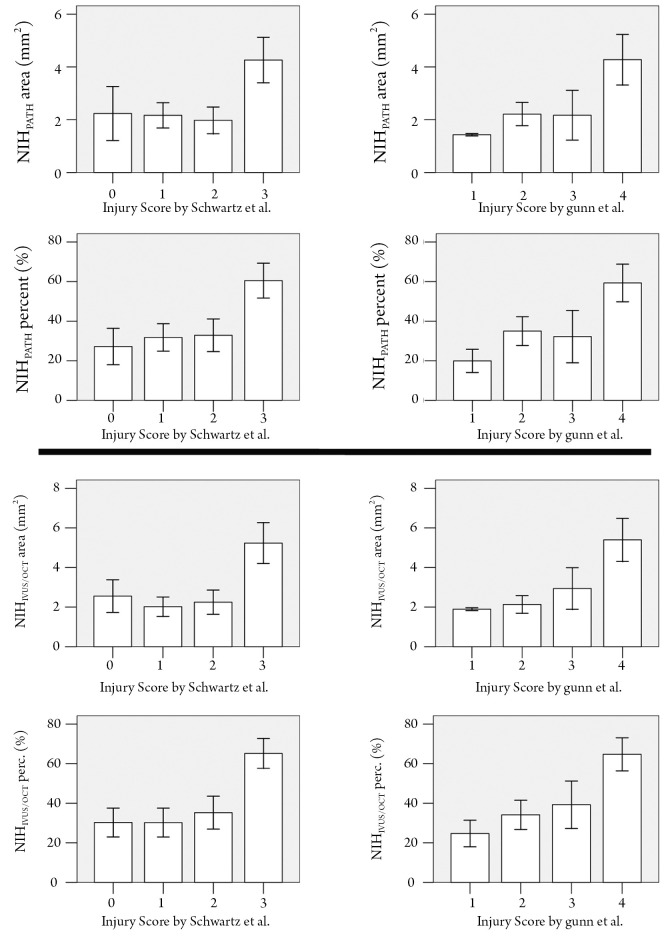
The degree of neointimal proliferation (area and percent obstruction) defined according to the traditional histopathological definitions significantly increased with increasing levels of vessel injury (Figure 4). Similarly, the amount of neointimal proliferation, quantified following the IVUS/OCT-like criteria, was positively related with the vessel injury scoring (Figure 4).
Figure 4.
Association between the degree of neointimal hyperplasia and the intensity of vessel injury. Neointimal parameters (area and percent obstruction) defined according to the usual histopathological definitions are shown in the upper panels and neointimal proliferation, quantified following the IVUS/OCT-like criteria, are shown in the lower panels. Vessel injury was semiquantified by the Schwartz et al. (4,5) and by the Gunn et al. (6) scoring systems (P<0.01 for all comparisons). For definitions see Figure 1. Bars are ± 2 standard errors
Discussion
The gold-standard histopathological analysis defines in-stent neointima as the interval encompassed by the internal elastic lamina and the lumen boundaries, as seen in vessel cross-sections. In IVUS and OCT imaging, the internal elastic lamina cannot be identified and the stent outline is used as a substitute for the calculation of neointimal parameters. In the present study, we show that the quantitative assessment of in-stent neointimal proliferation using IVUS & OCT-like definitions (i.e. replacing the measurement of IEL by the stent tracings) closely resembles the measurements from histopathological analysis. In particular, the percent neointimal obstruction calculated according to IVUS/OCT definitions followed an almost perfect correlation with the traditional pathological measurements (R2=0.95; P<0.01). Moreover, for both parameters, the average absolute difference between the measurements was close to zero. However, the some outliers were observed and resulted in a relatively wide range of the differences in measurements, particularly for the neointimal area.
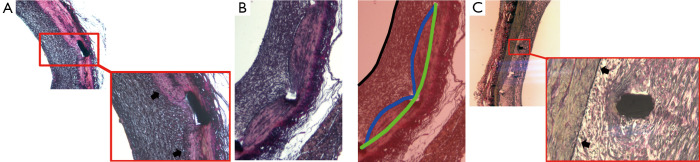
In our study, the stent area was larger than the internal elastic lamina area in approximately two thirds of all cross-sections. In practice, this difference in measurement might occur whenever the stent struts are deeply located beyond the intima layer (e.g., in sites with laceration of the IEL) (Figure 5A) or in areas where the media layer is compressed by the stent (Figure 5B). On the other hand, in one third of cross-sections, the measured stent area was smaller than the internal elastic lamina area, mostly as a consequence of non-apposition of the stent struts to the vessel wall after implantation (Figure 5C).
Figure 5.
Examples of discrepancies between the stent and the IEL boundaries. As illustrated in Figure 5A, increased stent areas occur where the stent struts are deeply located beyond the intima layer, with laceration of the IEL (in the detail, the intact IEL is indicated by black arrows). Also, Figure 5B shows an example where the stent area is larger than the IEL area due to compression of the media layer by the stent (the right panel depicts a schematic representation of the boundaries of the lumen [black line], IEL [blue line], and the stent [green line]). Conversely, the measured stent area may be smaller than the IEL area in regions of non-apposition of the stent struts to the vessel wall after implantation (Figure 5C)
In fact, the current analysis used the stent and lumen boundaries in histopathological vessel cuts to “simulate” the neointimal measurements that would be correspond to IVUS and OCT assessment. Crucial to the implications of our findings is the ability of IVUS and OCT to reliably reproduce both the stent and lumen borders. A recent comparative study has shown that OCT consistently overestimated the luminal and stent areas, compared with histology (9). Also, even though neointimal area and luminal area strictly correlated with pathology, the correlation for stent area was disappointing (9). Therefore, although our findings do support the use of stent area instead of IEL area for the calculation of neointimal parameters, the currently available technologies for intravascular imaging still seem to need fine tuning in terms of their capacity to quantify some vessel dimensions.
Previous histopathological studies have shown that the degree of neointimal proliferation is proportional to the level of vessel injury induced by the interventional procedure (4-6). Importantly, the present findings show that IVUS/OCT-like neointimal parameters also have a markedly significant relationship with vascular injury scoring, demonstrating that the biological information was not compromised by the change in definitions.
Overall, our findings indicate that IVUS/OCT-like neointimal parameters may be used instead of the traditional histopathology definitions to quantify neointimal area, in the context of normal vessels with experimentally induced neointimal proliferation. However, it must be understood that the present results cannot be directly extrapolated to the clinical practice. In theory, the discrepancies between IVUS/OCT-like and traditional histopathology neointimal parameters may be exacerbated by the presence, at the site of stent deployment, of atherosclerotic plaques with varying degrees of eccentricity, morphologies, tissue composition and calcification, or side branches.
Importantly, although good candidates to infer the degree of neointimal proliferation, neither IVUS nor OCT are complete substitutes for the histopathological analysis. The evaluation of vessel trauma, as well as other morphological parameters (e.g., inflammation, fibrin deposition, neovascularization, etc.) is not possible with IVUS and OCT, in the current stage of the development of their technologies. Particularly for pre-clinical validation studies, where safety assessment is generally pursued as a central point of interest, histopathology remains as the undisputable gold-standard analytic tool.
Conclusion
The quantitative assessment of in-stent neointimal proliferation using IVUS & OCT-like definitions (i.e. replacing the measurement of IEL by the stent tracings) closely correlates with the measurements obtained from traditional histopathological criteria. The present findings support the use of stent area in replacement to IEL area, as in IVUS & OCT imaging protocols, for the calculation of neointimal parameters in experimental studies.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Murata M, Matsubara Y, Kawano K, et al. Coronary artery disease and polymorphisms in a receptor mediating shear stress-dependent platelet activation. Circulation 1997;96:3281-6. [DOI] [PubMed] [Google Scholar]
- 2.Ahn YK, Jeong MH, Kim JW, et al. Preventive effects of the heparin-coated stent on restenosis in the porcine model. Catheter Cardiovasc Interv 1999;48:324-30. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz RS, Edelman E, Virmani R, et al. Drug-eluting stents in preclinical studies: updated consensus recommendations for preclinical evaluation. Circ Cardiovasc Interv 2008;1:143-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwartz RS, Edelman ER, Carter A, et al. Drug-eluting stents in preclinical studies: recommended evaluation from a consensus group. Circulation 2002;106:1867-73. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz RS, Chronos NA, Virmani R. Preclinical restenosis models and drug-eluting stents: still important, still much to learn. J Am Coll Cardiol 2004;44:1373-85. [DOI] [PubMed] [Google Scholar]
- 6.Gunn J, Arnold N, Chan KH, et al. Coronary artery stretch versus deep injury in the development of in-stent neointima. Heart 2002;88:401-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307-10. [PubMed] [Google Scholar]
- 8.Bland JM, Altman DG. Comparing methods of measurement: why plotting difference against standard method is misleading. Lancet 1995;346:1085-7. [DOI] [PubMed] [Google Scholar]
- 9.Murata A, Wallace-Bradley D, Tellez A, et al. Accuracy of optical coherence tomography in the evaluation of neointimal coverage after stent implantation. JACC Cardiovasc Imaging 2010;3:76-84. [DOI] [PubMed] [Google Scholar]