Abstract
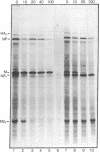
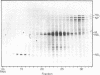
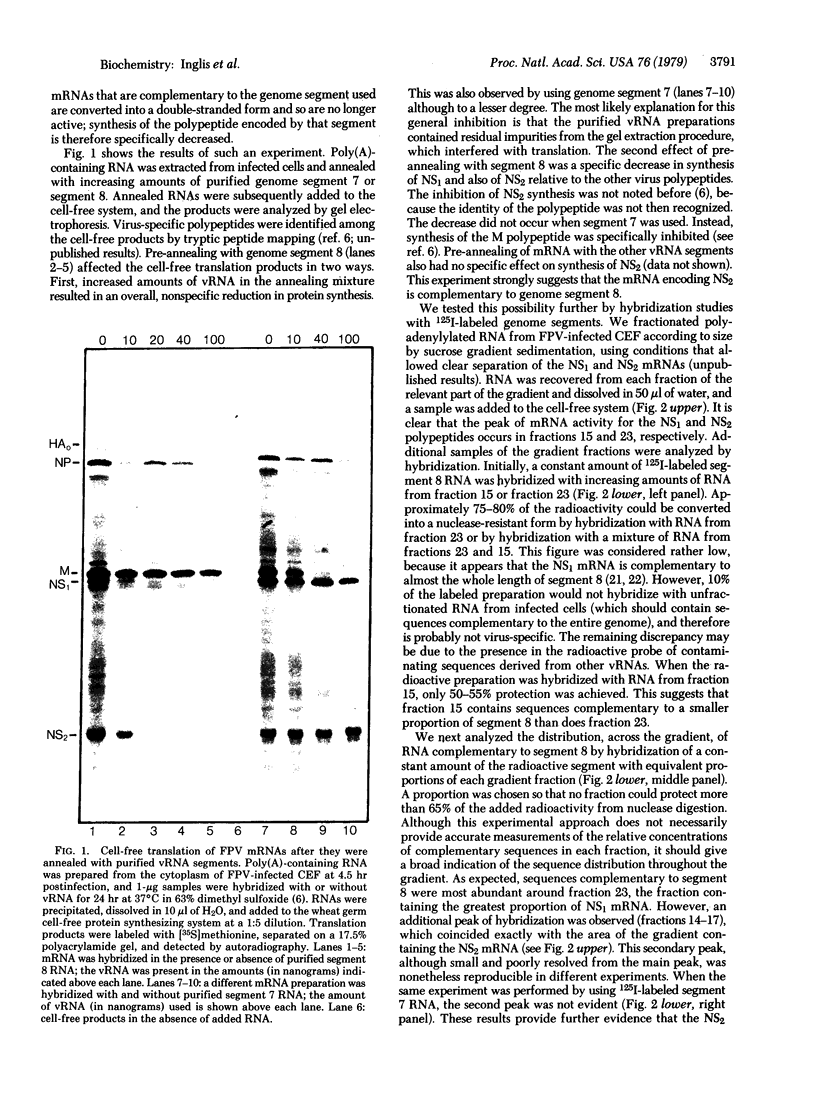
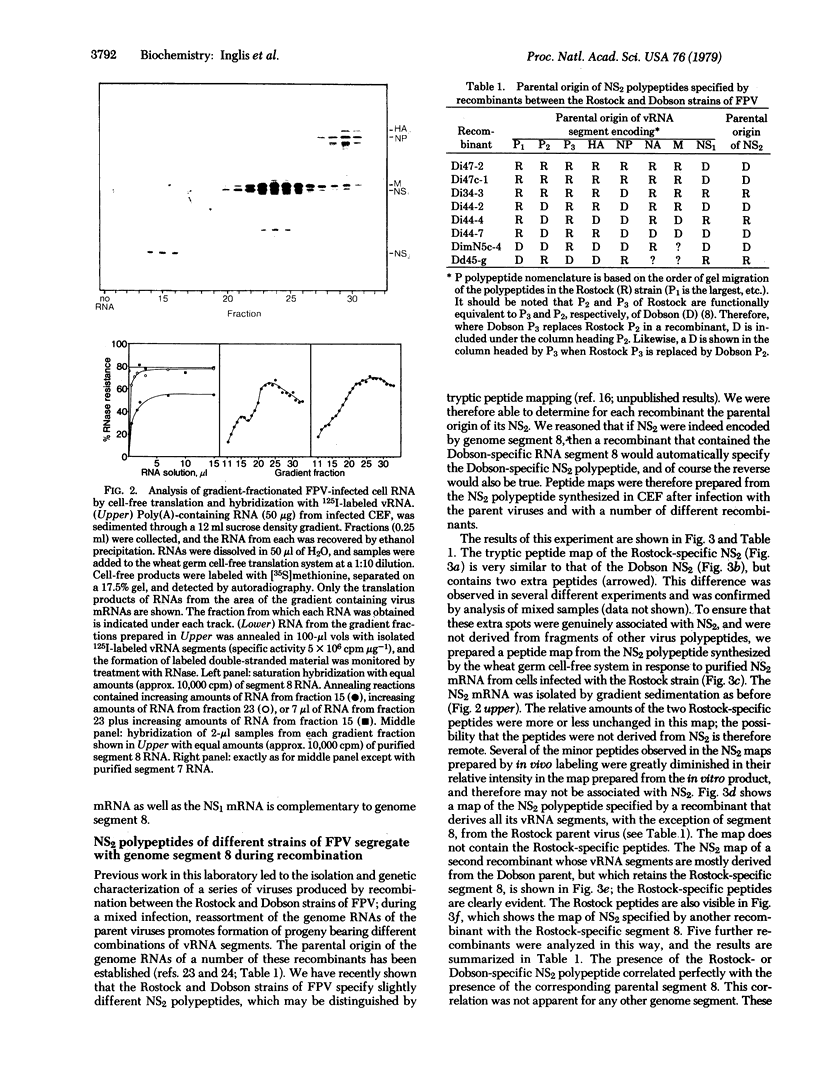
The genome of influenza virus consists of eight segments of single-stranded RNA, each of which encodes a different polypeptide. In addition to the eight recognized gene products, the virus specifies a distinct smaller nonstructural polypeptide (NS2), which is translated from a separate species of virus-specific mRNA. The location on the virus genome of the gene encoding this polypeptide was investigated by hybridization of the NS2 mRNA with isolated subgenomic RNA species, and by correlation of the inheritance of a strain-specific NS2 with inheritance of particular genome RNA segments during recombination between two different virus strains. The genetic information for NS2 was found to reside in the smallest genome RNA segment of the virion, which also encodes the NS1 polypeptide. Considering the sizes of the molecules involved, it is likely that the coding sequences for the two polypeptides overlap.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almond J. W. A single gene determines the host range of influenza virus. Nature. 1977 Dec 15;270(5638):617–618. doi: 10.1038/270617a0. [DOI] [PubMed] [Google Scholar]
- Almond J. W., Barry R. D. Genetic recombination between two strains of fowl plague virus: construction of genetic maps. Virology. 1979 Jan 30;92(2):407–415. doi: 10.1016/0042-6822(79)90145-4. [DOI] [PubMed] [Google Scholar]
- Almond J. W., McGeoch D., Barry R. D. Temperature-sensitive mutants of fowl plague virus: isolation and genetic characterization. Virology. 1979 Jan 30;92(2):416–427. doi: 10.1016/0042-6822(79)90146-6. [DOI] [PubMed] [Google Scholar]
- Barrell B. G., Air G. M., Hutchison C. A., 3rd Overlapping genes in bacteriophage phiX174. Nature. 1976 Nov 4;264(5581):34–41. doi: 10.1038/264034a0. [DOI] [PubMed] [Google Scholar]
- Borland R., Mahy B. W. Deoxyribonucleic acid-dependent ribonucleic acid polymerase activity in cells infected with influenza virus. J Virol. 1968 Jan;2(1):33–39. doi: 10.1128/jvi.2.1.33-39.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratosin S., Horowitz M., Laub O., Aloni Y. Electron microscopic evidence for splicing of SV40 late mRNAs. Cell. 1978 Apr;13(4):783–790. doi: 10.1016/0092-8674(78)90228-3. [DOI] [PubMed] [Google Scholar]
- Cancedda R., Villa-Komaroff L., Lodish H. F., Schlesinger M. Initiation sites for translation of sindbis virus 42S and 26S messenger RNAs. Cell. 1975 Oct;6(2):215–222. doi: 10.1016/0092-8674(75)90012-4. [DOI] [PubMed] [Google Scholar]
- Chow L. T., Gelinas R. E., Broker T. R., Roberts R. J. An amazing sequence arrangement at the 5' ends of adenovirus 2 messenger RNA. Cell. 1977 Sep;12(1):1–8. doi: 10.1016/0092-8674(77)90180-5. [DOI] [PubMed] [Google Scholar]
- Contreras R., Rogiers R., Van de Voorde A., Fiers W. Overlapping of the VP2-VP3 gene and the VP1 gene in the SV40 genome. Cell. 1977 Oct;12(2):529–538. doi: 10.1016/0092-8674(77)90129-5. [DOI] [PubMed] [Google Scholar]
- Etkind P. R., Krug R. M. Purification of influenza viral complementary RNA: its genetic content and activity in wheat germ cell-free extracts. J Virol. 1975 Dec;16(6):1464–1475. doi: 10.1128/jvi.16.6.1464-1475.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass S. E., McGeoch D., Barry R. D. Characterization of the mRNA of influenza virus. J Virol. 1975 Dec;16(6):1435–1443. doi: 10.1128/jvi.16.6.1435-1443.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T. R., Hunt T., Knowland J., Zimmern D. Messenger RNA for the coat protein of tobacco mosaic virus. Nature. 1976 Apr 29;260(5554):759–764. doi: 10.1038/260759a0. [DOI] [PubMed] [Google Scholar]
- Inglis S. C., Carroll A. R., Lamb R. A., Mahy B. W. Polypeptides specified by the influenza virus genome I. Evidence for eight distinct gene products specified by fowl plague virus. Virology. 1976 Oct 15;74(2):489–503. doi: 10.1016/0042-6822(76)90355-x. [DOI] [PubMed] [Google Scholar]
- Inglis S. C., Mahy B. W. Polypeptides specified by the influenza virus genome. 3. Control of synthesis in infected cells. Virology. 1979 May;95(1):154–164. doi: 10.1016/0042-6822(79)90410-0. [DOI] [PubMed] [Google Scholar]
- Inglis S. C., McGeoch D. J., Mahy B. W. Polypeptides specified by the influenza virus genoma. 2. Assignement of protein coding functions to individual genome segments by in vitro translation. Virology. 1977 May 15;78(2):522–536. doi: 10.1016/0042-6822(77)90128-3. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Choppin P. W. Synthesis of influenza virus proteins in infected cells: translation of viral polypeptides, including three P polypeptides, from RNA produced by primary transcription. Virology. 1976 Oct 15;74(2):504–519. doi: 10.1016/0042-6822(76)90356-1. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Etkind P. R., Choppin P. W. Evidence for a ninth influenza viral polypeptide. Virology. 1978 Nov;91(1):60–78. doi: 10.1016/0042-6822(78)90355-0. [DOI] [PubMed] [Google Scholar]
- McGeoch D., Fellner P., Newton C. Influenza virus genome consists of eight distinct RNA species. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3045–3049. doi: 10.1073/pnas.73.9.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor P. D., Dimmock N. J. Inhibition of synthesis of influenza virus proteins: evidence of two host-cell-dependent events during multiplication. Virology. 1975 Sep;67(1):114–123. doi: 10.1016/0042-6822(75)90409-2. [DOI] [PubMed] [Google Scholar]
- Palese P., Schulman J. L. Differences in RNA patterns of influenza A viruses. J Virol. 1976 Mar;17(3):876–884. doi: 10.1128/jvi.17.3.876-884.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson T., Harvey R., Smith A. E. The size of Rous sarcoma virus mRNAs active in cell-free translation. Nature. 1977 Aug 4;268(5619):416–420. doi: 10.1038/268416a0. [DOI] [PubMed] [Google Scholar]
- Plotch S. J., Bouloy M., Krug R. M. Transfer of 5'-terminal cap of globin mRNA to influenza viral complementary RNA during transcription in vitro. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1618–1622. doi: 10.1073/pnas.76.4.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons M. W. A reexamination of influenza single-and double-stranded RNAs by gel electrophoresis. Virology. 1976 Feb;69(2):789–792. doi: 10.1016/0042-6822(76)90508-0. [DOI] [PubMed] [Google Scholar]
- Ritchey M. B., Palese P., Schulman J. L. Mapping of the influenza virus genome. III. Identification of genes coding for nucleoprotein, membrane protein, and nonstructural protein. J Virol. 1976 Oct;20(1):307–313. doi: 10.1128/jvi.20.1.307-313.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholtissek C., Harms E., Rohde W., Orlich M., Rott R. Correlation between RNA fragments of fowl plague virus and their corresponding gene functions. Virology. 1976 Oct 15;74(2):332–344. doi: 10.1016/0042-6822(76)90340-8. [DOI] [PubMed] [Google Scholar]
- Shaw D. C., Walker J. E., Northrop F. D., Barrell B. G., Godson G. N., Fiddes J. C. Gene K, a new overlapping gene in bacteriophage G4. Nature. 1978 Apr 6;272(5653):510–515. doi: 10.1038/272510a0. [DOI] [PubMed] [Google Scholar]
- Shih D. S., Kaesberg P. Translation of brome mosaic viral ribonucleic acid in a cell-free system derived from wheat embryo. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1799–1803. doi: 10.1073/pnas.70.6.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skehel J. J., Hay A. J. Nucleotide sequences at the 5' termini of influenza virus RNAs and their transcripts. Nucleic Acids Res. 1978 Apr;5(4):1207–1219. doi: 10.1093/nar/5.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skehel J. J. Polypeptide synthesis in influenza virus-infected cells. Virology. 1972 Jul;49(1):23–36. doi: 10.1016/s0042-6822(72)80004-7. [DOI] [PubMed] [Google Scholar]
- Smith A. E., Kamen R., Mangel W. F., Shure H., Wheeler T. Location of the sequences coding for capsid proteins VP1 and VP2 on polyoma virus DNA. Cell. 1976 Nov;9(3):481–487. doi: 10.1016/0092-8674(76)90093-3. [DOI] [PubMed] [Google Scholar]
- Stephenson J. R., Hay A. J., Skehel J. J. Characterization of virus-specific messenger RNAs from avian fibroblasts infected with fowl plague virus. J Gen Virol. 1977 Aug;36(2):237–248. doi: 10.1099/0022-1317-36-2-237. [DOI] [PubMed] [Google Scholar]